Abstract
Background
Cognitive impairment affects 40%–68% of relapsing-remitting multiple sclerosis (RRMS) patients. Gray matter (GM) demyelination is complicit in cognitive impairment, yet cortical lesions are challenging to image clinically. We wanted to determine whether cortical cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) differences exist between cognitively impaired (CI) and unimpaired (NI) RRMS.
Methods
Prospective study of healthy controls (n = 19), CI (n = 20), and NI (n = 19) undergoing magnetic resonance imaging (MRI) and cognitive testing <1 week apart. White matter (WM) T2 hyperintense lesions and T1 black holes were traced. General linear regression assessed the relationship between lobar WM volume and cortical and WM CBF, CBV, and MTT. Relationship between global and lobar cortical CBF, CBV, and MTT and cognitive impairment was tested using a generalized linear model. Adjusted Bonferroni p < 0.005 was considered significant.
Results
No significant differences for age, gender, disease duration, and any fractional brain or lesion volume were demonstrated for RRMS subgroups. Expanded Disability Status Scale (EDSS) and Hospital Anxiety and Depression Scale–Depression (HADS-D) were higher in CI. Lobar cortical CBF and CBV were associated with cognitive impairment (p < 0.0001) after controlling for confounders. Cortical CBV accounted for 7.2% of cognitive impairment increasing to 8.7% with cortical CBF (p = 0.06), while WM and cortical CBF accounted for 8.2% of variance (p = 0.04).
Conclusion
Significant cortical CBF and CBV reduction was present in CI compared to NI in the absence of structural differences.
Keywords: Multiple sclerosis, relapsing/remitting, demyelination, MRI, quantitative MRI
Introduction
Cognitive impairment is present in 40%–68% of multiple sclerosis (MS) patients. A strong association exists between cortical lesion volume and cognitive1,2 and physical impairment.3 Cortical lesion burden may contribute to gray matter (GM) atrophy4 but may also occur independently,5 even preceding T2 hyperin-tense white matter (WM) lesion development in clinically isolated syndrome.6 Clinico-pathological correlation using high-field ex vivo magnetic resonance imaging (MRI) reveals relatively poor sensitivity of 46% for prospective cortical lesion identification using proteolipid protein staining as a reference standard.7 Significant progress in clinical imaging at high field strength has occurred, but such imaging is still challenged by prolonged scan times due to specific absorption rate considerations and is not yet available for routine clinical use.8 Furthermore, quantitative MRI at 3 T shows poor separation of MRI metrics between cortical lesions and normal-appearing gray matter (NAGM),9 underscoring the limitations of clinical MRI for direct lesion visualization. It is also clear that MRI-visualized cortical lesions represent the “tip of the iceberg” with visualization dependent on the volume of cortical disease burden and cortical lesion size.10 These limitations have encouraged the use of alternative MRI techniques as surrogates of cortical integrity or disease severity, including diffusion tensor imaging (DTI),11 magnetization transfer ratio (MTR),12 and perfusion imaging.13,14 Cortical perfusion assessment in particular shows promise, with a recent study demonstrating cerebral blood flow (CBF) reduction in early relapsing-remitting multiple sclerosis (RRMS) patients compared to healthy controls in both GM and WM in the absence of GM atrophy. Several clusters of CBF reduction distinguished RRMS from healthy controls, and a positive correlation was seen between CBF and objective memory tests in multiple regions.13 An earlier study in secondary progressive multiple sclerosis (SPMS) patients showed cortical cerebral blood volume (CBV) reduction in cognitively impaired (CI) compared to unimpaired (NI) patients after correcting for differences in WM T2 hyperintense lesion severity and brain atrophy.14 Finally, a recent study showed reduced CBF and CBV and prolonged mean transit time (MTT) in cortical lesions compared to NAGM.15 Building on these prior studies and the link between cortical disease and cognitive impairment, we sought to determine whether meaningful CBF, CBV, and MTT differences exist between age- and gender-matched RRMS patients without and with cognitive impairment and healthy controls. We hypothesized that RRMS patients with cognitive impairment would show greater cortical CBF and CBV reduction independent of common potential confounders including age, education, disease duration, anxiety or depressive scores, disability level, and structural differences including global GM and WM volume, T2 hyperintense WM lesion volume, T1 black hole volume, and cortical lesion volume.
