Abstract
Objective
To evaluate conditional disease-free survival (CDFS) for patients who underwent curative intent surgery for adrenocortical carcinoma (ACC).
Background
ACC is a rare but aggressive tumor. Survival estimates are usually reported as survival from the time of surgery. CDFS estimates may be more clinically relevant by accounting for the changing likelihood of disease-free survival (DFS) according to time elapsed after surgery.
Methods
CDFS was assessed using a multi-institutional cohort of patients. Cox proportional hazards models were used to evaluate factors associated with DFS. Three-year CDFS (CDFS3) estimates at “x” year after surgery were calculated as follows: CDFS3=DFS(x+3)/DFS(x).
Results
One hundred ninety-two patients were included in the study cohort; median patient age was 52 years. On presentation, 36% of patients had a functional tumor and median size was 11.5 cm. Most patients underwent R0 resection (75%) and 9% had N1 disease. Overall 1-, 3-, and 5-year DFS was 59%, 34%, and 22%, respectively. Using CDFS estimates, the probability of remaining disease free for an additional 3 years given that the patient had survived without disease at 1, 3, and 5 years, was 43%, 53%, and 70%, respectively. Patients with less favorable prognosis at baseline demonstrated the greatest increase in CDFS3 over time (eg, capsular invasion: 28%–88%, Δ60% vs no capsular invasion: 51%–87%, Δ36%).
Conclusions
DFS estimates for patients with ACC improved dramatically over time, in particular among patients with initial worse prognoses. CDFS estimates may provide more clinically relevant information about the changing likelihood of DFS over time.
Keywords: adrenocortical carcinoma, conditional probability, outcomes, surgery
Adrenocortical carcinoma (ACC) is a rare endocrine tumor that accounts for 0.2% of cancer-related deaths in the United States.1–3 Although complete surgical resection confers a 5-year survival of 32% to 50% in patients with localized ACC, the risk of recurrence after surgical resection for ACC can be as high as 50% to 85%.4–7 Of note, for patients who present with metastatic disease, the median survival is less than 1 year.8,9 Although several clinicopathologic factors are associated with risk of recurrence and survival, the specific set of factors that predict long-term prognosis have been reported to vary widely with a relative lack of consensus.4,10,11
Reliable prognostication at the initial time of treatment for any malignant disease aids both patients and physicians in decisions around adjuvant treatment, type and frequency of surveillance, as well as providing information regarding short- and long-term prognosis. The European Network for the Study of Adrenal Tumors (ENSAT) classification and the American Joint Committee on Cancer (AJCC)/International Union against Cancer (UICC) staging schema are the most widely used mechanisms to assess the prognosis of patients with ACC.1,12,13 Although the ENSAT and AJCC/UICC classification may offer important information for general prognostic assessment, survival estimates based on traditional survival curves may not provide accurate information for long-term prognosis as the risk of recurrence or death is often the highest during the initial years after surgery and diminishes with time.14
Conditional survival (CS) accounts for the changing likelihood of survival with increasing duration of follow-up after surgical resection. As such, CS estimates have been proposed as a more clinically relevant measures to predict long-term prognosis in patients having survived for a specific interval of time after surgery. In particular, CS estimates have been reported for patients with colorectal cancer, lung cancer, renal cancer, gastric cancer, and pancreatic adenocarcinoma, among others.15–25 Given that survival probabilities can change significantly when accounting for time elapsed after diagnosis, CS estimates may be a more useful way of predicting survival compared with conventional survival estimates15,19,22 To our knowledge, no previous study has assessed conditional disease-free survival (CDFS) among patients who underwent curative intent surgery for ACC. Therefore, the aim of the current study was to estimate CDFS among patients after resection of ACC, as well as stratify CDFS according to relevant patient- and disease-related characteristics.
