Abstract
Purpose of review
Urea is transported by urea transporter proteins in kidney, erythrocytes, and other tissues. Mice in which different urea transporters have been knocked-out have urine concentrating defects, which has led to the development and testing of UT-A and UT-B inhibitors as urearetics. This review summarizes the knowledge gained during the past year on urea transporter regulation and investigations into the clinical potential of urearetics.
Recent findings
UT-A1 undergoes several post-translational modifications that increase its function by increasing UT-A1 accumulation in the apical plasma membrane. UT-A1 is phosphorylated by PKA, Epac, PKCα, and AMPK, all at different serine residues. UT-A1 is also regulated by 14-3-3, which contributes to UT-A1 removal from the membrane. UT-A1 is glycosylated with various glycan moieties in animal models of diabetes mellitus. Transgenic expression of UT-A1 into UT-A1/UT-A3 knock-out mice restores urine concentrating ability. UT-B is present in descending vasa recta and urinary bladder, and is linked to bladder cancer. Inhibitors of UT-A and UT-B have been developed that result in diuresis with fewer abnormalities in serum electrolytes than conventional diuretics.
Summary
Urea transporters play critical roles in the urine concentrating mechanism. Urea transport inhibitors are a promising new class of diuretic agents.
Keywords: urea, urine concentrating mechanism, vasopressin, glucocorticoids, knock-out mice
Introduction
Urea is a small, highly polar molecule with low lipid solubility across artificial lipid bilayers (1). Although urea’s permeability across artificial lipid bilayers is quite low, it is not zero. A specific facilitated urea transport process was first proposed in kidney in 1987 (2). Subsequent physiologic studies led to the characterization of two families of urea transporters: Slc14A2 (UT-A) and Slc14A1 (UT-B) (reviewed in (3, 4)).
UT-A1 is the largest UT-A protein and is expressed in the apical plasma membrane in the inner medullary collecting duct (IMCD) (Figure 1). UT-A3 is also expressed in the IMCD, predominantly in the basolateral plasma membrane. UT-A2 is expressed in the thin descending limb (reviewed in (3, 4)). UT-B1 is expressed in the descending vasa recta and several extra-renal tissues. In people, UT-B1 protein is the Kidd antigen. UT-B2 mRNA is expressed in cow and sheep rumen (reviewed in (3, 4)).
Figure 1.
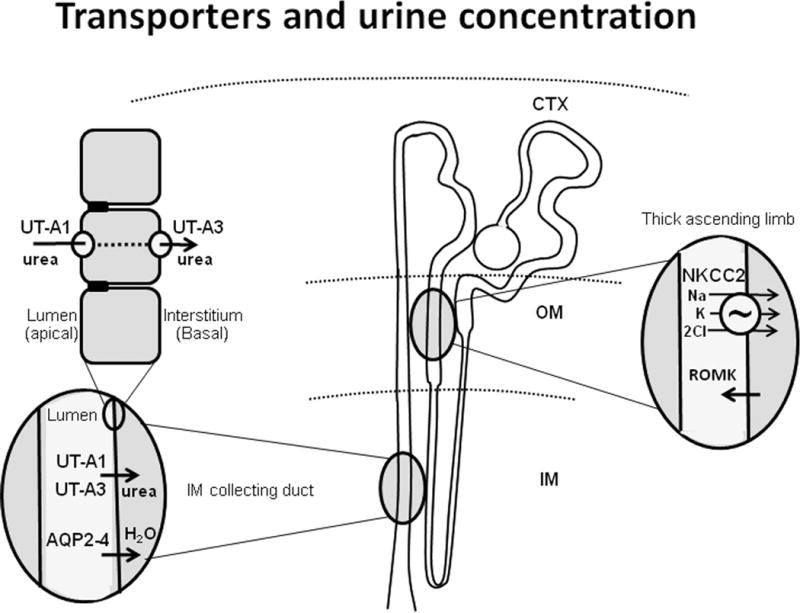
Diagram of transporters involved in urine concentration. In the center is a diagram of the nephron with cortex (CTX), outer medulla (OM) and inner medulla (IM) marked. Oval enlargements show locations of the sodium potassium 2 chloride cotransporter (NKCC2) and the renal outer medullary potassium channel (ROMK) in the thick ascending limb, aquaporins 2-4 (AQP2-4) in the inner medullary collecting duct (IMCD) and urea transporters (UT-A1 and UT-A3) in the IMCD. Top left enlargement shows IMCD cells with UT-A1 located apically and UT-A3 located basolaterally.
Urea transporters play an important role in the kidney under both normal and pathophysiological situations (reviewed in (3, 4)). Several knock-out mice lacking urea transporters, such as UT-A1 and UT-A3 (5–7), UT-A2 (8), UT-B1 (9–11), or UT-A2 and UT-B1 (12), have been generated and have decreased maximal urine concentrating ability, demonstrating that urea transporters play a major role in urinary concentration. They also raise the possibility that inhibition of urea transporters may be a target for the development of novel diuretics (urearetics).
In the past year, significant progress has been made in understanding the post-translational modifications that regulate UT-A1 in the IMCD and in the development and testing of UT-A and UT-B inhibitors as urearetics. This review summarizes the knowledge gained during the past year on urea transporter regulation and on the clinical potential of urearetics.
UT-A
Protein kinase A (PKA)
UT-A1 contains several consensus sites for phosphorylation (reviewed in (3, 4)) (Figure 2). Previous studies showed that S486 and S499 were PKA phosphorylation sites (13, 14). A polyclonal antibody was used to localize phospho-S486-UT-A1 to the apical plasma membrane in vasopressin-treated rat IMCD (15).
Figure 2.
