Abstract
The relationship of cerebral vessel pathology to brain microinfarcts is not fully understood. We examined associations of cerebral vessel pathology with microinfarcts among community‐dwelling persons who came to autopsy. Brain specimens were derived from 1,066 deceased subjects (mean age‐at‐death = 88 years, 65% women) participating in a cohort study of aging. Microinfarcts were classified by number, age and location. Severity of vessel pathologies was graded semi‐quantitatively. Almost a third of subjects (n = 300; 28%) had at least one chronic microinfarct, including 128 cortical only, 120 subcortical only, and 47 with both. Moderate‐to‐severe atherosclerosis was present in 430 (41%) subjects, arteriolosclerosis in 382 (36%), and amyloid angiopathy in 374 (35%). The odds of one or multiple microinfarct(s) was increased for more severe atherosclerosis (OR =1.22; 95%CI: 1.03–1.45), arteriolosclerosis (OR =1.18; 95%CI: 1.02–1.37) and amyloid angiopathy (OR =1.13; 95%CI: 1.00–1.28). Separately, the odds of subcortical microinfarct(s) was increased for atherosclerosis (OR =1.49; 95%CI: 1.20–1.84) and arteriolosclerosis (OR =1.39; 95%CI: 1.16–1.67) but not amyloid angiopathy; whereas the odds of cortical microinfarct(s) was increased for amyloid angiopathy (OR =1.26; 95%CI: 1.09–1.46) only. While cerebral vessel pathologies are associated with microinfarct burden, atherosclerosis and arteriolosclerosis are associated with subcortical microinfarcts, and amyloid angiopathy with cortical microinfarcts.
Keywords: atherosclerosis, brain, cerebrovascular disease, epidemiology, infarction, pathology
Introduction
Brain infarcts are a major pathologic substrate of cerebrovascular disease. While much effort has been spent on the study of large infarcts, data on small infarcts and on microinfarcts, in particular, are limited. Yet, brain microinfarcts are now recognized as common in aging, perhaps even more common than large infarcts, and have been shown to be associated with poor health outcomes, including impaired cognition and dementia 5, 24. A major reason that microinfarcts have been less well studied than larger infarcts is that they remain very challenging to identify during life, and definitive identification typically requires pathologic examination of brain tissue. To understand better the pathophysiologic mechanisms of microinfarcts, pathologic studies are needed, in particular studies which include data on other vascular pathologies such as cerebral vessel disease of both large and small vessels. Nonetheless, neuropathologic data on microinfarcts in humans remain sparse. A few recent studies on the relationship of cerebral vessel pathologies to microinfarcts suggest mixed results 14, 19, 32.
The overall goal of this study was to determine which cerebral vessel pathologies are independently associated with microinfarcts. A total of 1066 autopsied women and men, who were participating in one of two epidemiologic, clinical‐pathologic cohort studies of aging and came to autopsy, had complete cerebral vessel pathological data and were included in the analyses. We tested whether postmortem indices of three cerebral vessel pathologies, specifically atherosclerosis, arteriolosclerosis and amyloid angiopathy, are each separately associated with a higher odds of one or multiple microinfarct(s). We also examined associations of vessel pathologies with location of microinfarct, both cortical and subcortical.
Materials and Methods
Brain specimens
Brain specimens from deceased and autopsied older women and men were obtained from one of two ongoing epidemiologic, clinical‐pathologic cohort studies of aging: the Rush Memory and Aging Project and the Religious Orders Study. Both studies have Rush University Medical Center Institutional Review Board approval. Detailed methods of the studies were previously published 2, 3. Briefly, both studies were designed to have a common core of methods, including recruitment and specimen and data collection, which facilitates the merging of data to examine the relation of risk factors to neurological aspects of aging and brain pathology. At time of enrollment, each subject consented in writing to annual clinical evaluations, and signed an anatomical gift act for brain donation at time of death. Follow‐up and autopsy rates in both studies are high, and more than 80%.
The Rush Memory and Aging Project began subject enrollment in 1997. There were 1,736 community‐dwelling persons who underwent a baseline clinical evaluation by the time of analyses for the current study, and of these, 701 have died. Excluding those who withdrew prior to death (n = 10), 552 subjects underwent a brain autopsy (81% autopsy rate), thus providing the brain specimens necessary for the conduct of the current study. The first 513 subjects with complete neuropathology data available were included in analyses.
The Religious Orders Study Core started subject enrollment in 1994. There were 1,194 community‐dwelling, Catholic clergy women and men with a baseline clinical evaluation. Excluding subjects who withdrew (n = 2), 667 subjects have died, and of these, 622 have come to autopsy (93% autopsy rate). The first 553 subjects with complete neuropathology data available were included in analyses.
Clinical data
Baseline and annual follow‐up clinical evaluations were conducted in the community by the research team, and data were recorded on laptop computers, as previously described 2, 3. A range of clinical data was collected, including on vascular risk factors and diseases (such as stroke). Neuropsychological data were reviewed by a senior neuropsychologist. Each year, a clinician with expertise in dementia reviewed all data from the current evaluation cycle, blinded to previously collected data, and rendered the diagnostic classifications of cognitive, and other neurologic and medical conditions. In this study, we used the final diagnostic classification data rendered by an expert neurologist, based on data from across all study years and blinded to neuropathologic findings, including for the diagnosis of dementia and also specifically Alzheimer's disease (AD), and mild cognitive impairment (MCI). Details about the available clinical data are reported elsewhere 2, 3.