METHODS
Patient Population and Data Collection
Patients were identified from a multi-institutional database consisting of patients who underwent surgery for ACC between January 1993 and December 2014 at 13 major institutions in the United States (Johns Hopkins Hospital, Baltimore, MD; Emory University, Atlanta, GA; Stanford University, Palo Alto, CA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; The Ohio State University, Columbus, OH; Medical College of Wisconsin, Milwaukee, WI; New York University, New York, NY; University of California at San Diego, San Diego, CA; University of California at San Francisco, San Francisco, CA; University of Texas Southwestern Medical Center, Dallas, TX; and Vanderbilt University Medical Center, Nashville, TN). Information on disease characteristics, treatment, and long-term outcomes was extracted from medical records. Demographic and clinicopathologic data included age, sex, race, tumor size, laterality of tumor (ie, left, right), the presence or absence of capsular invasion, T, N, and M stage of disease, mitotic index, and functionality of tumor (ie, hormone-secreting, non-secreting). Tumor size was defined as the maximal diameter of the tumor in the resected specimen. Functional tumors were categorized as glucocorticoid, mineralocorticoid, or estrogen/ androgen hyper-secreting tumors vs nonsecreting tumors. Resection margin status (negative [R0], microscopically/macroscopically positive [R1/R2]) and lymph node status (no metastasis [N0], lymph node metastasis [N1]) were ascertained based on final pathologic assessment. Treatment and operative details included surgical approach (open abdominal or posterior, minimally invasive surgery [MIS], thoraco-abdominal surgery), as well as information on adjuvant chemotherapy, radiotherapy, and/or mitotane therapy. MIS was defined as robotic, laparoscopic, retroperitoneoscopic, or hand-assisted procedures. Only patients who underwent curative-intent surgery were included in the study group; patients with metastatic disease at presentation were excluded. Patients who were younger than 18 years of age or those with missing follow-up data were not included in the study. To avoid including deaths due to postoperative complications, patients who died within 30 days of surgery were excluded. After inclusion and exclusion criteria, 192 patients were included in the analytic cohort. The institutional review boards at each participating institution approved this study.
Statistical Methods
Summary statistics were presented as whole numbers and proportions for categorical variables or as medians with interquartile range (IQR) for continuous variables. Disease-free survival (DFS) estimates for the study population were generated using the Kaplan-Meier method calculated from the date of surgery to time of first documented recurrence, death, or censoring. Univariable analyses were performed using the log-rank test to assess differences in DFS between categorical groups. The association of relevant clinicopathologic variables with DFS was assessed using a Cox proportional hazards model; clinicopathological variables associated with prognosis were assessed a priori based on clinical importance, scientific knowledge, as well as predictors identified in previously published articles,4,10,26 including age, sex, mitotic index, surgical margin, tumor size, presence of capsular invasion, T stage, and functionality of the tumor. Variables that were statistically significant with P<0.05 in univariable analyses were assessed in a multiviariable model; histologic marker (ie, mitotic index) was not incorporated into multivariable analysis regardless of the statistical significance in univariable analysis because more than a half of the study sample had missing data. Multiple imputation and indicator terms were performed in the multivariable analysis for other missing covariates that were missing at less than 20%.
The 3-year conditional disease-free survival (CDFS3) estimates were defined as the probability of remaining disease free for an additional 3 years given that a patient had been disease free for x years, calculated as: CDFS3=DFS(x+3)/DFS(x).27 For example, CDFS3 among patients who had been disease free for 2 years from the date of surgery was calculated by dividing the 5-year DFS rate by the 2-year DFS rate. Changes in CDFS3 over time were assessed using a linear regression. DFS estimates were calculated at surgery and conditional on surviving 1, 2, 3, 4, and 5 years after surgery among the entire cohort and selected subgroups. Survival functions were stratified according to age (≤65 yrs vs >65 yrs), sex (female vs male), size (≤10 cm vs >10 cm), T stage (T1/T2 vs T3/T4), mitotic count in 50 high-power fields (HPF) (≤10 vs >10), functionality of tumors (nonfunctional vs functional), and the presence of capsular invasion (no capsular invasion vs capsular invasion). Standardized differences (d) were used to assess the differences of CS between subgroups based on the method described by Cucchetti et al,20 where the standardized difference is given by (P2–P1)/√[P(1–P)]. As an index to contrast 2 rates, d<|0.1| indicates very small differences between groups, |0.1| ≤ d < |0.3| indicates small differences, |0.3| ≤ d < |0.5| indicates moderate differences, and |0.5| ≤ d indicates considerable differences.20,24,28 All analyses were performed with STATA version 14.0 (StataCorp LP, College Station, TX), and R version 3.0.3 (http://www.r-project.org); all tests were 2-sided and a P < 0.05 was considered statistically significant.
RESULTS
Demographic and Clinicopathologic Characteristics
A total of 192 patients who underwent curative resection for ACC and met inclusion criteria were identified. Median age of the study population was 52 years (IQR: 43, 62) and 64.6% (n=124) of patients were female (Supplemental Table 1, http://links.lww.com/SLA/A924). A majority of patients was white (n=157, n=84.0%). Over one-third of patients had a functional tumor (n=65, 36.3%); most were either glucocorticoid (n=33, 18.4%) or estrogen/androgen (n=22, 12.3%) hyper-secreting tumors, whereas only 10 patients (5.6%) had a mineralocorticoid hormone-secreting type. ACC lesions were equally distributed on the left (n=105, 55.3%) and right (n=85, 44.7%) side. At the time of surgery, the majority of patients underwent an open abdominal or posterior procedure (n=125, 67.6%), whereas the remaining patients had an MIS (n=35, 18.9%) or thoraco-abdominal approach (n=25, 13.5%). On final pathology, median tumor size was 11.5 cm (IQR: 8.0, 15.0); most patients had an R0 margin (n=126, 75.0%), whereas 36 (21.4%) and 6 (3.6%) patients had an R1 and R2 margin, respectively. The majority of patients had capsular invasion (n=81, 60.0%). Approximately half of the tumors were advanced (T3 tumors: n=63, 35.8%; T4 tumors: n=22, 12.5%). About 1 in 3 patients received mitotane therapy postoperatively (n=57, 36.5%), whereas roughly 1 in 10 patients received adjuvant chemotherapy (n=23, 12.8%).