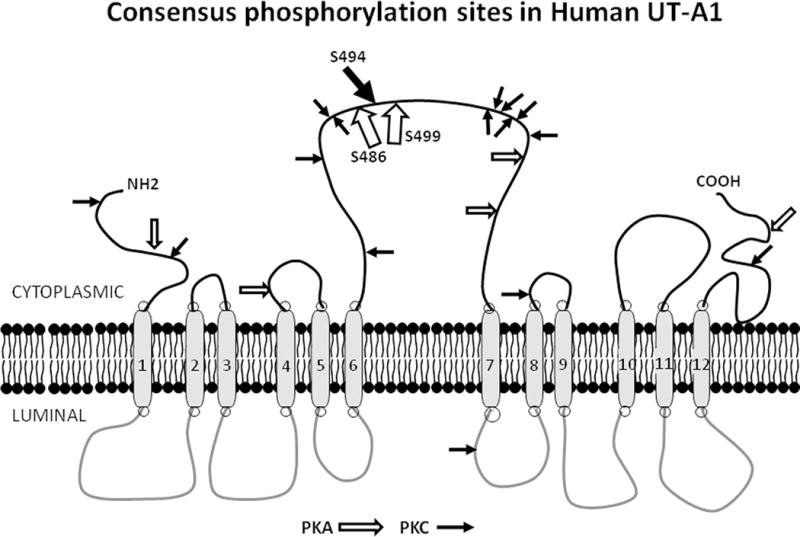
PKA and PKC consensus phosphorylation sites in UT-A1. This diagram presents UT-A1 in its theoretical 12 membrane spanning domain structure. Consensus phosphorylation sites for PKA are designated with open arrows; those for PKC are designated with closed arrows. The proven PKA (S486, S499) and PKC (Ser 494) sites are located in the large intracellular loop and designated by larger arrows.
These findings were recently extended by the generation of a polyclonal antibody to phospho-S499-UT-A1, which also permitted investigation into the relationship of these two PKA-sensitive sites (16). Like phospho-S486-UT-A1, phospho-S499-UT-A1 was primarily located to the apical plasma membrane, however, the two PKA phosphorylations were independent from one another (16). Finally, this study showed that activation of the Epac (exchange protein activated by cAMP) pathway, increases UT-A1 phosphorylation but not at either S486 or S499 (16).
Satavaptan, a selective inhibitor of the type 2 vasopressin receptor (V2R), was used in a phospho-proteomic study to determine a systems-wide analysis of the effect of vaptans (17). Satavaptan blocked V2R-mediated activation of basophilic kinases and V2R-mediated inhibition of proline-directed kinases (17). Thus, satavaptan affected many of the same signaling pathways that are affected by vasopressin (17).
The 14-3-3 proteins regulate protein function by binding to phosphorylated serine or threonine residues. 14-3-3γ was shown to bind to UT-A1, and activation of PKA enhanced this binding (18). 14-3-3γ decreased urea transport and increased UT-A1 ubiquitination and degradation by interacting with the E3 ubiquitin ligase, MDM2 (18). Thus, PKA activation increases UT-A1 phosphorylation, and the subsequent binding of 14-3-3γ leads to UT-A1 degradation. If these two opposing effects are separated temporally, they could provide a negative feedback mechanism to return UT-A1 function to its basal state following stimulation by vasopressin (18) (Figure 3). The data showing these opposite effects of PKA are established, but whether there is a negative feedback loop, or whether only one effect is dominant in vivo, remains to be determined.
Figure 3.
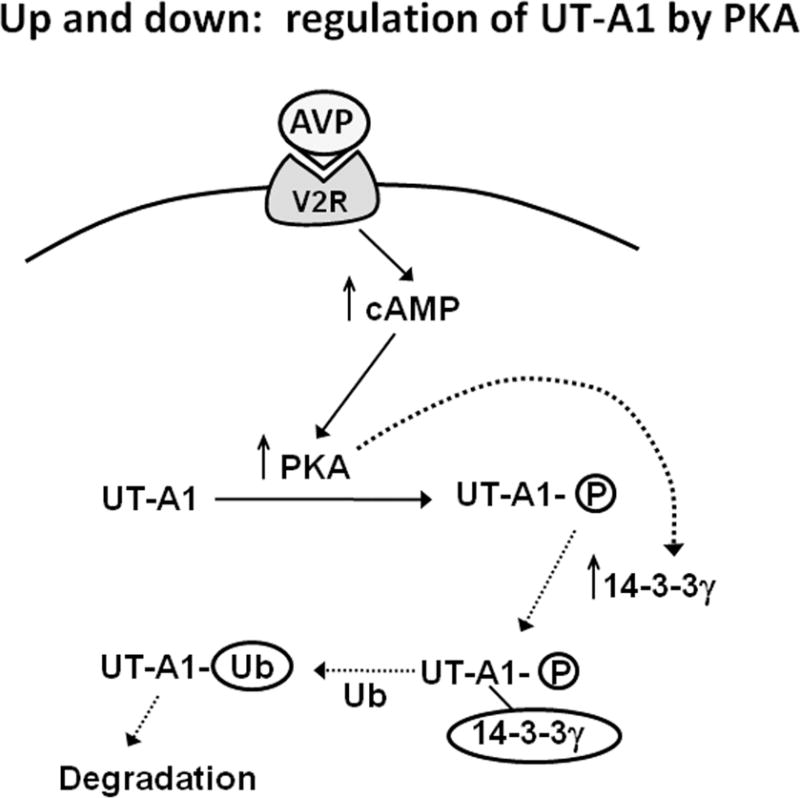
Proposed model for the negative feedback of vasopressin stimulated UT-A1 activity by 14-3-3ɣ. This schematic shows vasopressin (AVP) binding to the type 2 vasopressin receptor (V2R) and stimulating cAMP production, which stimulates PKA to activate/phosphorylate UT-A1. It also shows the PKA stimulation of 14-3-3ɣ which then binds to UT-A1 promoting ubiquitination (Ub) and subsequent degradation, effectively reversing the original PKA-mediated activation. The data showing these opposite effects of PKA are established, but whether there is a negative feedback loop, or whether only one effect is dominant in vivo, remains to be determined.