Neuropathologic data
Detailed and systematic neuropathologic evaluations of brain specimens were conducted, all blinded to clinical data, as reported elsewhere 23. In summary, each brain underwent a uniform gross and histologic evaluation for common age‐related pathologies including cerebrovascular disease.
Assessment for brain infarcts followed a multi‐step procedure. Gross examination documented number, volume and location of visualized infarcts (macroscopic or gross infarcts). Each gross infarct was then confirmed on microscopic examination and classified by age. Lacunar infarcts were defined as macroscopic infarcts in the subcortical gray or white matter which were 1cm in greatest dimension or less. Microscopic examination also allowed for the identification of microinfarcts, which were, by definition, not visible to the naked eye and identified only under microscopy 1. Blocks from a minimum of nine regions including midfrontal, middle temporal, entorhinal, hippocampal and inferior parietal cortices, anterior cingulate, thalamus, basal ganglia and midbrain, were paraffin embedded, cut, mounted on slides and stained with H&E for the purpose of documentation of presence and number (count) of microinfarcts. Location (cortical, subcortical, other) and age (chronic, subacute, acute) of microinfarcts were also recorded. For analyses in this study, only chronic infarcts were considered, and infarct outcomes were categorized into three levels according to number of infarcts identified: none, one, or multiple (more than one). Also, additional analyses considered location of microinfarcts, with number of cortical and of subcortical microinfarcts as separate outcomes.
Assessment for cerebral vessel pathology was also systematically conducted, including for large and small vessels. Assessment of large vessel atherosclerosis was performed by visual inspection after paraformaldehyde fixation, at the Circle of Willis at the base of the brain, and included evaluation of the vertebral, basilar, posterior cerebral, middle cerebral, and anterior cerebral arteries and their proximal branches. Severity was graded by visual examination of the extent of involvement of each artery and number of arteries involved. Arteries were bisected for evaluation of luminal narrowing when there was concern by appearance or palpation for occlusion; and degree of occlusion was also considered in the final score. We did not consider tortuosity or aneurysmal dilatation, and did not perform histologic evaluation. We used a semi‐quantitative scale from 0 (no atherosclerosis) to 6 (severe atherosclerosis), with a score of 1 indicating mild pathology and a score of 3 indicating moderate pathology. Mild atherosclerosis describes small amounts of atherosclerosis of up to several arteries (typically less than 25% vessel involvement) without significant occlusion; moderate atherosclerosis describes atherosclerosis in up to half of all visualized major arteries, with less than 50% occlusion of any single vessel; and severe atherosclerosis describes atherosclerosis in the more than half of all visualized arteries, and/or more than 75% occlusion of one or more vessels. Arteriolosclerosis was evaluated on histologic examination, on H&E stained sections of the anterior basal ganglia (including the anterior caudate, putamen, globus pallidus and internal capsule) 6. Severity of this pathology was graded based on concentric hyaline thickening of vessel walls. Emphasis was placed on evaluation of smaller arterioles, less than approximately 50 microns. The scale ranged from 0 if there was no arteriolosclerosis; 1 for mild arteriolosclerosis, if arteriole walls were minimally thickened; 3 for moderate, if arteriole walls were increased up to 2 × normal thickness; and 5 for severe, if arteriolar wall thickness was more than twofold greater in size than normal. A score of 6 reflected complete small vessel occlusion. For the purpose of analyses for this study, severity of each of these vessel pathologies was grouped into four levels: not present, mild, moderate and severe. Finally, the presence of cerebral amyloid angiopathy was determined on immunohistochemical examination of meningeal and parenchymal vessels in four neocortical regions (midfrontal, middle temporal, angular and calcarine cortices). Slides were cut from paraffin embedded blocks and stained with one of three antibodies to amyloid beta (10D5 Beta Amyloid, 17‐24 (4G8) Covance Madison, 1:9000; Anti‐Human Amyloid‐Beta 1‐16 (10D5) Elan Pharmaceuticals, San Francisco, 1:300; Anti‐Human Beta‐Amyloid, (6F/3D), DAKO North America, Carpinteria, 1:50). For each region, vascular deposition of amyloid beta was scored from 0 (none) to 4 (extensive circumferential deposition), similar to recently published guidelines 18. For analyses, regional amyloid angiopathy scores were averaged across the four neocortical regions to create a mean score of none, mild, moderate and severe for analyses. A separate binary measure of capillary amyloid angiopathy (present vs. absent) was determined based on presence of capillary amyloid angiopathy in any of the four regions.
Immunohistochemical methods were used to obtain markers of AD pathology, as described elsewhere 2, 3. Here, we used summary measures for the presence of amyloid and tangles separately.
Statistical analysis
We first obtained descriptive statistics for the entire autopsied group, and examined associations among age, microinfarcts, grades of cerebral vessel pathologies, and other clinical and pathologic variables, with odds ratios, chi squared tests and Spearman correlations. Analyses in this study considered chronic microinfarcts only. Next, we examine the relationship of cerebral vessel pathology to microinfarcts. All these and subsequent analyses included terms to adjust for age and sex. In the primary analysis, we used an ordinal logistic regression (proportional odds model) to examine the effects of severity of atherosclerosis, arteriolosclerosis and amyloid angiopathy on the odds of more microinfarcts being present. In this model, the primary predictors of interest were the severity grades of the three vessel pathologies, with no vessel pathology as the reference group. The key estimate from this model is an odds ratio which quantifies the shift in the likely burden of microinfarcts, because the odds ratio is interpretable both as the ratio of the odds of any microinfarct relative to none and as the odds of multiple microinfarcts relative to none. The outcome variable had three levels: microinfarcts not present (reference level), a single microinfarct recorded, or multiple microinfarcts recorded. We conducted similar analyses, with additional terms for the interaction of vessel pathologies with dementia, and other analyses with cortical and subcortical microinfarct outcomes separately (each outcome with three levels: none, single, multiple).