Methods
Patients and healthy controls
Clinically stable RRMS subjects (2010 revised McDonald criteria16) were prospectively recruited over 1 year from two tertiary referral MS clinics, with written consent obtained from all participants. Twenty CI RRMS patients were initially recruited and then age- and gender-matched with 19 NI RRMS patients and healthy controls, respectively. Healthy controls had no history of prior neurological disorder. RRMS patient exclusion criteria were relapse or corticosteroid use within the past 3 months, history of drug or alcohol abuse, premorbid (pre-MS) psychiatric history, head injury including loss of consciousness, concurrent morbidity such as cerebrovascular disease, and magnetic resonance (MR) imaging or gadolinium contraindications including impaired renal function. The study was approved by research ethics board at Sunnybrook and St. Michael’s hospitals.
Neuropsychological and clinical assessment
All patients underwent detailed clinical history including age, gender, education level, disease duration, medication and relapses, Expanded Disability Status Scale (EDSS), and neuropsychological assessment within 1 week of MR imaging. The minimal assessment of cognitive function in multiple sclerosis (MACFIMS) is a 90-minute cognitive battery recommended by an expert panel for clinical monitoring and research purposes comprising seven tests assessing five cognitive domains, including processing speed and working memory (Paced Auditory Serial Addition Test (PASAT), Symbol Digit Modalities Test (SDMT)), learning and memory (California Verbal Learning Test-II (CVLT2), Brief Visuospatial Test–revised (BVMT-R)), executive function (DKEFS Sorting Test (DST)), visuospatial perception, spatial processing (Judgment of Line Orientation Test (JLO)), and verbal fluency (Controlled Oral Work Association Test (COWAT)). The Hospital Anxiety and Depression Scale (HADS) was also administered. For each cognitive test, raw test scores were converted to Z scores using age- and gender-adjusted normative data. Impairment for a single test was defined as a Z score <−1.5. Patients with two or more test impairments within the MACFIMS battery were considered impaired.
MR imaging acquisition
MR imaging was performed on a 3 T MRI system (Achieva; Philips Healthcare, Best, The Netherlands) with a eight-channel phased array head coil receiver. Imaging parameters included axial volumetric (turbo spin-echo (TSE)) T1 (repetition time (TR)/echo time (TE)/flip angle: 9.5 ms/2.3 ms/12°; number of averages: 1; field of view (FOV): 24 cm; matrix size: 256 × 219; section thickness: 1.2 mm), axial proton density/T2 (TR/TE/flip angle: 2500 ms/10.7 ms/90°; FOV: 23 cm; matrix: 256 × 263; section thickness: 3 mm), axial phase-sensitive inversion recovery (PSIR) (TR/TE: 3374 ms/15 ms; FOV: 23 cm; matrix: 400 × 255; in-plane voxel size: 0.43 mm × 0.43 mm; section thickness: 3 mm), and axial field-echo echo-planar imaging (EPI) dynamic susceptibility contrast or DSC (TR/TE/flip angle: 1633 ms/30 ms/60°; FOV: 22 cm; section thickness: 4 mm; matrix: 96 × 93; in-plane voxel size: 1.72 mm × 1.72 mm; no gap; signal bandwidth: 1260 Hz/pixel; sections: 24). Ten milliliters of Gadobutrol (Gadovist; Bayer, Toronto, Canada) (1 mmol/mL) was administered by a power injector at a rate of 5 mL/s, followed by a 25-mL bolus of saline at 5 mL/s. A total of 60 images were acquired at 1.6-second intervals with the injection occurring at the fifth volume. An axial segmented inversion recovery look-locker EPI sequence was performed immediately before and after the DSC sequence (TR/TE/flip angle: 29 ms/14 ms/20°; inversion time: 15.8 ms; FOV: 22 cm; matrix: 128 × 126; 15 lines in k-space per acquisition; section thickness: 4 mm; 60 time points; scan time: 73 seconds) for the purposes of estimating T1 of WM. A 3000-ms delay was placed after the last imaging time point to facilitate longitudinal magnetization recovery.