Both UT-A1 and UT-A3 are expressed in the IMCD (reviewed in (3, 4)). To determine the effect of UT-A1 alone, a mouse expressing only UT-A1 was created by transgenic restoration of UT-A1 into a UT-A1/UT-A3 knock-out mouse (19). These UT-A1-only mice had normal basal urea permeability in the IMCD, but unlike wild-type mice, vasopressin did not stimulate urea permeability (19). Urine concentrating ability, absent in UT-A1/UT-A3 knock-out mice, was restored in the UT-A1 only mice (19).
Protein kinase C (PKC)
UT-A1 is also phosphorylated by PKCα (20–22). A recent study used mutagenesis to identify S494 as the PKC phosphorylation site (23) (Figure 2). A polyclonal antibody to phospho-S494-UT-A1 was generated and used to show that activators of PKC, phorbol dibutyrate and hypertonicity, increased UT-A1 phosphorylation at S494 while activators of either PKA or Epac did not (23). Activation of both PKA and PKC, but not PKC alone, increased the apical plasma membrane accumulation of UT-A1 (23). These findings suggest UT-A1 phosphorylation at S494 by PKC may increase vasopressin-stimulated urea transport by enhancing UT-A1 retention in the apical plasma membrane (23).
PKCα knock-out mice have a urine concentrating defect (20). Interestingly, PKCα knock-out mice are protected against lithium-induced nephrogenic diabetes insipidus (24). Compared to wild-type mice, lithium-treated PKCα knock-out mice had no polyuria after 5 days of lithium and significantly less polyuria after 6 weeks, and UT-A1 protein abundance was unchanged at either time point (24). Thus, knock-out of PKCα prevented the development of severe NDI in mice (24).
UT-A1 is a glycoprotein with two forms: 117 and 97 kDa (25). Over-expression or activation of PKCα increased UT-A1 sialylation and increased UT-A1 accumulation in the apical plasma membrane (26). UT-A1 sialylation is reduced in PKCα knock-out mice. Src kinase mediates the effect of PKCα UT-A1 sialylation (26). Since PKC inhibition blocked the induction of UT-A1 sialylation by high glucose, this pathway may be important in ameliorating the osmotic diuresis caused by glucosuria in patients with diabetes mellitus (26). PKC also enhances UT-A3 glycan sialylation, and this effect is mediated by ST6GalI (27).
Rats with streptozotocin-induced diabetes mellitus have an increase in the abundance of the larger glycoform of UT-A1 and an increase in urea transport (28). The carbohydrate structure of UT-A1 is also changed, with increased amounts of fucose, sialic acid, glycan branching, and changes in galectin proteins (29). RNA-seq and qPCR analysis showed changes in several glycosylation-associated genes in diabetic rats (29). Genes that were changed included some glycosyltransferases, sialylation enzymes, and glycan binding protein galectins (29). Thus, diabetes changed UT-A1 protein abundance and its glycan structure (29).
AMPK
AMPK activation increases UT-A1 and aquaporin-2 phosphorylation and urea and water transport in IMCDs (30). Thus, AMPK may represent a vasopressin-independent pathway to increase urine concentration and potentially treat congenital nephrogenic diabetes insipidus (30).
Other recent findings
Urea permeability was measured in inner medullary thin limb segments from Munich-Wistar rats (31). Urea permeability was lower in the upper portion of the thin descending limb than in the lower portion or the thin ascending limb. The urea permeability in the upper portion of the thin descending limb was not inhibited by phloretin, suggesting that it is not mediated by UT-A2 (31). A recent molecular cloning study identified two novel variants of UT-A2, named UT-A2c and UT-A2d, as well as a variant of the sodium-glucose transporter 1 named SGLT1a, that may mediate the high urea permeability in the lower portion of the thin descending limb and the thin ascending limb segments (32).
Two non-steroidal anti-inflammatory drugs (NSAIDs), meloxicam and ibuprofen, alter the phosphorylated forms of aquaporin-2 (33). However, neither medication altered total UT-A1 phosphorylation in rat inner medulla (33).
Succinylated gelatin was used to create a rat model of hepatorenal syndrome or abdominal compartment syndrome (34). UT-A2 and UT-A3 mRNA abundances were reduced in the renal medulla of rats with hepatorenal syndrome compared to those with cirrhosis (34). There was no difference in UT-A2, UT-A3, or UT-B mRNA abundances between rats with abdominal compartment syndrome and control rats (34). There was no change in UT-A1 protein abundance in any of the groups of rats (34).
LPS was used to create a mouse model of endotoxemia and UT-A3 was measured in brain (35). There was a significant decrease in UT-A3 protein abundance in the hippocampus and cortical astrocytes, and this decrease was partially restored by treatment with dexamethasone (35).
A recent review analyzed the evolution of urea transporters in vertebrates (36). This analysis showed that three homologues, UT-A, UT-C, and UT-D, evolved from a single ancestral UT in piscine lineages, followed by a reduction to a single UT-A (36). An internal tandem duplication led to UT-A1, and a second duplication led to UT-B (36). They propose that non-ornithine-urea cycle production of urea is important for the generation of polyamines and neurotransmitters, and provided an evolutionary driving force for urea transporter expression in extra-renal tissues (36). Another recent review suggests that urea transporters in skin may be involved in the development of uremic frost since both UT-A1 and UT-B are expressed in skin (37).
UT-B
Vasa recta
UT-B is expressed in erythrocytes and descending vasa recta (reviewed in (3, 4)). Rat outer medullary descending vasa recta have been successfully studied by perfusion (38). Recently, a technique was developed to perfuse rat inner medullary descending vasa recta (39). These inner medullary descending vasa recta also express UT-B (39). UT-B inhibition plays an important role in regulating blood pressure and vaso-relaxation via up-regulation of the L-arginine-eNOS-NO pathway (40). In human kidney, UT-B protein expression was found in descending vasa recta, with expression decreasing with depth below the outer medulla (41). UT-B protein expression was also found in the papillary surface epithelium in the lower inner medulla, providing a paracellular pathway for urea transport across this epithelium (41).
