Abstract
Background
Soy food intake may have protective effects against breast cancer risk, including estrogen receptor negative breast cancer. However, the underlying molecular mechanisms remain unclear.
Methods
To evaluate the association of soy intake with the expression of microRNAs and genes in the tumor tissue of triple-negative (lacking expression of estrogen receptor, progesterone receptor and HER2) breast cancer (TNBC) patients, a total of 800 microRNAs (miRNAs) and 302 gene expressions were measured by the NanoString nCounter Assays in formalin-fixed paraffin-embedded (FFPE) tumor tissues from 272 TNBC patients. Soy intake during the one-year period prior to cancer diagnosis was assessed by a validated food-frequency questionnaire. The association of soy intake with the expression of miRNAs and genes was evaluated by performed linear regression analysis with adjustments for patient age and TNM.
Results
A total of 14 miRNAs and 24 genes were significantly associated with soy food intake (p<0.05): 13 of the 14 miRNAs (92.9%) and 9 of 24 genes (37.5%), including tumor suppressors miR-29a-3p and IGF1R, showed over-expression for those women with a high soy intake, while the remaining miRNAs and genes, including oncogenes KRAS and FGFR4, showed under-expression. Furthermore, cell growth-related genes showed a predominant under-expression pattern when comparing tumor samples from women with a high soy food intake to samples from those who had a lower soy food intake.
Conclusions
Our study suggests that long-term pre-diagnosis soy intake may lead to increased expression of tumor suppressors and decreased expression of oncogenes, especially cell growth-related genes, in breast tumor tissues.
Keywords: triple-negative breast cancer, soy consumption, miRNA expression, gene expression
Introduction
Soy foods contain abundant amounts of isoflavones, which have estrogen-like structures and may act as natural estrogen receptor (ER) modulators that interact with ERs (1, 2). Soy foods have also been shown to have many non-estrogen related anti-cancer properties, such as inhibiting cell proliferation and inducing apoptosis (3). Previous epidemiological studies, including our own work, have found that soy intake was inversely associated with breast cancer risk (4-6), and post-diagnosis soy consumption was associated with a reduced risk of breast cancer recurrence and mortality (7, 8), including that of ER-negative breast cancer. These observations suggest that soy food may be a potential natural and alternative approach to the chemoprevention of breast cancer (8, 9). However, evidence of the potential anti-cancer benefit of soy food consumption is not entirely consistent (9, 10). Furthermore, the underlying molecular mechanisms of soy food intake on breast cancer risk and prognosis are still not fully understood.
Previous in vitro studies have shown that soy isoflavones may exhibit an anti-cancer effect by acting on specific miRNAs (11, 12). However, the effects of long-term soy food intake on miRNA expression in the tumor tissues of humans are not well understood. In addition, several experimental studies have suggested that soy isoflavones may also regulate genes that control cell cycle, apoptosis, and survival (13-15). Recently, Shike and colleagues reported that short-term (7-30 days) soy supplementation immediately prior to cancer surgery was associated with increased expression of cell-cycle genes in breast cancer tissue, provoking concerns of a potential cancer-promoting effect (16). However, the study was limited by its abbreviated window of exposure and sudden high dose of exposure (17). In the present study, we investigated the effects of long-term pre-diagnosis soy food intake on the expression of 800 miRNAs and 302 pre-selected genes in tumor tissues from 272 TNBC cases in a population-based cohort study of breast cancer patients.
Materials and methods
Study population
This study utilized the resources generated from the Shanghai Breast Cancer Survival Study (SBCSS), which is a longitudinal, population-based cohort study of breast cancer survival in Shanghai, China (details described elsewhere (8)). Briefly, the SBCSS included a total of 5,042 women with incident breast cancer, aged 20 to 75 years, who were recruited to the study between March 2002 and April 2006, approximately 6 months after diagnosis (response rate: 80%). Participants were followed up by in-person surveys at 18, 36, 60 and 120 months after cancer diagnosis, in combination with periodic record linkage with the Shanghai Vital Statistics Registry. Included in the current study are 272 TNBC cases who had both pre-diagnosis soy food intake information and sufficient tumor tissue RNA collected prior to any cancer therapy. Sample selection and preparation have been previously published (18) and a study flow chart can be found in the online supplementary materials. The study protocol was approved by the institutional review boards of Vanderbilt University and the Shanghai Municipal Center for Disease Control and Prevention and all participants provided written informed consent.