Quantitative MR perfusion
Quantitative cerebral blood volume (qCBV), quantitative cerebral blood flow (qCBF), and MTT were calculated using the bookend technique.17 Briefly, by carefully modeling the effects of intravascular-to-extravascular water exchange, the bookend technique utilizes pre- and post-gadolinium WM T1 values relative to T1 changes in the blood pool to quantify CBV in WM independent of an arterial input function (AIF). Quantification of qCBV and qCBF includes terms to account for compartmentalization effects, average brain density, and differences in hematocrit between large arteries and capillaries. Relative cerebral blood flow (rCBF) is computed by deconvolution of tissue concentration–time curves by the AIF using singular value decomposition and relative cerebral blood volume (rCBV) by calculating the ratio of area under the curve of the tissue concentration–time curve and AIF. Final qCBV and qCBF quantification is then performed as previously described. Finally, the MTT is calculated using the central volume principle. Perfusion quantification using bookend perfusion is previously validated against positron emission tomography (PET), demonstrating a test–retest intra-class coefficient of 0.90 and coefficient of variation of 0.09.18
Image processing
Structural T1-weighted and proton density/T2-weighted images are co-registered using linear registration (SPM 8, Wellcome Department of Imaging Neuroscience, London, UK). Intracranial tissue is automatically segmented by a validated technique using the structural T1 images into GM (including cortex, basal ganglia, and thalami), WM, and cerebrospinal fluid (CSF).19 Brain parenchymal fraction (BPF) was defined as the sum of all brain components including fractional cortical volume (fC), fractional white matter (fWM), fractional basal ganglia (fBG), and fractional thalamus (fTh). An experienced clinician (10 years’ experience) manually traced fractional cortical lesions (fCL) using PSIR, T2 hyperintense lesions (fT2h) on the proton density/T2 images, and T1 black holes (T1bh) on the structural T1 images using Analyze 8.0 (Mayo Clinic, Rochester, MN, USA) (Figure 1(a)). Cortical lesions were subtracted from the cortical segmentation to create an NAGM mask. Gadolinium enhancement of T2 hyper-intense lesions was determined from contrast-enhanced T1. Structural T1-weighted and proton density/T2-weighted images along with the segmented regions of interest (ROIs) are registered to the pre-gadolinium EPI sequence (which is intrinsically co-registered to quantitative perfusion maps) using a linear registration (FSL-FLIRT) followed by non-linear intensity modulation and multi-resolution non-linear registration with four subsampling levels (FSL-FLIRT: FMRIB Software Library v5.0) (Figure 1(b)). To better guide the alignment at each resolution level, the images are smoothed using full width half maximum (FWHM) Gaussian kernel. The respective kernel sizes (in mm) used at each resolution level are 6, 4, 2, 2 for the moving images and 4, 2, 0, 0 for the perfusion image. Similarly, ICBM’s lobar template (Laboratory of Neuroimaging Keck School of Medicine of USC, Los Angeles, CA, USA) for WM and MRIcro Brodmann template (Neuropsychology Laboratory, Columbia, SC, USA) for cortex are registered to the transformed T1 in EPI space. Average cortical and WM CBF, CBV, and MTT were measured within each lobar region.
Figure 1.
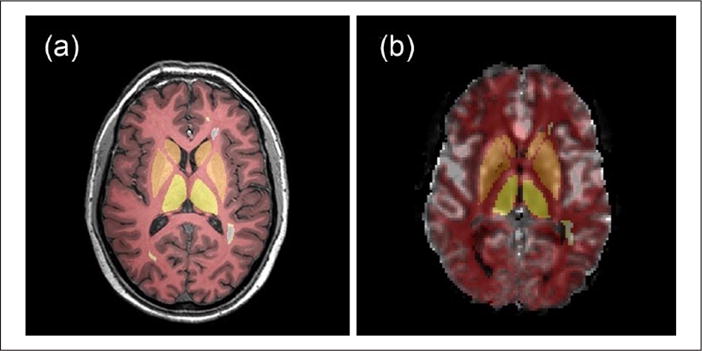
A representative slice of (a) T1-weighted MRI scan and (b) map of cerebral blood flow derived using the bookend technique with gray matter, white matter, basal ganglia, thalamus, and white matter lesion tissue regions of interest overlaid.