Bladder
Human bladder was shown to express UT-B (42). Bladder primarily expressed UT-B1 mRNA, although some UT-B2 was detected (42). UT-B protein was strongly expressed throughout all layers of the urothelium except the apical membrane of the outermost umbrella cells (42). A GWAS study found a link between a polymorphism in UT-B and bladder cancer in a Northern India population (43). An editorial suggested that the demonstration of UT-B protein in human bladder may explain the observation of a link between UT-B1 and bladder cancer (44). High protein diets can lead to high urinary urea concentrations, which may be carcinogenic in rat bladder (45). UT-B expression in the urothelium of the American black bear, an animal that hibernates, was similar to that found in other mammals (46).
Gastrointestinal tract/rumen
UT-B is important for salvaging urea nitrogen in the bovine gastrointestinal tract, especially in the rumen (47). The serosal-to-mucosal flux of urea across the isolated ruminal epithelium is mediated by UT-B, as well as aquaporins 3, 7, and 10, in Holstein calves (48). UT-B and aquaporin-3 protein abundances, but not aquaporin-7, were increased in calves during the transition from milk-feeding to solid-feeding (49). Sheep rumen expresses both UT-B1 and UT-B2 transcripts by PCR, and urea transport was regulated by changes in pH and ammonia concentrations (50). In primary cultures of goat rumen epithelial cells, short-chain fatty acids and acid pH increased UT-B mRNA and protein abundance (51). Ruminal UT-B protein was also increased when the goats were fed a diet with large amounts of nitrogen and non-fiber carbohydrates (51).
Other recent findings
A high salt diet (8% for 2 weeks) reduced UT-B mRNA and protein abundances in the choroid plexus of Dahl S, but not Dahl R, rats (52). UT-A mRNA abundance was not altered by the high salt diet in either Dahl S or Dahl R rats (52).
UT-B knock-out mice have an atrial-ventricular conduction block (53). A proteomics study found that 15 proteins involved in mitochondrial complexes I, III, IV, and V of the respiratory chain were down-regulated (54). These changes could reduce electron transport chain activity, lead to mitochondrial dysfunction, and explain the atrial-ventricular conduction block (54).
Mice were implanted with either UT-B expressing cells or control cells to investigate the utility of a gene reporter system based upon UT-B and C-13 hyperpolarized urea (55). UT-B lowered the apparent diffusion coefficient of hyperpolarized urea, suggesting that it has the potential to be used in vivo as a magnetic resonance-based gene reporter (55).
Urearetics
Urea transporter inhibitors are attractive targets for the development of novel diuretics (reviewed in (56, 57)). Since conventional diuretics target sodium transporters, agents inhibiting urea transporters could provide synergistic effects. Those that target UT-A1 are particularly attractive since UT-A1 is located in the last portion of the IMCD and thus may have less risk for side-effects, such as hypokalemia, than conventional diuretics (Figure 4).
Figure 4.
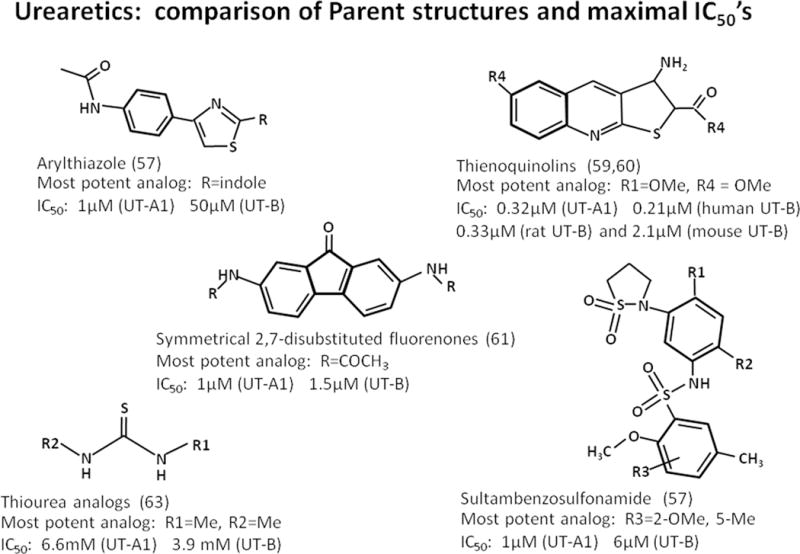
Urearetics: comparison of parent structures and maximal IC50’s. Shown are 5 classes of synthetic inhibitors of urea transporters. The IC50’s of the most potent of each of these “urearetics” is also provided.
A high-throughput assay was used to identify UT-A inhibitors (58). Compounds were identified using a structure-activity analysis that selectively inhibited UT-A by a non-competitive mechanism with an IC50 of 1 μM (58). Administering an indole thiazole or γ-sultambenzosulfonamide intravenously to rats resulted in diuresis with more urea than salt excretion, even when the rats were given dDAVP (58). Thus, these UT-A inhibitors may be clinically useful in patients with volume overload and high vasopressin levels, such as congestive heart failure or cirrhosis (58).
A thienoquinolin, PU-14, inhibits both UT-A and UT-B urea transporters and causes a diuresis (59). By inhibiting UT-B, PU-14 may be a novel therapy for hypertension (40). Structure-activity analysis was used to identify a thienoquinolin, PU-48, that was a more potent inhibitor of UT-A in the IMCD (60). PU-48 caused diuresis in both wild-type and UT-B knock-out mice, indicating that its effect was to inhibit UT-A. PU-48 inhibited urea permeability in perfused rat IMCDs (60). The diuresis induced by PU-48 did not change serum sodium, chloride, or potassium levels, suggesting that a UT-A inhibitor acting on the IMCD would have fewer side-effects on electrolyte homeostasis than conventional diuretics (60).