The measurement of Soy food intake
A validated food-frequency questionnaire (19) was used to assess usual soy and other dietary intake. Questions related to pre-diagnosis soy food intake were only administered to a sub-cohort of study participants, including 321 TNBC cases, per study design. Soy food intake during the year before cancer diagnosis was measured by soy protein intake, which was estimated by summing the product of individual soy food intake amount and its protein content based on the Chinese Food Composition Tables 2002 (8). Clinical information and tumor characteristics including patient age, TNM stage, tumor grade, chemotherapy usage, and ER/PR hormone receptor status were extracted from medical charts. HER2 status was measured in the Vanderbilt Molecular Epidemiology Core Laboratory (20).
miRNA and Gene Expression Analysis
Expression profiles of the 800 miRNAs and 302 pre-selected genes were measured by the NanoString nCounter Assays using total RNA isolated from formalin-fixed paraffin-embedded (FFPE) breast tumor tissues (details described elsewhere (18)). The Human v2 miRNA assay and custom-designed gene Expression Assay (NanoString) were used to measure the tumor tissue level of miRNA and gene expression following the NanoString standard protocol. The detailed normalization process in miRNA and gene expression and downstream statistical analysis are described in the Supplementary materials.
Results
Among the study participants, 272 had gene expression data, and 244 cases had miRNA expression data. The age at diagnosis for cases ranged from 26-75 years. The median soy protein intake assessed during one year period prior to diagnosis was 10.8 grams/day. We did not observe significant associations between soy food intake and demographics or clinicopathological characteristics, including patient age, tumor grade and TNM stage (Table 1).
Table 1. Soy Food Consumption by Demographic and Clinical Characteristics.
| Characteristic | N | soy food consumption mean (sd) | p value* |
|---|---|---|---|
| Age at diagnosis, yrs | |||
| < 46 | 68 | 12.12 (8.10) | 0.33 |
| 46-51 | 68 | 12.59 (7.25) | |
| 52-60 | 68 | 12.57(7.30) | |
| >60 | 68 | 12.12(6.80) | |
| TNM stage | |||
| 0 | 6 | 14.91(10.56) | 0.09 |
| I | 88 | 13.74(7.35) | |
| IIA | 92 | 11.11(6.9) | |
| IIB | 52 | 12.80(7.57) | |
| III, IV | 25 | 10.81(7.02) | |
| Unknown | 9 | 11.41(7.73) | |
| Grade | |||
| I | 34 | 13.98(9.24) | 0.36 |
| II | 86 | 12.11(7.08) | |
| III | 152 | 12.13(7.01) |
Spearman's rank correlation test was used for characteristic variables with soy food intake.
miRNAs significantly associated with soy food intake
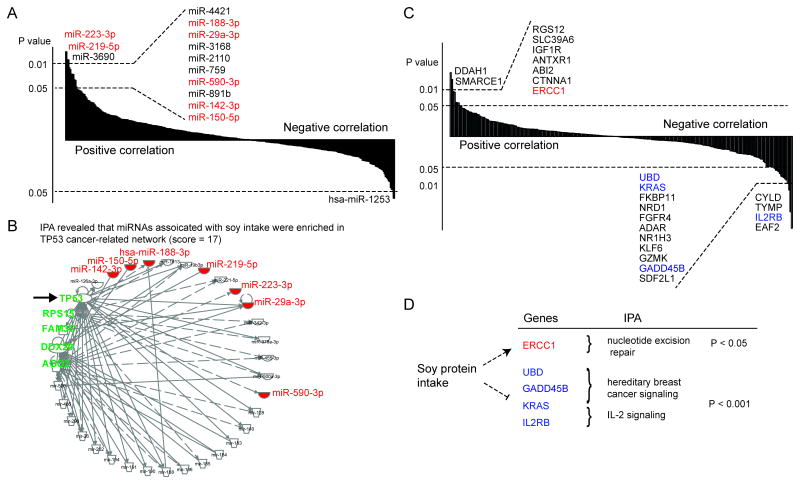
A total of 14 miRNAs showed a significant association pattern with soy food intake (p < 0.05; Figure 1A and Table 2). Of them, 13 miRNAs, (miR-223-3p, miR-219-5p, miR-3690, miR-4421, miR-188-3p, miR-3168, miR-29a-3p, miR-2110, miR-759, miR-590-3p, miR-891b, miR-142-3p and miR-150-5p) were positively correlated with soy intake; only one miRNA (miR-1253) showed an inverse association (Figure 1A, Table 2). Functional analysis using IPA revealed that seven miRNAs, including the four well-known cancer-related miRNAs described above, and another three miRNAs (miR188-3p, miR-219-5p and miR-5903p), are involved in the regulation of a TP53 cancer-related network (Figure 1B). This network includes several cancer-related genes, including TP53, AGO2 and DDX20. In addition, miR-150-5p and miR-142-3p were found to target downstream gene AGO2 (Figure 1B), which had been reported to lead to decreased tumor suppressor PTEN in hepatocellular carcinoma (47).