We checked for violations of the ordinal models using the score test for proportional odds and graphical analyses. All analyses were programmed in SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Demographic, clinical and neuropathologic characteristics
Demographic, clinical and neuropathologic characteristics of the 1,066 subjects studied are shown in Table 1. The mean age‐at‐death was 88.4 years and 65% were women. Vascular risk factors and diseases were common, as was dementia (with probable AD present in 390 subjects). Nearly half of the subjects (n = 517) had evidence of one or more brain infarcts (whether microinfarcts or gross infarcts) at postmortem, and most (n = 335) were not identified during the study (silent strokes). Almost a third of subjects (300/1,066) had one or more microinfarcts, of which about half also had gross infarcts (161/300). Of these 161 subjects, 117 had cortical infarcts and 150 had subcortical infarcts. Of those 150 with subcortical infarcts, 111 had lacunar infarct and 54 had non‐lacunar infarcts. Among all 300/1,066 subjects with microinfarcts, 114 had multiple microinfarcts. Microinfarcts were present in cortical and subcortical regions in similar percentages of subjects. There were 128 subjects with cortical microinfarcts without subcortical microinfarcts, 120 subjects with subcortical microinfarcts without cortical microinfarcts, 47 with both, and 5 with microinfarcts in other regions.
Table 1.
Demographic, clinical, and neuropathologic characteristics of subjects*
| Characteristics | Total n = 1,066 |
|---|---|
| Demographic | |
| Age‐at‐death, years (SD) | 88.4 (SD = 6.6) |
| Sex, female | 691 (65%) |
| Education, years (SD) | 16.3 (3.7) |
| Clinical | |
| Vascular risk factors (present at any time point) | |
| Hypertension | 695 (65%) |
| Diabetes | 224 (21%) |
| Vascular diseases (present at any time point) | |
| Stroke | 235 (22%) |
| Myocardial infarction | 236 (22%) |
| Cognitive conditions | |
| Dementia | 463 (43%) |
| Mild cognitive impairment | 258 (24%) |
| No cognitive impairment | 341 (32%) |
| Neuropathologic | |
| Chronic brain infarcts | |
| Any infarct present | 517 (49%) |
| Microinfarct burden | |
| Single | 186 (17%) |
| Multiple | 114 (11%) |
| Microinfarct region | |
| Cortical | 175 (16%) |
| Subcortical | 167 (15%) |
| Cerebral vessel pathology severity | |
| Atherosclerosis | |
| Low | 481 (45%) |
| Moderate | 338 (32%) |
| Severe | 92 (9%) |
| Arteriolosclerosis | |
| Low | 364 (34%) |
| Moderate | 273 (26%) |
| Severe | 109 (10%) |
| Amyloid Angiopathy | |
| Low | 469 (44%) |
| Moderate | 242 (23%) |
| Severe | 132 (12%) |
*N (%), unless otherwise specified
The frequencies of subcortical and cortical microinfarcts are shown for the 300 subjects with microinfarcts in Table 2. Cortical microinfarcts were single in 124 subjects, and multiple in 51 subjects. Subcortical microinfarcts were single in 123 subjects, and multiple in 44 subjects. A total of 47 persons had both cortical and subcortical microinfarcts, suggesting that the two brain regions only partially share pathophysiologic mechanisms for the development of microinfarcts. There were five persons with microinfarcts noted in the brainstem or cerebellum, but not in cortical or subcortical regions. However, we did not systematically evaluate the brainstem and cerebellum in all subjects.
Table 2.
Joint frequency of cortical and subcortical microinfarcts
| Subcortical Microinfarcts | ||||
|---|---|---|---|---|
| Cortical Microinfarcts | None | Single | Multiple | Total |
| None | 771 | 89 | 31 | 891 (83%) |
| Single | 96 | 22 | 6 | 124 (12%) |
| Multiple | 32 | 12 | 7 | 51 (5%) |
| Total | 899 (84%) | 123 (12%) | 44 (4%) | 1,066 (100%) |
Cerebral vessel pathologies were very common. A severity grade of moderate‐to‐severe was noted in about 41% of subjects for atherosclerosis, 36% for arteriolosclerosis, and 35% for amyloid angiopathy. Using the same cut‐off of the severity grade, 404 (38%) of subjects had only one vessel pathology, 277 (26%) had two vessel pathologies (most commonly atherosclerosis and arteriolosclerosis), and 76 (7%) had all three vessel pathologies. Figure 1 shows the number of subjects with each vessel pathology count by the presence of microinfarct(s). The distribution of the three cerebral vessel pathologies by cortical and subcortical microinfarcts is shown in Figure 2.
Figure 1.
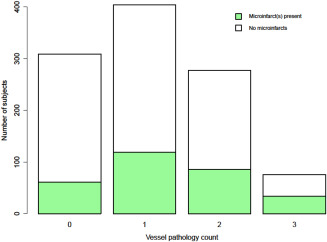
Number of subjects with no, one, two, or three cerebral vessel pathology(ies), with and without Microinfarcts.