Statistical analysis
To compare demographic, clinical, and volume data among three groups, univariate general linear and logistic regression were performed on all continuous outcomes and categorical variables, respectively. Correlation of T2 hyperintense lesions, cortical lesions, and T1 black hole with WM and cortical volumes was tested with Spearman correlation for all RRMS patients. CBF, CBV, and MTT differences between cortical lesions and NAGM were tested using Student’s t-test. General linear regression analyses were used to assess the relationship between WM and white matter lesion (WML) volumes and cortex and cortical lesion volumes. Similarly, we assessed the relationship between lobar WM and cortical CBF, CBV, and MTT. To search for potential confounding factors for cognitive impairment, the association between baseline variables and cognitive impairment was conducted using univariate general linear regression or logistic regression for continuous or categorical baseline outcome, respectively. Significant confounding factors with p < 0.10 in the univariate analysis were adjusted for in a generalized linear model with logit link function to explore the relationship between impairment and lobar cortical and WM CBF and CBV. SAS Proc GENMOD was performed to fit the model, with Bonferroni-adjusted p-value < 0.006 considered statistically significant. Quantification of model comparison was performed using the entropy r2 value, where the higher the r2 value the better the model. r2 was calculated from the equation r2 = (LO − LM)/LO, where LO and LM represent the log likelihood (maximized-2) of the null and the fitted model, respectively. Statistical analyses were conducted using SAS (version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
Baseline demographic data, individual neurocognitive scores from the MACFIMS battery, fractional brain volumes, and cortical and WM CBF, CBV, and MTT are presented in Table 1. No significant differences were found for age, gender, disease duration, and any fractional brain or lesion volume for NI versus CI patients. Compared to NI, CI demonstrated higher median Hospital Anxiety and Depression Scale–Depression (HADS-D), EDSS, and worse performance on all individual cognitive tests except for Delis–Kaplan Executive Function System (DKEFS) and JLO. All global CBF and CBV metrics were significantly lower in CI versus NI and healthy controls. Similarly, MTT was more prolonged. Compared to healthy controls, CI demonstrated higher education level (p = 0.004); lower BPF (p = 0.02), fWM (p = 0.008), and fTh (p = 0.01); and increased fractional cerebrospinal fluid (fCSF) (p = 0.007) volumes with a trend to fC reduction (p = 0.048). NI demonstrated no significant differences compared to healthy controls except for increased Hospital Anxiety and Depression Scale–Anxiety (HADS-A) (p = 0.01) and cortical and WM MTT prolongation (both <0.001).
Table 1.
Patient demographic data, neurocognitive scores, fractional brain volume, and cortical and WM perfusion.
| Variable | HC (n = 19) | NI (n = 19) | CI (n = 20) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 49.0 ± 7.1 | 46.4 ± 7.2 | 48.1 ± 4.7 |
| Female gender, n (%) | 14 (73.68) | 15 (78.95) | 12 (60) |
| Education (years) | 16.9 ± 2.9‡ | 16.1 ± 1.3 | 14.6 ± 1.9‡ |
| Disease duration (years) | 0.0 ± 0.0 | 11.8 ± 5.4 | 11.6 ± 4.9 |
| HADS-A (log) median (IQR) | 3 (1, 6)‡◊ | 6 (5, 7);◊ | 8 (7, 10)‡ |
| HADS-D (log) median (IQR) | 2 (1, 3)‡ | 3 (1, 5)* | 8 (6, 10)‡* |
| EDSS | NA | 1.5 (1, 2)* | 2.5 (2, 3)* |
| Z-score cog test | |||
| COWAT_FAS | −0.67 ± 0.83 | −0.26 ± 1.06* | −1.16 ± 0.89* |