Figure 1.
miRNAs and genes significantly associated with soy food intake. (A) A total of 14 miRNAs showed significant association with soy food intake. Of these, 13 miRNAs showed a significantly positive correlation pattern between their expression levels and soy intake, while one showed an inverse association. B) TP53 cancer-related network was shown for those significant miRNAs, including miR-233-3p, miR-219-5p, miR188-3p, miR-29a-3p, miR-5903p, miR-142-3p and miR-150-5p, which are highlighted in red. C) A total of 24 genes showed a significant association pattern with soy food intake. Of these, 15 genes showed an inverse correlation pattern between their expression levels and soy food intake, while 9 genes showed positive associations. D) Soy food intake and its association with the regulation of genes and pathways. The up-regulations and down-regulations of genes related to these pathways are highlighted in red and blue, respectively.
Table 2. miRNAs significantly associated with soy food intake.
| miRNA | Regression analysis* | Correlation analysis# | ||
|---|---|---|---|---|
|
| ||||
| Beta | p value | ρ | p value | |
| miR-223-3p | 0.025 | 0.005 | 0.172 | 0.007 |
| miR-219-5p | 0.023 | 0.008 | 0.142 | 0.027 |
| miR-3690 | 0.023 | 0.008 | 0.163 | 0.011 |
| miR-4421 | 0.022 | 0.013 | 0.158 | 0.013 |
| miR-188-3p | 0.021 | 0.015 | 0.149 | 0.020 |
| miR-3168 | 0.021 | 0.019 | 0.124 | 0.053 |
| miR-29a-3p | 0.021 | 0.019 | 0.154 | 0.016 |
| miR-2110 | 0.021 | 0.020 | 0.124 | 0.053 |
| miR-759 | 0.020 | 0.027 | 0.118 | 0.066 |
| miR-590-3p | 0.019 | 0.028 | 0.121 | 0.060 |
| miR-1253 | -0.019 | 0.030 | -0.108 | 0.092 |
| miR-891b | 0.018 | 0.042 | 0.116 | 0.072 |
| miR-142-3p | 0.017 | 0.049 | 0.087 | 0.177 |
| miR-150-5p | 0.017 | 0.050 | 0.107 | 0.096 |
Adjusted for patient age and TNM stage
Spearman's rank correlation analysis was performed between miRNA expression and soy food intake.
Genes significantly associated with soy food intake
Correlation analysis shows the expressions of 24 genes to be significantly associated with soy food intake (p < 0.05; Figure 1C and Table 3). Of these, 15 genes exhibited under-expression for women with a high soy intake, while 9 genes showed over-expression (Table 3). Of these identified genes, soy food intake was associated with decreasing expression of oncogenes KRAS and FGFR4 but with increasing expression of IGF1R, consistent with findings from previous in vitro studies (21-24). Functional enrichment analysis using IPA for the down-regulated genes revealed that the two most significantly-enriched pathways are hereditary breast cancer signaling (p < 1.08 × 10-4) and IL-2 signaling (p < 6.84 × 10-4), both of which were driven by oncogenes KRAS (25), IL2RB (26) and GADD45B (27) (Figure 1D). For up-regulated genes, the most significantly-enriched pathway was nucleotide excision repair (p < 1.54 × 10-2), which was driven by the tumor suppressor gene ERCC1 (28) (Figure 1D). It should be noted that we only included selected genes in the study which may have resulted in missing some significant soy-food intake associated genes and thus a reduced statistical power for the IPA enrichment analysis.