Figure 2.
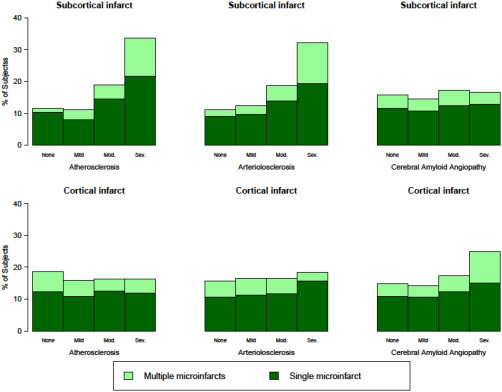
Distribution of cerebral vessel pathology by cortical and subcortical microinfarcts. Legend: Each panel shows % of subjects by level of vessel pathology severity (left most column: atherosclerosis, middle column: arteriolosclerosis, right most column: amyloid angiopathy) and burden of cortical (bottom row) or subcortical microinfarcts (top row). Light green represents multiple microinfarcts in the given location, and dark green represents a single microinfarct, and white represents no microinfarcts. Note the shift toward an increased burden of subcortical microinfarcts with increased atherosclerosis and arteriolosclerosis severity (top left and top middle panels), and shift toward an increased burden of cortical microinfarcts with increased amyloid angiopathy severity (bottom right panel).
We examined Spearman correlations among the three cerebral vessel measures and their correlation with age. Age was associated with microinfarct burden (r s = 0.08, P = 0.004), but the magnitude of the effect was relatively small. We also found associations of age with severity of each of the three vessel pathologies (atherosclerosis, arteriolosclerosis and amyloid angiopathy), with r s ranging from 0.14 to 0.17, and all P < 0.001. Atherosclerosis and arteriolosclerosis were associated (r s = 0.31, P <0.001). Amyloid angiopathy was not associated with either atherosclerosis or arteriolosclerosis (both r s <0.04 and P > 0.2).
Table 3 shows the distribution of the presence of clinical and pathologic variables for each of the three vessel pathologies by severity grade (not present‐to‐mild vs. moderate‐to‐severe). Severity of atherosclerosis was associated with hypertension and stroke. Severity of arteriolosclerosis was associated with stroke, with borderline associations noted for hypertension and myocardial infarction. Interestingly, diabetes was not found to be related to vessel disease severity. CAA was not related to vascular risk factors or diseases, but was associated with the brain accumulation of amyloid, as expected. All vessel diseases were related to dementia as a whole (with all three P < 0.01), and to AD dementia specifically (with a borderline association for arteriolosclerosis), and atherosclerosis and arteriolosclerosis were both associated with MCI. Additional analyses showed that microinfarcts were related to gross infarcts (r s =0.24; P < 0.01) and to lacunar infarcts (r s =0.20; P < 0.01). Also, dementia was associated with each of microinfarcts (χ2 = 6.9, DF = 1; P < 0.01), lacunar infarcts (χ2 = 16.8, DF = 2; P < 0.01), and gross infarcts (χ2 = 25.6, DF = 1; P < 0.01).
Table 3.
Distribution (number and percent) of clinical and pathologic characteristics of subjects, by severity of each of the three vessel pathologies
| Atherosclerosis | Arteriolosclerosis | Amyloid angiopathy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| None‐to‐mild | Moderate‐to‐severe | P value* | None‐to‐mild | Moderate‐to‐severe | P value * | None‐to‐mild | Moderate‐to‐severe | P value * | |
| Characteristic | n = 636 (60%) | n = 430 (40%) | n = 684 (64%) | n = 382 (36%) | n = 692 (65%) | n = 374 (35%) | |||
| Clinical Variables | |||||||||
| Vascular risk factors | |||||||||
| Hypertension | 397 (62%) | 298 (69%) | 0.02 | 460 (67%) | 235 (62%) | 0.07 | 463 (67%) | 232 (62%) | 0.10 |
| Diabetes | 136 (21%) | 88 (21%) | 0.75 | 149 (22%) | 75 (20%) | 0.41 | 153 (22%) | 71 (19%) | 0.22 |
| Vascular diseases | |||||||||
| Stroke | 113 (18%) | 122 (29%) | <0.01 | 135 (20%) | 100 (26%) | 0.01 | 150 (22%) | 85 (23%) | 0.74 |
| Myocardial infarction | 131 (21%) | 105 (24%) | 0.14 | 139 (20%) | 97 (25%) | 0.05 | 163 (24%) | 73 (20%) | 0.13 |
| Dementia status | |||||||||
| Dementia | 248 (39%) | 215 (50%) | <0.01 | 274 (40%) | 189 (49%) | <0.01 | 247 (36%) | 216 (58%) | <0.01 |
| AD dementia | 211 (35%) | 179 (46%) | <0.01 | 241 (37%) | 149 (44%) | 0.05 | 198 (31%) | 192 (55%) | <0.01 |
| Mild cognitive mpairment | 151 (39%) | 107 (50%) | 0.01 | 162 (40%) | 96 (50%) | 0.02 | 186 (42%) | 72 (46%) | 0.41 |
| Pathologic Variables | |||||||||
| Any infarct(s) | 245 (39%) | 272 (63%) | <0.01 | 293 (43%) | 224 (59%) | <0.01 | 327 (47%) | 190 (51%) | 0.27 |
| Microinfarct(s) | 151 (24%) | 149 (35%) | <0.01 | 170 (25%) | 130 (34%) | <0.01 | 186 (27%) | 114 (30%) | 0.21 |
| Lacunar infarct(s) | 104 (16%) | 145 (34%) | <0.01 | 126 (18%) | 123 (32%) | <0.01 | 162 (23%) | 87 (23%) | 0.95 |
| Gross infarct(s) | 163 (26%) | 215 (50%) | <0.01 | 203 (30%) | 175 (46%) | <0.01 | 240 (35%) | 138 (37%) | 0.47 |