Factors Associated With DFS
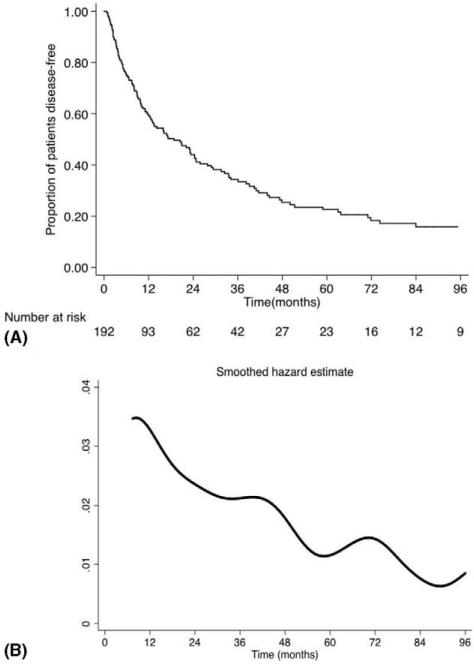
At a median follow-up of 24.3 months, 120 (62.5%) patients recurred and 73 (38.0%) patients had died. Median DFS was 18.8 months (95% confidence interval [CI]: 12.6–24.7) and 1-, 3-, and 5-year DFS was 59.5%, 34.3%, and 22.5%, respectively (Fig. 1A). There were several factors associated with DFS on univariable analysis (Supplemental Table 2, http://links.lww.com/SLA/A924). Specifically, higher mitotic index (>10/50 HPF: hazard ratio [HR]=1.82, 95% CI 1.08–3.08), positive surgical margin (R1/R2: HR=1.82, 95% CI 1.21–2.75), tumor size (>10 cm: HR=1.56, 95% CI 1.08–2.26), presence of capsular invasion (HR=2.03, 95% CI 1.28–3.21), advanced stage (T3/T4: HR=2.04, 95% CI 1.40–2.97), and functional tumors (HR=1.62, 95% CI 1.12–2.34) were associated with worse DFS (all P < 0.05). On multivariable analysis, after adjusting for potential risk factors, capsular invasion (HR=1.65; 95% CI 1.02–2.68; P=0.04) and functional tumors (HR=1.51, 95% CI 1.04–2.20; P=0.03) were independently associated with decreased DFS, whereas advanced stage (T3/T4: HR=1.46, 95% CI 0.95–2.25; P=0.08) also tended to be associated with prognosis.
FIGURE 1.
(A) Kaplan-Meier survival curve and (B) hazard estimate of recurrence or death among the entire cohort.
Comparison of DFS and CDFS
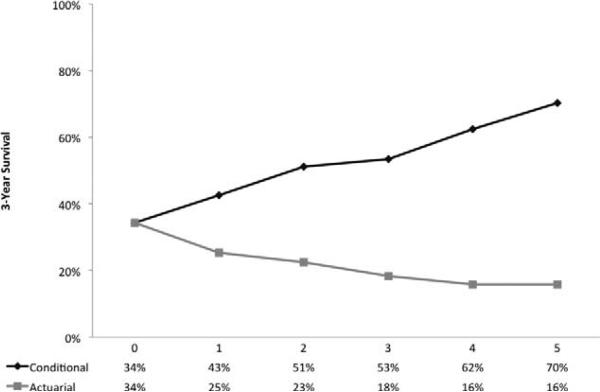
When assessed over time, the likelihood of recurrence or death peaked at 12 months after surgery and subsequently diminished onward (Fig. 1B), whereas the CDFS3 of the entire study population increased over the study period. In particular, the CDFS3 at 2 years (ie, the probability of being disease free at postoperative year 5 after having already been disease free to postoperative year 2) was 51% compared with an actuarial DFS at 5 years of 23% (Fig. 2). Similarly, the CDFS3 at 5 years (ie, the probability of being disease free at postoperative year 8 after having already been disease free to postoperative year 5) was 70% compared with an actuarial DFS at 8 years of 16%. CDFS3 increased over time from 34% to 70% (P<0.001), whereas actuarial DFS decreased over time from 34% at 3 years to 16% at 8 years (P<0.001). The probability of remaining disease free at 5 years after surgery increased as patients remained disease free for a certain amount of time; for example, if the patient was disease free at 12 months, 2 years, 3 years, and 4 years, the probability of remaining disease free at year 5 was 37.9%, 51.2%, 65.8%, and 88.9%, respectively (Table 1).