Table 3. Genes significantly associated with soy food intake.
| Genes | Regression analysis* | |
|---|---|---|
|
| ||
| Beta | p value | |
| DDAH1 | 0.025 | 0.002 |
| SMARCE1 | 0.024 | 0.004 |
| RGS12 | 0.021 | 0.012 |
| SLC39A6 | 0.02 | 0.013 |
| IGF1R | 0.017 | 0.035 |
| ANTXR1 | 0.017 | 0.036 |
| ABI2 | 0.017 | 0.037 |
| ERCC1 | 0.016 | 0.048 |
| CTNNA1 | 0.016 | 0.048 |
| EAF2 | -0.031 | 1.1×10-4 |
| UBD | -0.016 | 0.048 |
| KRAS | -0.017 | 0.044 |
| FKBP11 | -0.017 | 0.036 |
| NRD1 | -0.017 | 0.035 |
| FGFR4 | -0.018 | 0.031 |
| ADAR | -0.018 | 0.026 |
| NR1H3 | -0.019 | 0.020 |
| KLF6 | -0.019 | 0.019 |
| GZMK | -0.02 | 0.017 |
| GADD45B | -0.02 | 0.015 |
| SDF2L1 | -0.02 | 0.014 |
| CYLD | -0.021 | 0.010 |
| TYMP | -0.023 | 0.005 |
| IL2RB | -0.028 | 0.001 |
Adjusted for patient age and TNM stage.
Comparing differentially-expressed genes reported in the Shike et al study with our study
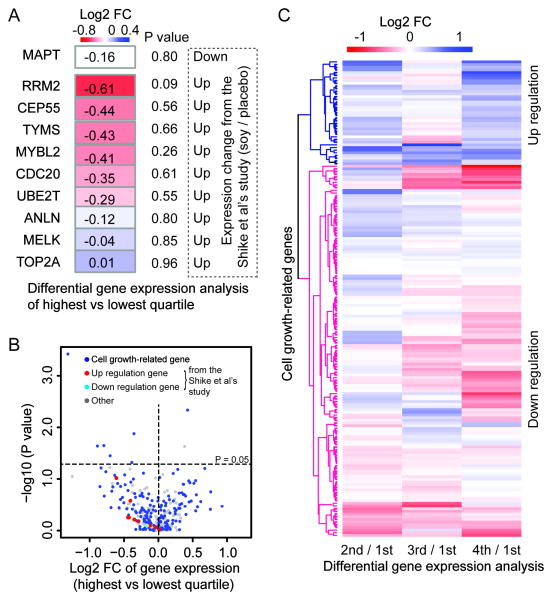
To further examine whether soy intake leads to over-expression of cell-cycle genes as recently reported by Shike et al (16), we carried out further analyses focusing on comparisons of the genes that differed significantly between soy supplement and control groups in their study. Notably, the medians of the lowest and highest quartiles of protein intake in our study were 4.9 and 21.2 (grams/day), respectively. The median protein intake in the soy protein supplement used in the Shike et al study was 25.8 grams/day. Thus, we applied the comparisons of the highest versus lowest quartile groups because intake level of the former is more comparable to the amount of soy supplement administered in their study. Of the 99 genes that differed significantly between soy supplement and control groups in the Shike et al study, 10 with > 2 fold change (FC), including one with under-expression and nine with over-expression, were included in our study (Figure 2A). We found that the gene MAPT, which was under-expressed in the Shike et al study, also had a lower expression in the tumor tissue of women in the highest soy protein intake group in our study, although the difference was not significant (p = 0.8, FC = 1.12, Figure 2A). Of the remaining nine over-expression genes, an opposite direction-of-expression difference pattern, i.e., lower gene expression in the higher soy protein intake group, was observed for all except one gene (TOP2A) in our study samples (Binomial test, p = 0.04), although none of the individual gene expression differences reached the significance threshold (Figure 2A). For example, cell-cycle genes MYBL2 and CDC20 exhibited under-expression with FCs of 1.33 and 1.27 respectively in our study, but showed over-expression with 2 and 3.07 FC in the soy supplementation group relative to the placebo group in Shike et al.
Figure 2.
Differentially-expressed genes in tumor tissue from women with different levels (quartiles) of soy food consumption. (A) Differential gene expression analysis comparing the highest and lowest quartiles of soy intake for 10 significant genes reported in the Shike et al study. P value denotes the significance of gene exposure differences across soy food intake. The dashed box lists the direction of gene expression change between soy protein intake and placebo reported in the Shike et al study. B) Heatmap of log2 FC of gene expression based on comparisons of the 4th, 3rd and 2nd vs the 1st (lowest) quartile of soy intake for cell growth genes. C) Distribution of log2 FC and gene expression significance, comparing the highest vs lowest quartiles of soy food intake.