| COWAT_Animals | −0.13 ± 1.14 | 0.41 ± 0.95* | −0.59 ± 1.18* |
| BVMT-R_IR | 0.37 ± 1.15‡ | −0.07 ± 1.04* | −1.68 ± 1.34‡* |
| BVMT-R_DR | 0.40 ± 1.14‡ | 0.42 ± 0.77* | −1.62 ± 1.48‡* |
| PASAT-2 | −0.21 ± 0.88‡ | −0.26 ± 0.66* | −1.80 ± 0.57‡* |
| JLO | 0.98 ± 0.19‡ | 0.83 ± 0.59 | 0.40 ± 0.67‡ |
| SDMT | −0.14 ± 0.92‡ | 0.02 ± 0.75* | −1.80 ± 1.17‡* |
| CVLT-II_IR | −0.25 ± 1.05‡ | −0.23 ± 1.04* | −1.94 ± 1.36‡* |
| CVLT-II_DR | −0.11 ± 0.66‡ | 0.21 ± 0.92* | −2.20 ± 1.61‡* |
| DKEFS | 0.51 ± 0.73‡ | 0.26 ± 0.61 | −0.20 ± 1.25‡ |
| Percent fractional brain volume | |||
| BPF | 00.79 ± 0.09‡ | 00.75 ± 0.06 | 00.72 ± 0.08‡ |
| fC | 45.15 ± 5.12 | 43.43 ± 3.91 | 41.87 ± 5.50 |
| fWM | 31.66 ± 4.07‡ | 29.61 ± 2.83 | 28.35 ± 3.51‡ |
| fCL | 00.00 ± 0.00‡ | 00.01 ± 0.01 | 00.01 ± 0.02‡ |
| fBG | 01.35 ± 0.19 | 01.31 ± 0.18 | 01.25 ± 0.22 |
| fTh | 00.68 ± 0.12‡ | 00.64 ± 0.14 | 00.55 ± 0.14‡ |
| fT2h | 00.00 ± 0.00‡◊ | 00.67 ± 0.74◊ | 00.92 ± 0.90‡ |
| fT1bh | 00.00 ± 0.00‡ | 00.23 ± 0.22 | 00.41 ± 0.51‡ |
| fCSF | 21.16 ± 8.78‡ | 24.10 ± 6.24 | 26.64 ± 7.32‡ |
| Perfusion | |||
| Cortical CBV | 02.78 ± 01.49‡ | 02.77 ± 01.14* | 02.29 ± 00.88‡* |
| Cortical CBF | 45.54 ± 16.90‡ | 44.80 ± 20.05* | 35.70 ± 15.40‡* |
| WM CBV | 01.66 ± 00.54‡ | 01.70 ± 00.64* | 01.42 ± 00.49‡* |
| WM CBF | 23.22 ± 06.87‡ | 24.05 ± 10.60* | 19.51 ± 07.89‡* |
| Cortical MTT | 03.80 ± 00.86‡◊ | 03.90 ± 00.83*◊ | 04.06 ± 00.86‡* |
| WM MTT | 04.53 ± 00.77‡◊ | 04.63 ± 00.79*◊ | 04.75 ± 00.87‡* |
HC: healthy controls; NI: non-impaired relapsing-remitting multiple sclerosis; CI: cognitively impaired relapsing-remitting multiple sclerosis; HADS-A: Hospital Anxiety and Depression Scale–Anxiety; HADS-D: Hospital Anxiety and Depression Scale–Depression; IQR: interquartile range; EDSS: Expanded Disability Status Scale; NA: not applicable; COWAT: Controlled Oral Work Association Test; BVMT-R: Brief Visuospatial Test–revised; PASAT: Paced Auditory Serial Addition Test; JLO: Judgment of Line Orientation Test; SDMT: Symbol Digit Modalities Test; CVLT-II: California Verbal Learning Test-II; IR: immediate recall; DR: delayed recall; DKEFS: Delis–Kaplan Executive Function System; BPF: brain parenchymal fraction; WM: white matter; CBF: cerebral blood flow; CBV: cerebral blood volume; MTT: mean transit time; fC: fractional cortical volume; fWM: fractional white matter; fCL: fractional cortical lesions; fBG: fractional basal ganglia; fTh: fractional thalamus; fT2h: fractional T2-hyperintensities; fT1bh: fractional T1 black holes; fCSF: fractional cerebrospinal fluid.
All values are mean ± SD unless specified.
Significance p < 0.05 (HC vs NI◊, HC vs CI‡, and NI vs CI*).
Association between regional WM volume, T2 hyperintense lesions, CBF, CBV, and MTT
T2 hyperintense lesions and T1 black holes demonstrated strong positive correlation with cortical lesions (0.82 and 0.84; p < 0.0001, respectively) and a moderate inverse correlation with cortical volume (−0.49 and −0.45; p < 0.0001, respectively). A negative correlation was seen between cortical lesions and volumes of both WM (−0.57; p < 0.0001) and cortex (−0.37; p = 0.005). Cortical CBF and CBV were unrelated to WM volume in any lobar region except the right occipital lobe (β, −0.47 to −0.111; standard error (SE), 0.02–0.051; p = 0.02 and 0.04, respectively). WM volume was associated with WM CBF and CBV in bilateral temporal lobes and right occipital and parietal lobes. Cortical and WM MTT were not associated with WM volume. Significant strong positive association (β = 0.83 and 0.99) between WM and cortical CBF, CBV, and MTT was seen for each lobar region (p < 0.0001 all regions).