Primary high-throughput virtual screening was used to identify four classes of compounds with UT-B inhibitory activity and to predict a human UT-B model (61). Comparing UT-B from different species allowed a novel inhibitory mechanism for UT-B to be postulated and suggested that phenylalanine198 in mouse and rat UT-B might impede inhibitor –UT-B interactions (55).
A cell-based high-throughput assay identified 2,7-distributed fluorenones as urea transporter inhibitors (62). The most potent compounds inhibited UT-A1 and UT-B with an IC50 of 1 μM (62). Computational docking to a UT-A1 homology model suggested that the inhibitor binds to the urea transporter cytoplasmic domain at a site away from the putative urea binding site (62).
Urea analogs, such as dimethylthiourea (DMTU), inhibit UT-A1 and UT-B (63, 64). Chronic treatment of rats with DMTU resulted in a sustained and reversible reduction in urine osmolality, a 3-fold increase in urine volume, and mild hypokalemia (64). Rats treated with DMTU had a greater diuresis and reduced urinary salt loss than rats treated with furosemide (64). DMTU treatment also prevented hyponatremia and water retention in a rat model of the syndrome of inappropriate antidiuretic hormone secretion (64). To identify more potent and selective inhibitors than DMTU, 36 thiourea analogs were tested (63). The most potent compound, 3-nitrophenyl-thiourea, inhibited both UT-A1 and UT-B with an IC50 of 0.2 mM. Other analogs were found that were relatively selective for UT-A1 or UT-B (63).
A computational and molecular dynamic simulation approach was used to investigate the structural characteristics of Desulfovibrio vulgaris UT (dvUT), a bacterial urea transporter (65). Three urea binding sites were identified. The simulations also suggested that dvUT is water permeable. DvUT was used as a model protein, along with the L-arginine/agmatin anti-porter and lactose permease from Escherichia coli, in a study to determine detergent-binding capacity and phospholipid content of membrane proteins with the ultimate goal of successfully crystallizing membrane proteins (66).
Conclusions
Additional research into the regulation of urea transporters, especially UT-A1, will be important for the development of urearetic agents. If these agents can be developed for clinical use, they would add a novel class of diuretic that may have fewer side-effects on serum electrolytes than conventional diuretics. Urearetics work by increasing luminal urea concentration and causing an osmotic diuresis, so one might expect them to cause hypernatremia. However, the studies reviewed herein have not reported hypernatremia. Urea transport inhibitors may be especially useful in conditions associated with reduced circulating volume and hyponatremia, such as congestive heart failure or cirrhosis.
Elucidating non-vasopressin-mediated pathways to stimulate UT-A1 function may be important for the treatment of patients with nephrogenic diabetes insipidus. Activating alternate pathways to increase UT-A1 function could reduce the severity of the urine concentrating defects.
Key Points.
PKA can activate UT-A1 but also promote UT-A1 degradation through 14-3-3ɣ, providing a feedback mechanism to return UT-A1 function to its basal state following stimulation by vasopressin.
PKC may promote sialyation of UT-A1 and inhibition of PKC may be important for ameliorating the osmotic diuresis caused by glucosuria in patients with diabetes mellitus.
Human kidney UT-B is localized to descending vasa recta, with expression decreasing with depth below the outer medulla, and also to the papillary surface epithelium providing a paracellular pathway for urea transport across this epithelium.
Since UT-A1 is located in the last portion of the IMCD, urearetics that target UT-A1 may have less risk for side-effects, such as hypokalemia, than conventional diuretics.
Acknowledgments
None
Financial support and sponsorship
This work was supported by NIH grants DK41707 and DK89828.
Footnotes
Conflicts of interest
None
Reference section
- 1.Galluci E, Micelli S, Lippe C. Non-electrolyte permeability across thin lipid membranes. Arch Int Physiol Biochim. 1971;79:881–7. doi: 10.3109/13813457109104847. [DOI] [PubMed] [Google Scholar]
- 2.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987;253:F823–F32. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- 3.Klein JD, Blount MA, Sands JM. Urea transport in the kidney. Compr Physiol. 2011;1(2):699–729. doi: 10.1002/cphy.c100030. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Sands JM. In: Urea Transporters. Harris R, editor. New York: Springer; 2014. p. 265. [Google Scholar]
- 5.Fenton RA, Chou C-L, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA. 2004;101(19):7469–74. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16(6):1583–92. doi: 10.1681/ASN.2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob VA, Harbaugh CM, Dietz JR, Fenton RA, Kim SM, Castrop H, et al. Magnetic resonance imaging of urea transporter knockout mice shows renal pelvic abnormalities. Kidney Int. 2008;74(9):1202–8. doi: 10.1038/ki.2008.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol. 2005;25(16):7357–63. doi: 10.1128/MCB.25.16.7357-7363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem. 2002;277:10633–7. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Verkman AS. Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B. J Biol Chem. 2002;277(39):36782–6. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- 11.Klein JD, Sands JM, Qian L, Wang X, Yang B. Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol. 2004;15(5):1161–7. doi: 10.1097/01.asn.0000125617.19799.72. [DOI] [PubMed] [Google Scholar]