Under-expression of cell growth-related genes associated with soy food consumption
We also found that eight cell growth-related genes were significantly and differentially expressed in the tumor tissue (Wilcoxon rank-sum test, p < 0.05; Table 4) in the group comparisons. Among them, growth factor TYMP and five cell growth-related genes, including KLF6, IL2RB, NR1H3, ABCB1 and EAF2, had a lower expression in tumor tissue from women with high soy food intake, whereas transporter SNX13 and cell-growth gene DDAH1 had a higher expression in the high soy food intake group (Figure 2B, Table IV). Most genes that were significant in the group differential analyses were also significantly correlated with soy intake in the regression analysis (Table 4). In addition, breast cancer tissue from women in the highest quartile of soy protein intake had an overall slightly higher proportion of cell growth-related genes that were under-expressed (57.8 % of total 166 cell-growth genes, Figure 2C) as compared to women in the lowest quartile of intake (Fold change - FC 4th/1st > 1). The proportion of under-expressed genes in the high soy protein intake group increased when a higher FC cutoff was applied (e.g., 60.6% or 78.9% cell growth-related genes were under-expressed when the FC cutoff was 1.2 or 1.5, respectively). We further performed differential gene expression analyses for the second and third quartiles versus the lowest quartile of protein intake for cell-growth genes. A similarly predominant down-regulation pattern for these genes was observed based on a hierarchical cluster analysis (Figure 2C). In general, the cell growth-related genes are necessary in the control of cell growth and division, while down-regulation of those genes can lead to reduced cancer cell proliferation.
Table 4. Genes differentially expressed in tumor tissue based on comparison of the lowest vs highest quartile of soy food intake.
| Gene | Quartile analysis | Regression analysis* | Cell growth gene | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2nd/1st& | 3rd/1st& | 4th/1st& | p value (4th/1st) | Beta | p value | ||
| EAF2 | -0.291 | -0.561 | -1.318 | 3.7×10-4 | -0.031 | 1.1×10-4 | yes |
| DDAH1 | 0.402 | -0.107 | 0.426 | 0.005 | 0.025 | 0.003 | yes |
| KLF6 | -0.329 | -0.353 | -0.355 | 0.013 | -0.019 | 0.019 | yes |
| SNX13 | 0.295 | 0.144 | 0.383 | 0.023 | 0.013 | 0.103 | |
| NR1H3 | -0.329 | -0.267 | -0.794 | 0.023 | -0.019 | 0.020 | yes |
| ABCB1 | -0.115 | -0.600 | -0.887 | 0.023 | -0.013 | 0.106 | yes |
| IL2RB | -0.110 | -0.649 | -0.702 | 0.036 | -0.028 | 0.001 | yes |
| TYMP | -0.161 | -0.222 | -0.386 | 0.048 | -0.023 | 0.005 | |
Per Log2 fold change.
Adjusted for patient age and TNM stage.
Discussion
In this cohort of 272 TNBC patients, we found that soy food intake during the one-year period prior to cancer diagnosis was associated with increasing expression of tumor-suppressor miRNAs and genes, but with decreasing expression of oncogenes, especially cell growth-related genes, in TNBC tumor tissue. These results reveal possible molecular mechanisms by which soy food intake influences the risk and prognosis of breast cancer.
Although soy isoflavones are known as estrogen receptor modulators, there are also other non-estrogen mediated anti-cancer mechanisms which may explain the associations between soy food intake and breast cancer risk and prognosis. Our findings are consistent with previous studies suggesting that soy consumption may have anti-cancer effects beyond its estrogen-related effect (29). One of the potential mechanisms is via epigenetic modification (32, 33). Previous studies have shown that isoflavones are capable of demethylating CpG sites in the promoter region of tumor-suppressor miRNAs and genes (14, 34). Altered miRNA expression may in turn influence their downstream-regulated genes and affect cancer risk and prognosis. Genisteins, major isoflavones in soy food, have been found to increase tumor-suppressors miR-34a and miR-574-3p in pancreatic cancer cells, resulting in inhibition of cell growth and induction of apoptosis (11, 13). In the prostate cancer cell line, isoflavones were shown to increase miR-29a and miR-1256, leading to the inhibition of cell growth and invasion (30). Genisteins were shown to inhibit the oncogenes miR-1260b and miR-151, leading to the suppression of cancer cell growth (15, 31). In addition, soy foods are rich in folate, anti-oxidants and other phytochemicals that have known anti-cancer properties (35). The associations observed in our study could also be attributed to these soy constituents instead of soy isoflavones.