FIGURE 2.
Three-year conditional disease-free survival relative to actuarial survival among the entire cohort.
TABLE 1.
Percent of Patients Who Have Reached a Specific Time Point Given That They Have Already Remained Disease Free a Certain Amount of Time Among Patients With Adrenocortical Carcinoma Undergoing Surgical Resection
| If the Patient Has Remained Disease Free to |
||||||||
|---|---|---|---|---|---|---|---|---|
| 12 mo | 2 yrs | 3 yrs | 4 yrs | 5 yrs | 6 yrs | 7 yrs | 8 yrs | |
| 12 mo | 100.0 | |||||||
| 2 yrs | 73.9 | 100.0 | ||||||
| 3 yrs | 57.6 | 77.9 | 100.0 | |||||
| 4 yrs | 42.6 | 57.6 | 74.0 | 100.0 | ||||
| 5 yrs | 37.9 | 51.2 | 65.8 | 88.9 | 100.0 | |||
| 6 yrs | 30.7 | 41.6 | 53.4 | 72.2 | 81.2 | 100.0 | ||
| 7 yrs | 26.6 | 36.0 | 46.2 | 62.4 | 70.2 | 86.5 | 100.0 | |
| 8 yrs | 26.6 | 36.0 | 46.2 | 62.4 | 70.2 | 86.5 | 100.0 | 100.0 |
When DFS was assessed relative to clinically important variables, mitotic index (>10/50 HPF), size (>10 cm), capsular invasion, advanced stage (T3/T4), positive margin (R1/R2), and functional tumors were each associated with prognosis (all P<0.05), whereas there were no association with age and sex (both P>0.05) (Table 2). The role of relevant clinical and tumor characteristics at different time points during follow-up was then examined conditioned on the intervals that had already been accrued by estimating CDFS3 for strata of different clinicopathologic variables (Table 3). CDFS3 in each subgroup increased after surgery (all P<0.05). Of note, the CDFS3 of patients with advanced T stages (ie, T3 and T4), high mitotic index (ie, >10/50 HPF), as well as positive margin (ie, R1 and R2) remained worse during the first 3 years. In contrast, tumor factors such as size, capsular invasion, and functionality of tumors had an impact on prognosis for the first 2 years after surgery, but had only a small impact from the second year onward. For example, patients with capsular invasion initially had a lower CDFS3 of 27.7% vs 50.6% for patients who did not have capsular invasion; however, at 5 years from the time of surgery, CDFS3 of patients with and without capsular invasion was very similar.
TABLE 2.
Actuarial Disease-free Survival Rates of Patients in Relationship to Clinicopathologic Features
| Patient Disease-free Survival |
||||
|---|---|---|---|---|
| Variables | 3 yrs, % | 5 yrs, % | 8 yrs, % | P |
| All patients | 34.3 | 22.5 | 15.8 | — |
| Age | 0.39 | |||
| ≤65 yrs | 37.2 | 23.3 | 15.3 | |
| >65 yrs | 18.4 | 18.4 | 18.4 | |
| Sex | 0.74 | |||
| Female | 33.6 | 24.0 | 19.1 | |
| Male | 35.4 | 19.6 | 10.5 | |
| Mitotic index | 0.02 | |||
| ≤10/50 HPF | 33.2 | 29.5 | 20.2 | |
| >10/50 HPF | 20.9 | 7.8 | 7.8 | |
| Size | 0.02 | |||
| ≤10 cm | 43.8 | 28.9 | 22.3 | |
| >10 cm | 28.9 | 19.4 | 12.4 | |
| Capsular invasion | 0.002 | |||
| No | 50.6 | 31.9 | 27.9 | |
| Yes | 27.7 | 19.3 | 16.9 | |
| T stage | <0.001 | |||
| I & II | 45.0 | 35.9 | 27.3 | |
| III & IV | 21.6 | 9.1 | 7.2 | |
| Margin | 0.004 | |||
| R0 | 37.2 | 26.8 | 21.3 | |
| R1/R2 | 20.0 | 10.0 | — | |
| Functional tumors | 0.009 | |||
| No | 43.5 | 28.5 | 22.2 | |
| Yes | 19.5 | 14.7 | 12.2 | |
TABLE 3.