| AD pathology | |||||||||
| Amyloid | 553 (87%) | 265 (85%) | 0.35 | 597 (88%) | 321 (84%) | 0.11 | 559 (81%) | 359 (96%) | <0.01 |
| Tangles | 634 (100%) | 427 (100%) | 0.78 | 681 (100%) | 380 (100%) | 0.68 | 688 (100%) | 373 (100%) | 0.30 |
*Chi squared test, comparing persons with moderate‐to‐severe vessel pathology to those with none‐to‐mild pathology (reference group)
Relationship of cerebral vessel pathology and microinfarcts
The overall goal of this study was to examine the relationship of cerebral vessel pathology and brain microinfarcts. We examined the effects of the three cerebral vessel pathologies, atherosclerosis, arteriolosclerosis and amyloid angiopathy, on the burden of microinfarcts (odds of one or multiple microinfarcts). We first conducted ordinal logistic regression analyses limited to one vessel pathology predictor at a time, in models controlling for age and sex. Using three separate models, the result for atherosclerosis was OR =1.29; 95%CI: 1.10–1.52, for arteriolosclerosis was OR =1.25; 95%CI: 1.10–1.44, and for amyloid angiopathy was OR =1.14; 95%CI: 1.01–1.28. Next, in the main analysis, we used a single ordinal logistic regression, adjusted for age and sex and including all three vessel pathologies as predictors (Table 4). In model 1 where the outcome was microinfarcts anywhere in the brain, each of the three vessel pathologies (atherosclerosis, arteriolosclerosis and amyloid angiopathy all included in a single model) independently increased the odds of having one or multiple microinfarct(s) by 13 to 22%. The associations for atherosclerosis and arteriolosclerosis were stronger than for amyloid angiopathy (Table 4). Compared to the analyses of the three vessel pathologies examined separately (above), the associations between vessel pathologies and microinfarcts in a single model (Table 4) showed lower, but still significant effect sizes for atherosclerosis and arteriolosclerosis, and essentially no change in effect size for amyloid angiopathy. These findings are not surprising given the relationship between atherosclerosis and arteriolosclerosis, but are important in demonstrating that each vessel pathology has a separate relationship with microinfarcts even after accounting for the other related pathology. In a separate analysis with all three vessel pathologies included in a single model, also controlling for age and sex, the number of vessel pathologies (one, two, or three) was also associated with an increased odds of having one or multiple microinfarct(s) (OR =1.36; 95%CI: 1.17–1.58).
Table 4.
Relationship of cerebral vessel pathologies to brain microinfarcts*
| Cerebral vessel pathology (predictor) | Odds Ratio (95% confidence interval) | P value |
|---|---|---|
| Any microinfarct (Model 1) | ||
| Atherosclerosis | OR =1.22; 95%CI: 1.03‐1.45 | 0.023 |
| Arteriolosclerosis | OR =1.18; 95%CI: 1.02‐1.37 | 0.022 |
| Amyloid angiopathy | OR =1.13; 95%CI: 1.00‐1.28 | 0.048 |
| Subcortical microinfarcts (Model 2) | ||
| Atherosclerosis | OR =1.49; 95%CI: 1.20‐1.84 | <0.001 |
| Arteriolosclerosis | OR =1.39; 95%CI: 1.16‐1.67 | <0.001 |
| Amyloid angiopathy | OR =1.04; 95%CI: 0.89‐1.22 | 0.596 |
| Cortical microinfarcts (Model 3) | ||
| Atherosclerosis | OR =0.90; 95%CI: 0.73‐1.11 | 0.305 |
| Arteriolosclerosis | OR =1.02; 95%CI: 0.86‐1.22 | 0.789 |
| Amyloid angiopathy | OR =1.26; 95%CI: 1.09‐1.46 | 0.002 |
*Ordinal logistic regression models for 3‐level microinfarct outcomes (none, single, multiple), adjusted for age and sex. For each outcome, a single ordinal logistic regression model was run including all three cerebral vessel pathologies.
We next examined the associations of vessel pathologies with burden of microinfarcts by location of microinfarcts, with separate outcomes for cortical and subcortical locations. Atherosclerosis and arteriolosclerosis each independently increased the odds of one or multiple subcortical microinfarct(s) by about 40‐50%, while amyloid angiopathy was not related to subcortical microinfarcts (Table 4, Model 2). Conversely, amyloid angiopathy increased the odds of one or multiple cortical microinfarct(s) by about 25%, but atherosclerosis and arteriolosclerosis were not related to cortical microinfarcts (Table 4, Model 3). In separate analyses considering capillary amyloid angiopathy specifically (present in 16%), we did not find associations of capillary amyloid angiopathy with the overall burden of microinfarcts (P = 0.61), nor with subcortical (P = 0.34) or cortical microinfarcts (P = 0.78).