The majority of our identified miRNAs associated with soy intake may play regulatory roles in the inhibition of carcinogenesis. For example, miR-29a-3p has been previously shown in an in vitro study to inhibit prostate cancer cell growth and invasiveness (14). No in vitro study has been conducted for evaluation of the association between any other miRNA and soy intake. MiR-29a-3p, miR-223-3p, miR-142-3p and miR-150-5p are well-known for their involvement in the process of cancerogenesis. Previous studies have shown that miR-223-3p may inhibit cell proliferation by targeting IGF-1R in endometrial carcinoma (36), and is possibly associated with both recurrence and survival in TNBC (37). Both miR-142-3p and miR-150-5p have been suggested to play a dual role in the promotion and inhibition of carcinogenesis (38-41). As soy intake was associated with over-expression of specific tumor-suppressor miRNAs, one might expect soy food consumption to be related to their downstream target genes. In fact, we did find in our study that soy food intake was also associated with a decreasing expression of these miRNA targets, including cell-growth genes CYLD (miR-590-3p), KLF6 (miR-590-3p), GADD45B (miR-219-5p), and IL2RB (miR-150-5p). These results lend strong support to the hypothesis that down-regulations of cell growth-related genes mediated by their upstream miRNAs may be one of the mechanisms by which soy food influences breast cancer etiology and prognosis.
Of the total of 24 identified genes significantly associated with soy food intake, we examined whether those genes function as either tumor suppressor or oncogenes based on a literature review and the Cancer Gene Census catalogue (http://cancer.sanger.ac.uk/census/). Several genes, including KRAS, FGFR4, IL2RB and GADD45B, function as oncogenes, while ERCC1 is reported to function as a tumor suppressor. Correlations for those genes observed in our study are in the expected direction of the hypothesized mechanisms. The functional roles for the remaining genes are either not well known or bidirectional in carcinogenesis. For example, KLF6 plays an important role in the regulation of cell proliferation and apoptosis, and as such has been considered a tumor suppressor. However, a recent report suggests that an oncogenic splice variant (KLF6-SV1) of KLF6 could lead to increased progression and metastasis of breast cancer (42). In our study, soy protein intake was inversely associated with the expression of KLF6.
Our study does not support the finding of Shike et al that soy food intake is associated with over-expression of cell growth-related genes in tumor tissue. However, it should be noted that our study differs from Shike et al in several aspects. First, our study population is Chinese women, almost all of whom have had some soy food exposure during their lifetime, while women in the Shike et al study had only consumed soy for one to four weeks immediately prior to surgery. Sudden exposure to a high dose of soy at a vulnerable window may lead to a quite different consequence than that produced from long-term exposure (17). Second, because almost none of the women in our study were non-soy food eaters, we had to rely on comparisons between highest and lowest quartile. Thus, a stronger association would be expected had the comparisons been made between high soy food consumers and non-consumers. Third, our study included only TNBC cases, while most women in the Shike et al study were diagnosed with ER-positive breast cancer. Thus, our findings may not directly apply to ER or PR positive breast cancers. However, previous studies have suggested that soy intake is associated with breast cancer risk and prognosis irrespective of hormone receptor status (43-45).
It should also be noted that our findings are based on an observational study. Soy food consumption was not randomly assigned. Thus, potential confounding from factors related to self-selection of high/low soy food consumption cannot be ruled out. In addition, the sample size of our study is relatively small, and assessment of dietary intake is inherently prone to misclassification–both of these factors can compromise statistical power. Furthermore, because a large number of genes and miRNAs were evaluated in the study, some of the findings could be due to chance. Only the gene E2F2 reaches the threshold of significance if a false discovery rate P < 0.05 is applied. Another limitation of our study is that we included only 302 genes, selected either to define the intrinsic subtype of breast cancer or for their potential to predict TNBC outcomes. Although a large proportion of these genes are related to cell-cycle regulation, they represent only a small proportion of genes in the relevant pathways. Thus, more studies with increased sample sizes and comprehensive evaluations of gene expression are warranted.
Conclusions
We found that high pre-diagnosis soy food consumption may prevent breast carcinogenesis through the increased expression of tumor-suppressor miRNAs and genes, and decreased expression of oncogenes, especially cell-growth genes, in TNBC tumor tissue.
Supplementary Material
Acknowledgments
This study was supported by grants from the Department of Defense Breast Cancer Research Program (DAMD 17-02-1-0607) and the National Institutes of Health (R01CA118229; P50CA098131). RNA sample preparation was conducted at the Survey and Biospecimen Shared Resources facility that is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA068485).