Three-year Conditional Disease-free Survival Rates of Patients in Relationship to Clinicopathologic Features
| Conditional 3-yr Disease-free Survival, % |
|||||
|---|---|---|---|---|---|
| Variables | At Surgery | 1 yr After Surgery | 2 yrs After Surgery | 3 yrs After Surgery | 5 yrs After Surgery |
| All patients | 34.3% | 42.6% | 51.2% | 53.4% | 70.2% |
| Age | |||||
| ≤65 yrs | 37.2% | 44.6% | 50.8% | 49.0% | 65.5% |
| >65 yrs | 18.4% | 31.5% | 53.6% | 100.0% | 100.0% |
| |d| | 0.39 | 0.27 | 0.06 | 1.04 | 0.75 |
| Sex | |||||
| Female | 33.6% | 43.3% | 55.9% | 56.8% | 79.5% |
| Male | 35.4% | 40.8% | 42.9% | 47.6% | 53.6% |
| |d| | 0.04 | 0.05 | 0.26 | 0.18 | 0.61 |
| Mitotic Index | |||||
| ≤10/50 HPR | 33.2% | 50.0% | 59.5% | 76.2% | 68.5% |
| >10/50 HPR | 20.9% | 34.1% | 23.4% | 37.5% | 100.0% |
| |d| | 0.27 | 0.32 | 0.73 | 0.84 | 0.74 |
| Size | |||||
| ≤10cm | 43.8% | 50.5% | 49.8% | 50.8% | 77.1% |
| >10 cm | 28.9% | 38.7% | 54.9% | 55.3% | 64.2% |
| |d| | 0.31 | 0.24 | 0.10 | 0.09 | 0.28 |
| Capsular invasion | |||||
| No | 50.6% | 47.9% | 47.3% | 55.2% | 87.5% |
| Yes | 27.7% | 45.0% | 54.8% | 60.9% | 87.5% |
| |d| | 0.47 | 0.06 | 0.15 | 0.12 | 0 |
| T stage | |||||
| I & II | 45.0% | 59.8% | 62.8% | 68.4% | 76.2% |
| III & IV | 21.6% | 21.9% | 30.6% | 33.5% | 79.9% |
| |d| | 0.49 | 0.76 | 0.65 | 0.71 | 0.09 |
| Margin | |||||
| R0 | 37.2% | 45.2% | 54.7% | 63.1% | 79.6% |
| R1/R2 | 20.0% | 31.2% | 37.5% | 33.4% | — |
| |d| | 0.36 | 0.28 | 0.35 | 0.60 | |
| Functional tumors | |||||
| No | 43.5% | 47.6% | 56.4% | 56.0% | 77.8% |
| Yes | 19.5% | 33.6% | 46.0% | 62.5% | 83.4% |
| |d| | 0.49 | 0.28 | 0.21 | 0.13 | 0.14 |
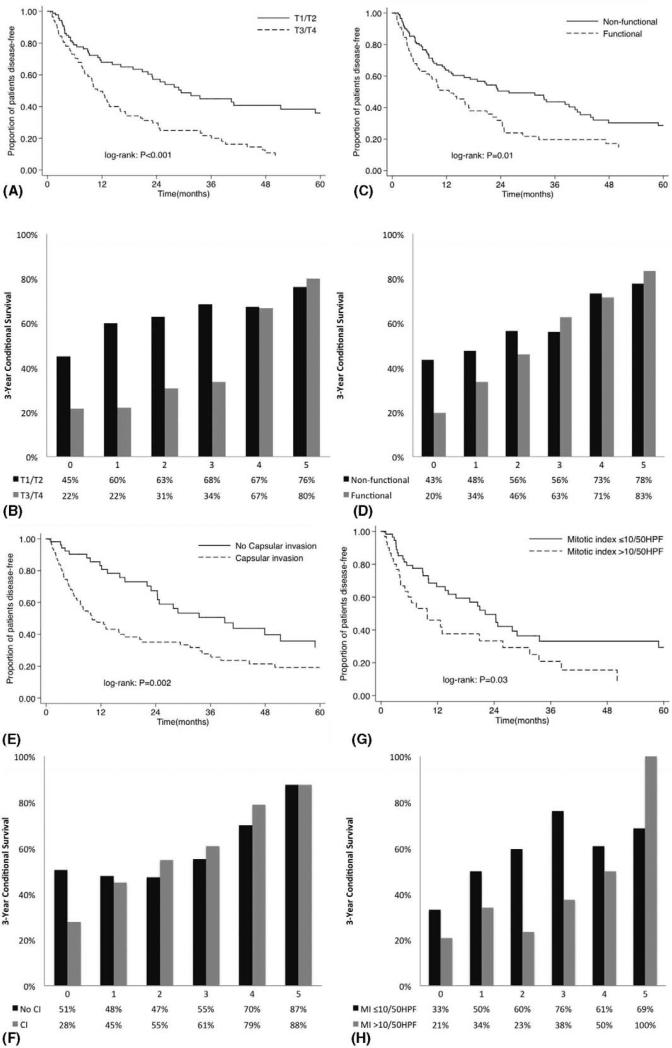
When comparing calculated CDFS3 vs actuarial DFS, CDFS3 exceeded the actuarial survival for all subgroups examined (Tables 2 and 3). However, the changes in CDFS3 were more pronounced in subgroups of patients who initially had the least favorable tumor characteristics (Fig. 3). For example, larger changes in CDFS3 were seen among patients with capsular invasion (28%–88%, Δ60%) vs without capsular invasion (51%–87%, Δ36%). Similarly, patients with functional tumors (20%–83%, Δ63%) had larger differences in CDFS3 vs patients without functional tumors (43%–78%, Δ35%). These patterns were also seen in CDFS3 among patients with T3/T4 (22%–80%, Δ58%) vs T1/T2 (45%–76%, Δ31%) tumors, as well as among patients with high mitotic index (>10/50 HPF) (21%–100%, Δ79%) vs low mitotic index (≤10/50 HPF) (33%–69%, Δ36%) disease (all P < 0.001).