- 12.Lei T, Zhou L, Layton AT, Zhou H, Zhao X, Bankir L, et al. Role of thin descending limb urea transport in renal urea handling and the urine concentrating mechanism. American Journal of Physiology - Renal Physiology. 2011;301(6):F1251–F9. doi: 10.1152/ajprenal.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, et al. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol. 2008;295(1):F295–F9. doi: 10.1152/ajprenal.00102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffert JD, Pisitkun T, Wang G, Shen R-F, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103(18):7159–64. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein JD, Blount MA, Frohlich O, Denson CE, Tan X, Sim JH, et al. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol. 2010;298(4):F935–40. doi: 10.1152/ajprenal.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Hoban CA, Black LN, O RJ, Gumina DL, Pulous FE, Sim JH, et al. Vasopressin regulation of multisite phosphorylation of UT-A1 in the inner medullary collecting duct. Am J Physiol Renal Physiol. 2015;308:F49–F55. doi: 10.1152/ajprenal.00642.2013. This paper reports a new phospho-specific antibody to S499 in UT-A1 and compares phosphorylation at S486 and S499 in response to vasopressin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffert JD, Pisitkun T, Saeed F, Wilson JL, Knepper MA. Global analysis of the effects of the V2 receptor antagonist satavaptan on protein phosphorylation in collecting duct. Am J Physiol Renal Physiol. 2014;306(4):410–21. doi: 10.1152/ajprenal.00497.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Feng X, Li Z, Du Y, Fu H, Klein JD, Cai H, et al. Downregulation of urea transporter UT-A1 activity by 14-3-3 protein. Am J Physiol Renal Physiol. 2015;309(1):F71–8. doi: 10.1152/ajprenal.00546.2014. This paper is the first to show that UT-A1 interacts with 14-3-3ɣ, which then recruits MdM2 and stimulates UT-A1 ubiquitination and degradation. These findings suggest a novel regulatory mechanism by which vasopressin both inceases urea transport, and then stimulates UT-A1 degradation, thereby returning the cell to its basal state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Klein JD, Wang Y, Mistry A, LaRocque LM, Molina PA, Rogers RT, et al. Transgenic Restoration of Urea Transporter A1 Confers Maximal Urinary Concentration in the Absence of Urea Transporter A3. J Am Soc Nephrol. 2016;27(5):1448–55. doi: 10.1681/ASN.2014121267. This paper reports a new transgenic mouse in which UT-A1 is reintroduced into a UT-A1/UT-A3 knock-out mouse. The approach is novel and may be useful for the creation of other transgenic animals. The findings suggest that expression of UT-A1 alone restores urine concentrating ability and basal urea permeability, but not vasopressin-stimulated urea permeability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein JD, Martin CF, Kent KJ, Sands JM. Protein kinase C-alpha mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol. 2012;302(9):F1098–103. doi: 10.1152/ajprenal.00664.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Liedtke CM, Klein JD, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2010;299(6):F1401–F6. doi: 10.1152/ajprenal.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Klein JD, Froehlich O, Sands JM. Role of protein kinase C-alpha in hypertonicity-stimulated urea permeability in mouse inner medullary collecting ducts. Am J Physiol Renal Physiol. 2013;304(2):F233–8. doi: 10.1152/ajprenal.00484.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Blount MA, Cipriani P, Redd SK, Ordas RJ, Black LN, Gumina DL, et al. Activation of protein kinase Calpha increases phosphorylation of the UT-A1 urea transporter at serine 494 in the inner medullary collecting duct. 2015;309(9):C608–15. doi: 10.1152/ajpcell.00171.2014. This paper identifies S494 as the site in UT-A1 that is phosphorylated by PKC and also reports on a new phospho-specific antibody to S494-UT-A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim JH, Himmel NJ, Redd SK, Pulous FE, Rogers RT, Black LN, et al. Absence of PKC-alpha attenuates lithium-induced nephrogenic diabetes insipidus. PloS one. 2014;9(7):e101753. doi: 10.1371/journal.pone.0101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford AD, Terris J, Ecelbarger CA, Klein JD, Sands JM, Chou C-L, et al. 97 and 117 kDa forms of the collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol. 2001;281(1):F133–F43. doi: 10.1152/ajprenal.2001.281.1.F133. [DOI] [PubMed] [Google Scholar]
- *26.Li X, Yang B, Chen M, Klein JD, Sands JM, Chen G. Activation of protein kinase C-alpha and Src kinase increases urea transporter A1 alpha-2, 6 sialylation. J Am Soc Nephrol. 2015;26(4):926–34. doi: 10.1681/ASN.2014010026. This paper reports on a novel mechanism by which PKC regulates UT-A1 function by increasing glycan sialyation through the Src kinase pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Qian X, Sands JM, Song X, Chen G. Modulation of kidney urea transporter UT-A3 activity by alpha2,6-sialylation. Pfluegers Arch. 2016:468. doi: 10.1007/s00424-016-1802-0. in press. This paper reports on a novel mechanism by which PKC regulates UT-A3 function by increasing glycan sialyation through ST6GalI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pech V, Klein JD, Kozlowski SD, Wall SM, Sands JM. Vasopressin increases urea permeability in initial IMCDs from diabetic rats. Am J Physiol Renal Physiol. 2005;289(3):F531–F5. doi: 10.1152/ajprenal.00125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Qian X, Li X, Ilori TO, Klein JD, Hughey RP, Li CJ, et al. RNA-seq analysis of glycosylation related gene expression in STZ-induced diabetic rat kidney inner medulla. Front Physiol. 2015;6:274. doi: 10.3389/fphys.2015.00274. This paper reports on an RNA-seq analysis of the changes in glycosylation genes that may contribute to changes in UT-A1 glycan structure. It shows that PKC may promote sialyation of UT-A1 and inhibition of PKC may be important for ameliorating the osmotic diuresis caused by glucosuria in patients with diabetes mellitus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Klein JD, Wang Y, Blount MA, Molina PA, LaRocque LM, Ruiz JA, et al. Metformin, an AMPK activator, stimulates the phosphorylation of aquaporin 2 and urea transporter A1 in inner medullary collecting ducts. Am J Physiol Renal Physiol. 