The authors thank participants and research team members of the Shanghai Breast Cancer Survival Study for their dedication to the study; Ms. Regina Courtney and Dr. Bo Huang for their help with RNA sample preparation; and Ms. Nancy Kennedy for assisting with manuscript preparation and submission.
Footnotes
Conflict of Interest Statement: None declared.
Author Contributions: Xingyi Guo: Methodology, software, validation, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, visualization, and supervision. Qiuyin Cai: Investigation, writing – review and editing, and supervision. Pingping Bao: Investigation, resources, data curation, and writing – review and editing. Jie Wu: Methodology and investigation. Wanqing Wen: Methodology and formal analysis. Fei Ye: Formal analysis and writing – review and editing. Wei Zheng: Investigation, resources, writing – review and editing, and funding acquisition. Ying Zheng: Investigation, resources, data curation, writing – review and editing, and project administration. Xiao-Ou Shu: Conceptualization, methodology, investigation, resources, writing – original draft, writing – review and editing, supervision, and funding acquisition.
References
- 1.Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29(11):2162–8. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer research. 2001;61(13):5045–50. [PubMed] [Google Scholar]
- 3.Douglas CC, Johnson SA, Arjmandi BH. Soy and its isoflavones: the truth behind the science in breast cancer. Anti-cancer agents in medicinal chemistry. 2013;13(8):1178–87. doi: 10.2174/18715206113139990320. [DOI] [PubMed] [Google Scholar]
- 4.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. The American journal of clinical nutrition. 2009;89(6):1920–6. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. Journal of the National Cancer Institute. 2006;98(7):459–71. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. British journal of cancer. 2008;98(1):9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nechuta SJ, Caan BJ, Chen WY, Lu W, Chen Z, Kwan ML, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. The American journal of clinical nutrition. 2012;96(1):123–32. doi: 10.3945/ajcn.112.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. Jama. 2009;302(22):2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. Journal of the National Cancer Institute. 2006;98(18):1275–84. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 10.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. The Journal of nutrition. 2001;131(11 Suppl):3095S–108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 11.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, Saini S, et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PloS one. 2013;8(3):e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Xiang J, Shen J, Zou X, Zhai S, Yin Y, et al. Oncogenic MicroRNA-27a is a target for genistein in ovarian cancer cells. Anti-cancer agents in medicinal chemistry. 2013;13(7):1126–32. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 13.Xia J, Duan Q, Ahmad A, Bao B, Banerjee S, Shi Y, et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Current drug targets. 2012;13(14):1750–6. doi: 10.2174/138945012804545597. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Kong D, Ahmad A, Bao B, Dyson G, Sarkar FH. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics : official journal of the DNA Methylation Society. 2012;7(8):940–9. doi: 10.4161/epi.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, Shahryari V, et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PloS one. 2012;7(8):e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shike M, Doane AS, Russo L, Cabal R, Reis-Filho JS, Gerald W, et al. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. Journal of the National Cancer Institute. 2014;106(9) doi: 10.1093/jnci/dju189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan VC. Avoiding the bad and enhancing the good of soy supplements in breast cancer. Journal of the National Cancer Institute. 2014;106(9) doi: 10.1093/jnci/dju233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baglia ML, Cai Q, Zheng Y, Wu J, Su Y, Ye F, et al. Dual specificity phosphatase 4 gene expression in association with triple-negative breast cancer outcome. Breast cancer research and treatment. 2014;148(1):211–20. doi: 10.1007/s10549-014-3127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. European journal of clinical nutrition. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 20.Su Y, Zheng Y, Zheng W, Gu K, Chen Z, Li G, et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC cancer. 2011;11:292. doi: 10.1186/1471-2407-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark JW, Santos-Moore A, Stevenson LE, Frackelton AR., Jr Effects of tyrosine kinase inhibitors on the proliferation of human breast cancer cell lines and proteins important in the ras signaling pathway. International journal of cancer Journal international du cancer. 1996;65(2):186–91. doi: 10.1002/(SICI)1097-0215(19960117)65:2<186::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Nakata T, Kuzumaki T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer letters. 2002;176(1):17–23. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- 23.Munoz R, Klingenberg O, Wiedlocha A, Rapak A, Falnes PO, Olsnes S. Effect of mutation of cytoplasmic receptor domain and of genistein on transport of acidic fibroblast growth factor into cells. Oncogene. 1997;15(5):525–36. doi: 10.1038/sj.onc.1201226. [DOI] [PubMed] [Google Scholar]