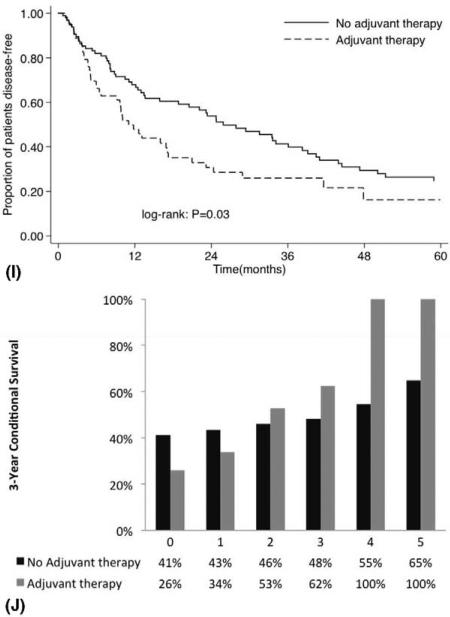
FIGURE 3.
Overall actuarial disease-free survival stratified by: (A) T stage, (C) functionality, (E) capsular invasion, (G) mitotic index, and (I) adjuvant therapy vs conditional disease-free survival relative to actuarial survival stratified by: (B) T stage, (D) functionality, (F) capsular invasion, (H) mitotic index, and (J) adjuvant therapy.
Of note, patients who initially had the least favorable tumor characteristics had the most pronounced CDFS3 changes; differences in long-term prognosis between calculated CDFS3 and actuarial DFS were again most pronounced among patients who would have been initially predicted to have the worst survival (Tables 3 and 4). For example, although the 8-year actuarial DFS for patients with T3/T4 disease was only 7.2%, the CDFS3 at 5 years was 79.9% (Δ72.7%). In contrast, patients with favorable tumor characteristics at baseline had smaller differences in actuarial and CDFS estimates. Specifically, the 8-year actuarial DFS for patients with T1/T2 disease was 27.3% and the CDFS3 at 5 years was 76.2 (Δ48.9%).
DISCUSSION
Although surgical resection remains the treatment of choice for ACC, recurrence is common with as many as 50% to 85% of patients experiencing recurrence.4–7 The overall 5-year survival of patients with ACC after surgery varies widely ranging from 15% to 80% depending on a number of factors determined at the time of diagnosis, reflecting the prognostic heterogeneity associated with the disease.6,12,29 With such a wide range of survival estimates, traditional actuarial survival estimates based on data around the time of diagnosis or resection may not be accurate. CS estimates take into account this “accrued” survival time, and provides clinically relevant survival estimates of long-term outcome for patients who return to clinic years after surgery inquiring about further prognosis.30 The current study is important because, for the first time, it defines CS for long-term DFS after curative intent surgery for ACC in a large, multi-institutional cohort of patients. On average, CDFS estimates increased over time and were higher than traditional overall survival (OS) estimates. More importantly, the magnitude of difference between CS and actuarial OS estimates was highest among patients with worse prognostic features. These data suggest that the prognostic importance of these factors decreased as time after surgery increased. Perhaps more importantly, current data provide important information for patients who are alive after resection of ACC and are seeking information about future prognosis.
As prognostic factors reported to be associated with outcomes have varied widely, the optimal method for risk stratification of patients with ACC remains unclear.4,10,11,13,31 Although several studies have reported that age, sex, grade of tumors, functionality, or size of tumor were associated with poor prognosis in ACC patients,4,8,11,31–36 other study groups have reported no correlation between age, sex, tumor size, or functionality and long-term survival.7,10,37–40 In the current study, we similarly did not demonstrate an association between age, sex, and tumor size with DFS (Supplementary Table 2, http://links.lww.com/SLA/A924). Although several studies on ACC have demonstrated surgical margin (ie, R0 vs R1/R2) and tumor stage at diagnosis were associated with worse prognosis,1,4,11,35,36 we failed to find an association of these factors with prognosis on standard survival analyses (Supplementary Table 2, http://links.lww.com/SLA/A924). In contrast, T stage and surgical margin were associated with outcome in conditional DFS estimates among subgroups at all time points until 3 years after surgery. Similarly, although capsular invasion and functionality of the tumor were found to be associated with worse DFS on conventional survival metrics, these factors predicted DFS only in the first 2 years on CS analyses. These data are of note as we were able to demonstrate how the prognostic impact of different clinicopathologic factors after resection of ACC changed over time.