2016:310. doi: 10.1152/ajprenal.00102.2016. in press. This paper suggests that AMPK may be a vasopressin-independent pathway to phosphorylate UT-A1 and aquaporin-2, thereby increasing urine concentration and potentially treating congenital nephrogenic diabetes insipidus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nawata CM, Evans KK, Dantzler WH, Pannabecker TL. Transepithelial water and urea permeabilities of isolated perfused Munich-Wistar rat inner medullary thin limbs of Henle’s loop. Am J Physiol Renal Physiol. 2014;306(1):F123–F9. doi: 10.1152/ajprenal.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Nawata CM, Dantzler WH, Pannabecker TL. Alternative channels for urea in the inner medulla of the rat kidney. Am J Physiol Renal Physiol. 2015;309(11):F916–24. doi: 10.1152/ajprenal.00392.2015. This paper identifies some novel molecular isoforms of UT-A2 in the rat medulla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren H, Yang B, Molina PA, Sands JM, Klein JD. NSAIDs alter phosphorylated forms of AQP2 in the inner medullary tip. PloS one. 2015;10(10):e0141714. doi: 10.1371/journal.pone.0141714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song W, Qi X, Zhang W, Zhao Y, Cao Y, Wang F, et al. Abnormal Expression of Urea Transporter Protein in a Rat Model of Hepatorenal Syndrome Induced by Succinylated Gelatin. Medical science monitor : international medical journal of experimental and clinical research. 2015;21:2905–11. doi: 10.12659/MSM.894232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Y, Meng Y, Lv X, Guo L, Wang X, Su Z, et al. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins, ameliorating brain endotoxemia in mice. International journal of clinical and experimental pathology. 2014;7(12):8443–52. [PMC free article] [PubMed] [Google Scholar]
- 36.LeMoine CM, Walsh PJ. Evolution of urea transporters in vertebrates: adaptation to urea’s multiple roles and metabolic sources. The Journal of experimental biology. 2015;218(Pt 12):1936–45. doi: 10.1242/jeb.114223. [DOI] [PubMed] [Google Scholar]
- 37.Saardi KM, Schwartz RA. Uremic frost: a harbinger of impending renal failure. International journal of dermatology. 2016;55:17. doi: 10.1111/ijd.12963. [DOI] [PubMed] [Google Scholar]
- 38.Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest. 1994;93:212–22. doi: 10.1172/JCI116948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Evans KK, Nawata CM, Pannabecker TL. Isolation and perfusion of rat inner medullary vasa recta. Am J Physiol Renal Physiol. 2015;309(4):F300–4. doi: 10.1152/ajprenal.00214.2015. This paper is the first report of a method to perfuse inner medullary vasa recta and shows that UT-B is expressed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Sun Y, Lau CW, Jia Y, Li Y, Wang W, Ran J, et al. Functional inhibition of urea transporter UT-B enhances endothelial-dependent vasodilatation and lowers blood pressure via L-arginine-endothelial nitric oxide synthase-nitric oxide pathway. Scientific reports. 2016;6:18697. doi: 10.1038/srep18697. This paper shows that inhibiting UT-B with PU-14 can regulate blood pressure and vaso-relaxation via up-regulation of the L-arginine-eNOS-NO pathway. Thus, UT-B inhibition by the urearetic PU-14 may be a novel therapy for hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Wei G, Rosen S, Dantzler WH, Pannabecker TL. Architecture of the human renal inner medulla and functional implications. Am J Physiol Renal Physiol. 2015;309(7):F627–37. doi: 10.1152/ajprenal.00236.2015. This paper describes the architecture of human inner medulla and discusses similarities and differences from rodent inner medulla. UT-B is abundantly expressed in long-loop descending thin limbs and vasa recta, similar to rodents, but in contrast to rodents, interstitial nodal spaces are rare in the human inner medulla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Walpole C, Farrell A, McGrane A, Stewart GS. Expression and localization of a UT-B urea transporter in the human bladder. Am J Physiol Renal Physiol. 2014;307(9):F1088–F94. doi: 10.1152/ajprenal.00284.2014. This paper confirms that UT-B1 is expressed in the human bladder. This finding is important since a link has been suggested between UT-B allelic variation and human bladder cancer risk. [DOI] [PubMed] [Google Scholar]
- 43.Singh V, Jaiswal PK, Mittal RD. Replicative study of GWAS TP63C/T, TERTC/T, and SLC14A1C/T with susceptibility to bladder cancer in North Indians. Urologic oncology. 2014;32(8):1209–14. doi: 10.1016/j.urolonc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Atala A. Re: Expression and Localization of a UT-B Urea Transporter in the Human Bladder. J Urol. 2015;194(2):592. doi: 10.1016/j.juro.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Liu M, Li M, Liu J, Wang H, Zhong D, Zhou H, et al. Elevated urinary urea by high-protein diet could be one of the inducements of bladder disorders. Journal of translational medicine. 2016;14(1):53. doi: 10.1186/s12967-016-0809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spector DA, Deng J, Coleman R, Wade JB. The urothelium of a hibernator: the American black bear. Physiological reports. 2015;3(6) doi: 10.14814/phy2.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coyle J, McDaid S, Walpole C, Stewart GS. UT-B Urea Transporter Localization in the Bovine Gastrointestinal Tract. J Membr Biol. 2015 doi: 10.1007/s00232-015-9850-5. [DOI] [PubMed] [Google Scholar]
- 48.Walpole ME, Schurmann BL, Gorka P, Penner GB, Loewen ME, Mutsvangwa T. Serosal-to-mucosal urea flux across the isolated ruminal epithelium is mediated via urea transporter-B and aquaporins when Holstein calves are abruptly changed to a moderately fermentable diet. J Dairy Sci. 2015;98(2):1204–13. doi: 10.3168/jds.2014-8757. [DOI] [PubMed] [Google Scholar]