- 24.Chen WF, Gao QG, Wong MS. Mechanism involved in genistein activation of insulin-like growth factor 1 receptor expression in human breast cancer cells. The British journal of nutrition. 2007;98(6):1120–5. doi: 10.1017/S0007114507777139. [DOI] [PubMed] [Google Scholar]
- 25.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Molecular cancer research : MCR. 2007;5(2):195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Interleukin-2 and its receptor complex (alpha, beta and gamma chains) in in situ and infiltrative human breast cancer: an immunohistochemical comparative study. Breast cancer research : BCR. 2004;6(1):R1–7. doi: 10.1186/bcr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Xiao X, Li D, Chi Y, Wei P, Wang Y, et al. Abnormal expression of GADD45B in human colorectal carcinoma. Journal of translational medicine. 2012;10:215. doi: 10.1186/1479-5876-10-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libert F, Passage E, Lefort A, Vassart G, Mattei MG. Localization of human thyrotropin receptor gene to chromosome region 14q3 by in situ hybridization. Cytogenetics and cell genetics. 1990;54(1-2):82–3. doi: 10.1159/000132964. [DOI] [PubMed] [Google Scholar]
- 29.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutrition reviews. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 30.Ling C, Su VM, Zuo D, Muller WJ. Loss of the 14-3-3sigma tumor suppressor is a critical event in ErbB2-mediated tumor progression. Cancer discovery. 2012;2(1):68–81. doi: 10.1158/2159-8290.CD-11-0189. [DOI] [PubMed] [Google Scholar]
- 31.Hirata H, Ueno K, Nakajima K, Tabatabai ZL, Hinoda Y, Ishii N, et al. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. British journal of cancer. 2013;108(10):2070–8. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. The Journal of steroid biochemistry and molecular biology. 2014;140:116–32. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molinie B, Georgel P. Genetic and epigenetic regulations of prostate cancer by genistein. Drug news & perspectives. 2009;22(5):247–54. doi: 10.1358/dnp.2009.22.5.1378633. [DOI] [PubMed] [Google Scholar]
- 34.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. International journal of cancer Journal international du cancer. 2008;123(3):552–60. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 35.Kang J, Badger TM, Ronis MJ, Wu X. Non-isoflavone phytochemicals in soy and their health effects. Journal of agricultural and food chemistry. 2010;58(14):8119–33. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- 36.Huang K, Dong X, Sui C, Hu D, Xiong T, Liao S, et al. MiR-223 suppresses endometrial carcinoma cells proliferation by targeting IGF-1R. American journal of translational research. 2014;6(6):841–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Kleivi Sahlberg K, Bottai G, Naume B, Burwinkel B, Calin GA, Borresen-Dale AL, et al. A Serum MicroRNA Signature Predicts Tumor Relapse and Survival in Triple-Negative Breast Cancer Patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(5):1207–14. doi: 10.1158/1078-0432.CCR-14-2011. [DOI] [PubMed] [Google Scholar]
- 38.Isobe T, Hisamori S, Hogan DJ, Zabala M, Hendrickson DG, Dalerba P, et al. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. eLife. 2014:3. doi: 10.7554/eLife.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen WW, Zeng Z, Zhu WX, Fu GH. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. Journal of molecular medicine. 2013;91(8):989–1000. doi: 10.1007/s00109-013-1037-x. [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Chen Y, Wu W, Ouyang N, Chen J, Li H, et al. miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PloS one. 2013;8(12):e80707. doi: 10.1371/journal.pone.0080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe A, Tagawa H, Yamashita J, Teshima K, Nara M, Iwamoto K, et al. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia. 2011;25(8):1324–34. doi: 10.1038/leu.2011.81. [DOI] [PubMed] [Google Scholar]
- 42.Hatami R, Sieuwerts AM, Izadmehr S, Yao Z, Qiao RF, Papa L, et al. KLF6-SV1 drives breast cancer metastasis and is associated with poor survival. Science translational medicine. 2013;5(169):169ra12. doi: 10.1126/scitranslmed.3004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, et al. Population-based case-control study of soy food intake and breast cancer risk in Shanghai. British journal of cancer. 2001;85(3):372–8. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T, Matsuo K, Tsunoda N, Hirose K, Hiraki A, Kawase T, et al. Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. International journal of cancer Journal international du cancer. 2008;123(7):1674–80. doi: 10.1002/ijc.23644. [DOI] [PubMed] [Google Scholar]
- 45.Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. British journal of cancer. 2008;99(1):196–200. doi: 10.1038/sj.bjc.6604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.