Several investigators, including our own group, have argued that CS estimates provide more accurate prognostic information than standard survival estimates because CS takes into account a patient's changing likelihood of survival over time.15,19 Traditionally, the outcome of patients with ACC has only been reported as 5-year DFS or OS based on AJCC/UICC or ENSAT staging system or predictive nomograms.13,41 Although these tools provide important prognostic information, factors identified at the time of surgery do not take into account time actually accrued and therefore do not reflect how the “prognostic power” of different clinicopathologic variables may change over time after initial diagnosis or treatment. Such information is important to patients who are seeking to be informed of their updated prognosis as time passes from diagnosis and treatment. For example, we noted that patients who remained disease free at 3 years had a 53.4% chance of staying disease free for an additional 3 years, whereas the actuarial DFS at 6 years predicted at the time of surgery was only 18.3%. As demonstrated (Fig. 1), the risk of death was not uniform over time and the majority of recurrences and deaths (60.0%) occurred within the first 3 years after surgical resection for ACC with a steady decrease thereafter. In fact, risk predictors identified at the time of surgery did not take into account time already passed, and patients who survived without relapsing for a certain period had a better prognosis despite their initial higher risk. For example, 5-year actuarial OS predicted at the time of surgery was only 23% vs a CDFS3 of 51% for patients who survived the first 2 years after surgery. In other words, if you survived to year 2, your chances of now surviving to year 5 was 48%—not the initially predicted 25%. Furthermore, continuous improvement in CS estimates during the study period indicated that the underlying hazard of death continued to change over time. As such, by considering the time already passed from surgery, CS estimates can provide critical quantitative prognostic information for survivors.
Several studies have demonstrated that the greatest increases in CS were among patients diagnosed with advanced stage cancers.3,17,18,21,42 Data from the current study similarly noted that the most important gains in survival estimates were among patients with aggressive disease. In particular, patients with worse prognostic features had a higher increase in CDFS based on actual time survived (Table 3 and Fig. 3). In line with previous studies,15–25 we noted that the greatest increase in CDFS3 was among patients who were initially predicted to have the poorest prognosis. For example, patients without capsular invasion showed only a 36% increase in CDFS3 vs a 60% increase among patients with capsular invasion (Fig. 3). Given that recurrence is common after curative intent surgery among patients with ACC, standard survival curves calculated at the time of diagnosis or surgery of ACC are relatively pessimistic. As time passes, even patients who were initially predicted to have a poor prognosis “out run” the impact of any specific clinicopathologic factor. In turn, patients with the least favorable prognosis at the time of surgery showed the greatest increase in conditional DFS after 5 years. As such, CS estimates may be most clinically relevant to high-risk patients who have survived a period of time after ACC resection.
Several limitations should be considered when interpreting our data. Despite being a large series of nonmetastatic ACCs combining the experience of 13 large health care centers, the study still had a relatively small size. As such, some analyses may have been limited. Although collaborating with multiple institutions may have caused heterogeneity regarding the specific diagnostic and/or treatment protocols utilized, the multi-institutional nature of the current study supports the generalizability of our findings. Finally, the current study specifically examined CS probability relating to initial recurrence. As such, the data do not inform the likelihood of survival after a recurrence has occurred. Patients with recurrence are often treated with multiple modalities of standard and experimental therapies, and the current CS model does not provide insight into long-term prognosis after a recurrence.
In conclusion, the current study demonstrated that DFS estimates after surgical resection of ACC were dynamic as the likelihood of DFS changed over time after surgery. Specifically, the probability of remaining disease free for an additional 3 years given that a patient was disease free at 1 year, 3 years, and 5 years was 42.6%, 53.4%, and 70.2%, respectively. In fact, DFS estimates for patients with ACC improved dramatically over time, in particular among patients with initial worse prognoses. CDFS estimates may provide more clinically relevant information about the changing likelihood of DFS in the subset of patients who survive over time. CDFS estimates may provide more accurate prognostic DFS information for patients enrolled in long-term surveillance programs after resection of ACC.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
Disclaimers: The authors declare no conflict of interests.