Finally, we conducted an additional analysis to determine whether the presence of dementia affected the relationship of vessel pathology and microinfarcts. Similar to the model 1 of Table 4, we conducted an ordinal logistic regression analysis but now adding terms for dementia and the interaction of dementia by each of the three vessel pathologies. We found no evidence for an interaction of any of the vessel pathologies by dementia (all P > 0.13), suggesting that the relationship of vessel pathologies and microinfarcts is not modified by dementia status.
Discussion
In this study of 1,066 community‐dwelling subjects who came to autopsy, results show a separate relationship of each of the three major cerebral vessel pathologies to the presence of brain microinfarcts. In addition, moderate‐to‐severe vessel pathologies and chronic microinfarcts were found to be very common in aging, affecting about of third of subjects. Furthermore, a higher severity of each of the cerebral vessel pathologies was associated with a greater burden of microinfarcts. Specifically, we found that higher severity levels of both atherosclerosis and arteriolosclerosis increase the odds of one or multiple microinfarct(s) by about 20%, and that amyloid angiopathy increases the odds by 13%. In analyses examining location of microinfarcts, atherosclerosis and arteriolosclerosis were both associated with subcortical microinfarcts, whereas amyloid angiopathy was with cortical microinfarcts.
The importance of research on brain microinfarcts stems from several factors. First, and most importantly, microinfarcts have been shown to be associated with impaired neurologic function, including cognitive impairment even among persons without dementia, and with dementia itself 4, 5, 6, 7, 13, 24, 25, 27, 31. Second, and in keeping with the findings presented here, data have now consistently shown that microinfarcts are common in the aged brain, with a frequency ranging from 16% to as high as 64% 1, 16, 17, 32. A recent study suggests that the observation of even a few microinfarcts on pathologic assessment may be indicative of a much larger burden of unrecognized pathology 30. Indeed, our assessment of the number of microinfarcts in this study is based on observations of select brain regions, and does not capture a true count of the total number of microinfarcts present, but rather provides an estimate of the burden of this pathology. Third, the mechanisms by which microinfarcts impair neurologic function are unclear and further research on mechanisms leading to cognitive impairment and dementia is needed. Finally, the pathogenesis of brain microinfarcts itself remains to be elucidated. While largely assumed to be similar to the pathogenesis of larger infarcts, the pathogenesis of microinfarcts has actually not been well studied. Importantly in this study, we found that even among subjects without or with little of the three major cerebral vessel pathologies, about 15% had microinfarcts (as shown in Figure 1), suggesting that mechanisms other than vessel disease are also at play. These and other factors contribute to the mounting effort researchers are investing into the study of microinfarcts.
Because microinfarcts are common in aging, are associated with neurologic impairment, and are potentially preventable, it is critical for researchers to unravel the pathophysiologic mechanisms involved in microinfarct genesis. While systemic risk factors and diseases (e.g., hypertension and myocardial infarction) have been shown to be associated with an increased the likelihood of brain microinfarcts, less is known about central (brain) processes which may play a pathophysiologic role in microinfarcts 9, 22, 28. Several recent studies have suggested a link between microinfarcts and brain atrophy 16, 21. And some data are available on microinfarcts and cerebral vessel disease, mostly for cerebral amyloid angiopathy 20. Indeed, several small studies of autopsied persons with dementia, found associations of amyloid angiopathy with microinfarcts 11, 26. A recent study used both human and animal brain tissue to examine the relationship of two vessel diseases to microinfarcts 19. In that study, first using 31 postmortem human brains, severity of amyloid angiopathy, but not atherosclerosis, was associated with microinfarcts, and an experiment in mice exposed to chronic hypoperfusion (via bilateral common carotid artery stenosis) showed that both amyloid angiopathy and microinfarcts were increased in postmortem mouse brain 19. In contrast, in a subsequent larger study of 91 autopsy cases, authors found that while brain microinfarcts were observed more commonly among cases with amyloid angiopathy, statistical analyses did not confirm a correlation 14. Finally, in the largest and most comprehensive published study on cerebral vessel diseases and microinfarcts, with 163 autopsied elderly persons, authors found a twofold increased odds of microinfarcts in persons with more severe atherosclerosis 32. While important study design differences are present with the current study (including recruitment of mostly persons with cognitive impairment from a memory clinic setting, and considering microinfarcts only as a dichotomous measure of present vs. not), the study by Zheng et al also assessed the same three vessels pathologies and similarly analyzed, using ordinal logistic regression analyses, grade of severity of these pathologies in relation to microinfarcts 32. Unlike the current study, they found no relationships of arteriolosclerosis or amyloid angiopathy to microinfarcts, although authors raised the possibility that their sample size may have affected results 32.