- 49.Berends H, van den Borne JJ, Rojen BA, van Baal J, Gerrits WJ. Urea recycling contributes to nitrogen retention in calves fed milk replacer and low-protein solid feed. The Journal of nutrition. 2014;144(7):1043–9. doi: 10.3945/jn.114.191353. [DOI] [PubMed] [Google Scholar]
- 50.Lu Z, Stumpff F, Deiner C, Rosendahl J, Braun H, Abdoun K, et al. Modulation of sheep ruminal urea transport by ammonia and pH. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(5):R558–70. doi: 10.1152/ajpregu.00107.2014. [DOI] [PubMed] [Google Scholar]
- 51.Lu Z, Gui H, Yao L, Yan L, Martens H, Aschenbach JR, et al. Short-chain fatty acids and acidic pH upregulate UT-B, GPR41, and GPR4 in rumen epithelial cells of goats. American journal of physiology Regulatory, integrative and comparative physiology. 2015;308(4):R283–93. doi: 10.1152/ajpregu.00323.2014. [DOI] [PubMed] [Google Scholar]
- *52.Guo L, Meng J, Xuan C, Ge J, Sun W, O’Rourke ST, et al. High salt-diet reduces SLC14A1 gene expression in the choroid plexus of Dahl salt sensitive rats. Biochem Biophys Res Commun. 2015;461(2):254–9. doi: 10.1016/j.bbrc.2015.04.010. This paper shows that UT-B is expressed in the choriod plexus and suggests that altered UT-B expression may contribute to abnormalities in blood pressure in salt-sensitive rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng Y, Zhao C, Zhang X, Zhao H, Guo L, Lu B, et al. Surface electrocardiogram and action potential in mice lacking urea transporter UT-B. Sci China C Life Sci. 2009;52(5):474–8. doi: 10.1007/s11427-009-0047-y. [DOI] [PubMed] [Google Scholar]
- 54.Du Y, Meng Y, Zhu J, Kang L, Jia X, Gui L, et al. Quantitative proteomic study of myocardial mitochondria in urea transporter B knockout mice. Proteomics. 2014;14(17–18):2072–83. doi: 10.1002/pmic.201400123. [DOI] [PubMed] [Google Scholar]
- 55.Patrick PS, Kettunen MI, Tee SS, Rodrigues TB, Serrao E, Timm KN, et al. Detection of transgene expression using hyperpolarized 13C urea and diffusion-weighted magnetic resonance spectroscopy. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2015;73(4):1401–6. doi: 10.1002/mrm.25254. [DOI] [PubMed] [Google Scholar]
- 56.Esteva-Font C, Anderson MO, Verkman AS. Urea transporter proteins as targets for small-molecule diuretics. Nature reviews Nephrology. 2015;11(2):113–23. doi: 10.1038/nrneph.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sands JM. Urea transporter inhibitors: en route to new diuretics. Chem Biol. 2013;20:1201–2. doi: 10.1016/j.chembiol.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esteva-Font C, Cil O, Phuan PW, Su T, Lee S, Anderson MO, et al. Diuresis and reduced urinary osmolality in rats produced by small-molecule UT-A-selective urea transport inhibitors. Faseb J. 2014;28(9):3878–90. doi: 10.1096/fj.14-253872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F, Lei T, Zhu J, Wang W, Sun Y, Chen J, et al. A novel small-molecule thienoquinolin urea transporter inhibitor acts as a potential diuretic. Kidney Int. 2013;83(6):1076–86. doi: 10.1038/ki.2013.62. [DOI] [PubMed] [Google Scholar]
- *60.Ren H, Wang Y, Xing Y, Ran J, Liu M, Lei T, et al. Thienoquinolins exert diuresis by strongly inhibiting UT-A urea transporters. Am J Physiol Renal Physiol. 2014;307(12):F1363–72. doi: 10.1152/ajprenal.00421.2014. This paper identified a potent thienoquinolin-inhbitor of UT-A in the inner medullary collecting duct and showed that induces diuresis, and suggests that this class of molecule could be a developed as diuretics with fewer side effects than current diuretics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Tou WI, Zhou H, Li F, Ren H, Chen CY, et al. Developing hypothetical inhibition mechanism of novel urea transporter B inhibitor. Scientific reports. 2014;4:5775. doi: 10.1038/srep05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S, Esteva-Font C, Phuan PW, Anderson MO, Verkman AS. Discovery, synthesis and structure-activity analysis of symmetrical 2,7-disubstituted fluorenones as urea transporter inhibitors. Med Chem Comm. 2015;6:1278–84. doi: 10.1039/C5MD00198F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Esteva-Font C, Phuan PW, Lee S, Su T, Anderson MO, Verkman AS. Structure-activity analysis of thiourea analogs as inhibitors of UT-A and UT-B urea transporters. Biochimica et biophysica acta. 2015;1848(5):1075–80. doi: 10.1016/j.bbamem.2015.01.004. This paper identified thiourea analogs that were potent inhibitors of UT-A1 and/or UT-B, and that may have potential application as novel salt-sparing diuretics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Cil O, Esteva-Font C, Tas ST, Su T, Lee S, Anderson MO, et al. Salt-sparing diuretic action of a water-soluble urea analog inhibitor of urea transporters UT-A and UT-B in rats. Kidney Int. 2015;88(2):311–20. doi: 10.1038/ki.2015.138. This paper studies the utility of dimethylthiourea (DMTU) as a reversible inhibitor of UT-A1 and UT-B in rats. Compared to rats treated with a loop diuretic, DMTU-treared rats had a larger diuresis and less urinary salt loss, demonstrating the efficacy of urea transporter inhibition as a diuretic. In addition, DMTU prevented hyponatremia in a rat model of the syndrome of inappropriate antiduretic hormone secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Yu T, Sang JP, Zou XW, Yan C, Zou X. Computation and simulation of the structural characteristics of the kidney urea transporter and behaviors of urea transport. The journal of physical chemistry B. 2015;119(16):5124–31. doi: 10.1021/jp511300u. [DOI] [PubMed] [Google Scholar]
- 66.Ilgu H, Jeckelmann JM, Gachet MS, Boggavarapu R, Ucurum Z, Gertsch J, et al. Variation of the detergent-binding capacity and phospholipid content of membrane proteins when purified in different detergents. Biophys J. 2014;106(8):1660–70. doi: 10.1016/j.bpj.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