REFERENCES
- 1.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adreno-cortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 2.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 3.Sun M, Abdollah F, Bianchi M, et al. Conditional survival of patients with urothelial carcinoma of the urinary bladder treated with radical cystectomy. Eur J Cancer. 2012;48:1503–1511. doi: 10.1016/j.ejca.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 5.Terzolo M, Baudin AE, Ardito A, et al. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169:263–270. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 6.Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol. 2014;21:3509–3514. doi: 10.1245/s10434-014-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 8.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu S, Sywak M, Robinson B, et al. Adrenocortical cancer: recent clinical and molecular advances. Curr Opin Oncol. 2004;16:13–18. doi: 10.1097/00001622-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Fassnacht M, Libe R, Kroiss M, et al. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol. 2011;7:323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 11.Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 12.Asare EA, Wang TS, Winchester DP, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery. 2014;156:1378–1385. doi: 10.1016/j.surg.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46:713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mertens AC, Yong J, Dietz AC, et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2015;121:1108–1117. doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764. 764–766. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76:237–242. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. 2003;21:3035–3040. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 18.Wang SJ, Emery R, Fuller CD, et al. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10:153–158. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 19.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucchetti A, Piscaglia F, Cescon M, et al. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res. 2012;18:4397–4405. doi: 10.1158/1078-0432.CCR-11-2663. [DOI] [PubMed] [Google Scholar]
- 21.Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66:361–370. doi: 10.1016/j.eururo.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Kurta ML, Edwards RP, Moysich KB, et al. Prognosis and conditional disease-free survival among patients with ovarian cancer. J Clin Oncol. 2014;32:4102–4112. doi: 10.1200/JCO.2014.55.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee M, Muenz DG, Worden FP, et al. Conditional survival in patients with thyroid cancer. Thyroid. 2014;24:1784–1789. doi: 10.1089/thy.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facciorusso A, Del Prete V, Antonino M, et al. Conditional survival analysis of hepatocellular carcinoma patients treated with radiofrequency ablation. Hepatol Res. 2015;45:E62–E72. doi: 10.1111/hepr.12458. [DOI] [PubMed] [Google Scholar]
- 25.Polley MY, Lamborn KR, Chang SM, et al. Conditional probability of survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2011;29:4175–4180. doi: 10.1200/JCO.2010.32.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson LA, Rivera M, Zhang J. Adrenocortical carcinoma: review and update. Adv Anat Pathol. 2014;21:151–159. doi: 10.1097/PAP.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 27.Bischof DA, Kim Y, Dodson R, et al. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors: a multi-institutional analysis of 502 patients. JAMA Surg. 2015;150:299–306. doi: 10.1001/jamasurg.2014.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnand B, Kernan WN, Feinstein AR. Indexes and boundaries for “quantitative significance” in statistical decisions. J Clin Epidemiol. 1990;43:1273–1284. doi: 10.1016/0895-4356(90)90093-5. [DOI] [PubMed] [Google Scholar]
- 29.Carnaille B. Adrenocortical carcinoma: which surgical approach? Langenbecks Arch Surg. 2012;397:195–199. doi: 10.1007/s00423-011-0852-1. [DOI] [PubMed] [Google Scholar]
- 30.Dikken JL, Baser RE, Gonen M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann Surg Oncol. 2013;20:1623–1630. doi: 10.1245/s10434-012-2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison LE, Gaudin PB, Brennan MF. Pathologic features of prognostic significance for adrenocortical carcinoma after curative resection. Arch Surg. 1999;134:181–185. doi: 10.1001/archsurg.134.2.181. [DOI] [PubMed] [Google Scholar]
- 32.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 33.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro RC, Michalkiewicz EL, Figueiredo BC, et al. Adrenocortical tumors in children. Braz J Med Biol Res. 2000;33:1225–1234. doi: 10.1590/s0100-879x2000001000013. [DOI] [PubMed] [Google Scholar]
- 35.Icard P, Louvel A, Chapuis Y. Survival rates and prognostic factors in adrenocortical carcinoma. World J Surg. 1992;16:753–758. doi: 10.1007/BF02067377. [DOI] [PubMed] [Google Scholar]
- 36.Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: surgical progress or status quo? Arch Surg. 2001;136:543–549. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 37.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20:941–950. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 38.Canter DJ, Mallin K, Uzzo RG, et al. Association of tumor size with metastatic potential and survival in patients with adrenocortical carcinoma: an analysis of the National Cancer Database. Can J Urol. 2013;20:6915–6921. [PubMed] [Google Scholar]
- 39.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 40.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92:1113–1121. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 41.Zini L, Capitanio U, Jeldres C, et al. External validation of a nomogram predicting mortality in patients with adrenocortical carcinoma. BJU Int. 2009;104:1661–1667. doi: 10.1111/j.1464-410X.2009.08660.x. [DOI] [PubMed] [Google Scholar]
- 42.Ploussard G, Xylinas E, Lotan Y, et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur Urol. 2015;67:803–812. doi: 10.1016/j.eururo.2014.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.