Perfusion differences between cortical lesions and NAGM
There was no significant CBF, CBV, or MTT difference between cortical lesions seen in NI versus CI. Cortical lesions demonstrated significant mean CBF (31.96 vs 39.32 mL/100 g/min; p = 0.04) and CBV (1.96 vs 2.47 mL/100 g; p = 0.009) reduction compared to mean NAGM. No MTT differences were detectable.
Global cortical and WM perfusion group differences
Cortical and WM global CBF, CBV, and MTT values are presented in Table 1. Cortex and WM demonstrated an 18% and 20% CBV and 20% and 19% CBF reduction in CI compared to NI, respectively. A modest but significant MTT prolongation was present in CI compared to NI for both cortex and WM (4% and 3%, respectively). Although a small but significant 3% and 2% global MTT prolongation was present in NI compared to healthy controls, no significant CBV and CBF differences were detected.
Regional perfusion associations with cognitive impairment
Variables associated with cognitive impairment on univariate analysis included EDSS, HADS-D, and regional cortical and WM CBF and CBV but not MTT (Table 2; all p < 0.001). There was a borderline association between years of education and cognitive impairment. After controlling for EDSS, HADS-D, education, and lobar cortical CBF and CBV but not cortical MTT or WM CBF, CBV or MTT demonstrated significant association with cognitive impairment (Table 3). Regional structural and lesional volumetric data and perfusion data for each lobe are presented in the supplementary table for healthy controls and NI and CI patients. Cortical CBV alone accounted for 7.2% of variance associated with cognitive impairment compared to 6.6% for cortical CBF alone. WM CBF and CBV, respectively, accounted for 4.5% and 5.3% of variance. The addition of WM CBV to the cortical CBV model increased the variance from 7.2% to 8.7%, trending to improvement over cortical CBV alone (p = 0.06). WM CBF, however, significantly added to a model of cortical CBF together accounting for 8.2% of variance (p = 0.04). Comparing NI to healthy controls, no significant lobar CBF, CBV, and MTT reduction was present for any variable.
Table 2.
Variables associated with cognitive impairment defined as two or more test impairments within the MACFIMS battery.
| Parameter | p-value |
|---|---|
| Age | 0.5101 |
| Gender | 0.3732 |
| Education | 0.0696 |
| Disease duration | 0.9091 |
| HADS-A (log) | 0.2567 |
| HADS-D (log) | 0.0001 |
| EDSS | 0.0144 |
| Overall cortical CBF (regional level) | <0.0001 |
| Overall WM CBF (regional level) | <0.0001 |
| Overall cortical CBV (regional level) | <0.0001 |
| Overall WM CBV (regional level) | <0.0001 |
| Overall cortical MTT (regional level) | 0.0887 |
| Overall WM MTT (regional level) | 0.2101 |
| BPF | 0.2058 |
| fC (%) | 0.3451 |
| fWM (%) | 0.3041 |
| fT2h (%) | 0.3667 |
| fT1bh (%) | 0.2205 |
| fCSF (%) | 0.2004 |
HADS-A: Hospital Anxiety and Depression Scale–Anxiety; HADS-D: Hospital Anxiety and Depression Scale–Depression; EDSS: Expanded Disability Status Scale; WM: white matter; CBF: cerebral blood flow; CBV: cerebral blood volume; MTT: mean transit time; BPF: brain parenchymal fraction; fC: fractional cortical volume; fWM: fractional white matter; fT2h: fractional white matter T2-hyperintensities; fT1bh: fractional T1 black holes; fCSF: fractional cerebrospinal fluid. p-value < 0.05 was considered statistically significant.
Table 3.
Generalized linear model of perfusion parameters nested by lobe demonstrating the association of each location and confounding variable with cognitive impairment.