Our study provides new data on a large community sample that extends the results of these previous studies. First, while severity of atherosclerosis increases the odds of one or multiple microinfarcts, atherosclerosis appears to be specifically associated with subcortical microinfarcts in particular. Second, perhaps with the increased number of subjects in our study, we found that arteriolosclerosis was also associated with microinfarcts and subcortical microinfarcts in particular. Lastly, we found a weak but positive association of amyloid angiopathy with any microinfarcts, and this vessel pathology was strongly associated specifically with cortical microinfarcts. Considering the vessel pathology and microinfarct literature as a whole, our study further refined the recently published finding with atherosclerosis by Zheng et al 32, clarified the previously mixed results for amyloid angiopathy, and identified a new relationship for arteriolosclerosis. Our results suggest that different vessel pathologies, including those affecting large, mid‐, and small‐sized vessels and including different pathologic features (atheroma, amyloid deposition, others), are associated with microinfarcts. Further, an increase in the number of cerebral vessel pathologies is associated with an increased brain microinfarct burden. We also found that certain vessel pathologies seem to have a predilection for microinfarcts in certain brain regions, with atherosclerosis and arteriolosclerosis being associated with microinfarcts in subcortical regions, and amyloid angiopathy in cortical regions. The additional analysis taking dementia status into account does not suggest that the relationship of vessel pathologies and microinfarcts differ among those with and without dementia. While the regional findings for arteriolosclerosis and amyloid angiopathy are perhaps not very surprising given where these two vessel pathologies tend to occur in the brain 29, the finding for atherosclerosis is more surprising. On the one hand, several possibilities should be considered for why atherosclerosis may be related to subcortical microinfarcts. First, large vessel disease at the base of the brain may lead to microemboli lodging in the deep penetrating arteries in subcortical regions, and subsequently may lead to local ischemia and ultimately subcortical microinfarction 12. Second, atherosclerosis may affect not only larger arteries but also smaller arteries and arterioles, and these may be specifically affected in the subcortical parenchyma 15. On the other hand, explanations for why atherosclerosis was not found to be associated with cortical microinfarcts should also be considered. For instance, may there be more developed vascular collaterals in the cortex or meninges, or other factors, which yield a “relative protection” of the cortical regions from atherosclerosis? These and alternate possibilities need further exploration. Separately, while the literature has pointed to capillary amyloid angiopathy as playing a role in certain brain pathologies 8, our study did not find associations of this specific vessel pathology with microinfarcts.
More research is needed, to identify specific upstream and downstream factors linking the different cerebral vessel pathologies to microinfarcts. And, with advancing techniques in neuroimaging, associations of in vivo vessel pathology with microinfarcts can be examined 10. Additional clinical‐pathologic studies are also needed to examine the relationship of microinfarcts to larger infarcts, and other vascular and non‐vascular pathologies 17, 20, 32. Central processes, with or without coexisting atherosclerosis and arteriolosclerosis, may also be important, such as defects in the blood brain barrier and inflammation.
Finally, research will need to take into account not only central (brain), but also systemic variables and risk factors and diseases. Here, we show that specific vascular risk factors and diseases are related to atherosclerosis and arteriolosclerosis. Further work is needed to address whether there are additional risk factors and diseases that are related to microinfarcts, including acute and chronic impairment in cardiac output and other cardiac processes, sleep apnea and other sleep related disturbances, and chronic renal disease, and others.
There are several limitations to our study. Importantly, our study did not provide a more detailed assessment of vessel pathology, thus limiting our ability to elucidate additional aspects of pathogenesis of microinfarcts and of potential therapeutic targets. For instance, atherosclerosis may have been systematically assessed using more sophisticated techniques such as digitally analyzed photography, and by documenting additional features such as vessel tortuosity and aneurysmic dilatations. Similarly, the measure of arteriolosclerosis could have captured information on hyalinization and changes in the smooth muscle. Also, because the assessment of vessel pathology was conducted only in select brain regions (e.g., arteriolosclerosis in one region), we may have misclassified some cases. Furthermore, we may be misclassifying atherosclerosis and arteriolosclerosis because of a more limited sampling of these pathologies as compared to amyloid angiopathy. Indeed, atherosclerosis was only evaluated in the circle of Willis and not assessed in larger (e.g., internal carotid arteries) or smaller (e.g., intraparenchymal) vessels. Similarly, arteriolosclerosis was only evaluated in the basal ganglia. Evaluation of other subcortical regions, especially white matter may be informative. Further, we sampled only a limited number of brain regions for microinfarcts and thereby are likely underestimating the number of cases with microinfarcts. Finally, while this single study with extensive data and detailed analyses presents substantial complexity, several questions remain unanswered and will need to be addressed in future studies. Nonetheless, while there are limitations to our study, there are also important strengths to note. We examined a very large number of brain specimens, with a sample size of more than 1,000 brains. Subjects were derived from a community‐based setting, rather than an autopsy series or clinic‐based setting, favoring results in our study to be more generalizable to the population. The parent studies (Rush Memory and Aging Project; Religious Orders Study) each have a high autopsy rate, lending further internal validity to our findings. And, the neuropathology data were systematically collected, and this was achieved while being blinded to clinical data.
Conflict of Interest
Authors have no conflict of interest to declare.
Acknowledgments
The study was funded by the National Institutes of Health: P30 AG10161, R01 AG15819, R01 AG17917, R01 AG40039, R01 NS084965, and R01 AG043379.
Authors thank participants enrolled in the Rush Memory and Aging Project and Religious Orders Study Core.
Authors are grateful to the Rush Alzheimer's Disease Center staff, in particular Karen Skish for laboratory coordination, John Gibbons for data management, Donna Esbjornson for statistical analyses, and Traci Colvin for study coordination of the cohorts.
References
- 1. Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA (2011) Microinfarct pathology, dementia, and cognitive systems. Stroke 42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS (2012) Overview and findings from the religious orders study. Curr Alzheimer Res 9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS (2012) Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al (2009) Neuropathological correlates of dementia in over‐80‐year‐old brain donors from the population‐based Cambridge city over‐75s cohort (CC75C) study. J Alzheimers Dis 18:645–658. doi: 10.3233/JAD-2009-1182 [DOI] [PubMed] [Google Scholar]
- 5. Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ (2012) Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 32(3):425–436. doi: 10.1038/jcbfm.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA (2011) Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 42:3183–3189. doi: 10.1161/STROKEAHA.111.623462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchman AS, Yu L, Boyle PA, Levine SR, Nag S, Schneider JA, et al (2013) Microvascular brain pathology and late‐life motor impairment. Neurology 80(8):712–718. doi: 10.1212/WNL.0b013e3182825116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carrano A, Hoozemans JJ, van der Vies SM, van Horssen J, de Vries HE, Rozemuller AJ (2011) Amyloid Beta induces oxidative stress‐mediated blood‐brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal 15:1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 9. Chester EM, Agamanolis DP, Banker BQ, Victor M (1978) Hypertensive encephalopathy: a clinicopathologic study of 20 cases. Neurology 28(9 Pt 1):928–939. [DOI] [PubMed] [Google Scholar]
- 10. Dieleman N, van der Kolk AG, van Veluw SJ, Frijns CJ, Harteveld AA, Luijten PR, et al (2014) Patterns of intracranial vessel wall changes in relation to ischemic infarcts. Neurology 83:1316–1320. doi: 10.1212/WNL.0000000000000868 [DOI] [PubMed] [Google Scholar]
- 11. Haglund M, Passant U, Sjöbeck M, Ghebremedhin E, Englund E (2006) Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia. Int J Geriatr Psychiatry 21:681–687. [DOI] [PubMed] [Google Scholar]
- 12. Kalaria RN, Perry RH, O'Brien J, Jaros E (2012) Atheromatous disease in small intracerebral vessels, microinfarcts and dementia. Neuropathol Appl Neurobiol 38:505–508. doi: 10.1111/j.1365-2990.2012.01264.x [DOI] [PubMed] [Google Scholar]
- 13. Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al (2004) Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke 35:410–414. [DOI] [PubMed] [Google Scholar]
- 14. Kövari E, Herrmann FR, Hof PR, Bouras C (2013) The relationship between cerebral amyloid angiopathy and cortical microinfarcts in brain ageing and Alzheimer's disease. Neuropathol Appl Neurobiol 39:498–509. doi: 10.1111/nan.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lammie GA (2002) Hypertensive cerebral small vessel disease and stroke. Brain Pathol 12:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Launer LJ, Hughes TM, White LR (2011) Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 70:774–780. doi: 10.1002/ana.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longstreth WT Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ (2009) Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord 23:291–294. doi: 10.1097/WAD.0b013e318199fc7a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K, et al (2014) Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post‐mortem brain tissue. Am J Neurodegener Dis 3:19–32. [PMC free article] [PubMed] [Google Scholar]
- 19. Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, et al (2012) Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 123:381–394. doi: 10.1007/s00401-011-0925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olichney JM, Hansen LA, Lee JH, Hofstetter CR, Katzman R, Thal LJ (2000) Relationship between severe amyloid angiopathy, apolipoprotein E genotype, and vascular lesions in Alzheimer's disease. Ann N Y Acad Sci 903:138–143. [DOI] [PubMed] [Google Scholar]
- 21. Raman MR, Preboske GM, Przybelski SA, Gunter JL, Senjem ML, Vemuri P, et al (2014) Antemortem MRI findings associated with microinfarcts at autopsy. Neurology 82:1951–1958. doi: 10.1212/WNL.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, Esiri MM (2012) The neuropathology of vascular disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Curr Alzheimer Res 9:687–696. [DOI] [PubMed] [Google Scholar]
- 23. Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, et al (2003) Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology 60:1082–1088. [DOI] [PubMed] [Google Scholar]
- 24. Smith EE, Schneider JA, Wardlaw JM, Greenberg SM (2012) Cerebral microinfarcts: the invisible lesions. Lancet Neurol 11:272–282. doi: 10.1016/S1474-4422(11)70307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al (2007) Pathological correlates of dementia in a longitudinal, population‐based sample of aging. Ann Neurol 62:406–413. [DOI] [PubMed] [Google Scholar]
- 26. Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, et al (2010) Cerebral microinfarcts associated with severe cerebral beta‐amyloid angiopathy. Brain Pathol 20:459–467. doi: 10.1111/j.1750-3639.2009.00322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ (2008) Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol 64:168–176. doi: 10.1002/ana.21413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Dalen JW, Scuric EE, van Veluw SJ, Caan MW, Nederveen AJ, Biessels GJ, et al (2015) Cortical microinfarcts detected in vivo on 3 Tesla MRI: clinical and radiological correlates. Stroke 46:255–257. doi: 10.1161/STROKEAHA.114.007568 [DOI] [PubMed] [Google Scholar]
- 29. Vinters HV, Gilbert JJ (1983) Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 14:924–928. [DOI] [PubMed] [Google Scholar]
- 30. Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM (2013) Estimating cerebral microinfarct burden from autopsy samples. Neurology 80:1365–1369. doi: 10.1212/WNL.0b013e31828c2f52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White L (2009) Brain lesions at autopsy in older Japanese‐American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu‐Asia aging study. J Alzheimers Dis 18:713–725. doi: 10.3233/JAD-2009-1178 [DOI] [PubMed] [Google Scholar]
- 32. Zheng L, Vinters HV, Mack WJ, Zarow C, Ellis WG, Chui HC (2013) Cerebral atherosclerosis is associated with cystic infarcts and microinfarcts but not Alzheimer pathologic changes. Stroke 44:2835–2841. doi: 10.1161/STROKEAHA.113.001945 [DOI] [PMC free article] [PubMed] [Google Scholar]