| Parameter | GM
|
WM
|
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | χ2 | p-value | Estimate | SE | χ2 | p-value | |
| CBF | ||||||||
| Intercept | 2.3143 | 2.3980 | 00.93 | 0.3345 | 0.0210 | 2.1547 | 00.00 | 0.9922 |
| Cortical CBF (frontal-left) | −2.1446 | 0.5521 | 15.09 | 0.0001 | −1.4243 | 0.5350 | 07.09 | 0.0078 |
| Cortical CBF (frontal-right) | −2.1600 | 0.5565 | 15.06 | 0.0001 | −1.4233 | 0.5345 | 07.09 | 0.0078 |
| Cortical CBF (occipital-left) | −2.2025 | 0.5666 | 15.11 | 0.0001 | −1.3602 | 0.5107 | 07.09 | 0.0077 |
| Cortical CBF (occipital-right) | −2.1914 | 0.5642 | 15.08 | 0.0001 | −1.3398 | 0.5035 | 07.08 | 0.0078 |
| Cortical CBF (parietal-left) | −2.2287 | 0.5734 | 15.11 | 0.0001 | −1.4598 | 0.5499 | 07.05 | 0.0079 |
| Cortical CBF (parietal-right) | −2.1935 | 0.5645 | 15.10 | 0.0001 | −1.4258 | 0.5375 | 07.04 | 0.0080 |
| Cortical CBF (temporal-left) | −2.1737 | 0.5593 | 15.11 | 0.0001 | −1.3691 | 0.5141 | 07.09 | 0.0077 |
| Cortical CBF (temporal-right) | −2.1423 | 0.5506 | 15.14 | <0.0001 | −1.3618 | 0.5113 | 07.09 | 0.0077 |
| HADS-D (log) | 2.9478 | 0.4093 | 51.88 | <0.0001 | 2.7505 | 0.3870 | 50.51 | <0.0001 |
| EDSS | 2.1434 | 0.3593 | 35.58 | <0.0001 | 2.1094 | 0.3467 | 37.01 | <0.0001 |
| Education (years) | −0.2779 | 0.1235 | 05.06 | 0.0245 | −0.3419 | 0.1199 | 08.13 | 0.0044 |
| CBV | ||||||||
| Intercept | −1.3645 | 2.0484 | 00.44 | 0.5053 | −2.5647 | 1.9427 | 01.74 | 0.1868 |
| Cortical CBF (frontal-left) | −3.1343 | 0.9007 | 12.11 | 0.0005 | −2.3492 | 1.4153 | 02.76 | 0.0969 |
| Cortical CBF (frontal-right) | −3.2215 | 0.9307 | 11.98 | 0.0005 | −2.2947 | 1.3921 | 02.72 | 0.0993 |
| Cortical CBF (occipital-left) | −2.5862 | 0.7393 | 12.24 | 0.0005 | −1.5052 | 0.8617 | 03.05 | 0.0807 |
| Cortical CBF (occipital-right) | −2.6050 | 0.7530 | 11.97 | 0.0005 | −1.3976 | 0.8280 | 02.85 | 0.0914 |
| Cortical CBF (parietal-left) | −3.2543 | 0.9306 | 12.23 | 0.0005 | −2.1827 | 1.4041 | 02.42 | 0.1201 |
| Cortical CBF (parietal-right) | −3.1189 | 0.8935 | 12.19 | 0.0005 | −1.9961 | 1.3076 | 02.33 | 0.1269 |
| Cortical CBF (temporal-left) | −2.7647 | 0.7928 | 12.16 | 0.0005 | −1.5703 | 0.9419 | 02.78 | 0.0955 |
| Cortical CBF (temporal-right) | −2.6541 | 0.7669 | 11.98 | 0.0005 | −1.5059 | 0.9248 | 02.65 | 0.1034 |
| HADS-D (log) | 2.9385 | 0.4101 | 51.34 | <0.0001 | 2.7336 | 0.3837 | 50.76 | <0.0001 |
| EDSS | 2.1445 | 0.3649 | 34.54 | <0.0001 | 2.0472 | 0.3438 | 35.46 | <0.0001 |
| Education (years) | −0.3812 | 0.1182 | 10.40 | 0.0013 | −0.3923 | 0.1158 | 11.48 | 0.0007 |
GM: gray matter; WM: white matter; SE: standard error; CBF: cerebral blood flow; CBV: cerebral blood volume; HADS-D: Hospital Anxiety and Depression Scale–Depression; EDSS: Expanded Disability Status Scale.
Bonferroni-adjusted p-value<0.005 was considered statistically significant (shown in bold).
Discussion
We demonstrate significant cortical CBF and CBV differences between NI and CI RRMS patients in the absence of structural or demographic group differences after considering confounding variables of EDSS, HADS, and education. Global and regional cortical CBF and CBV reduction was significantly associated with cognitive impairment independent of the underlying WM volume in all but one lobar region.
Similar to Debernard et al.13 who used pseudo-continuous arterial spin labeling (pCASL) MRI to demonstrate CBF differences between healthy controls and early RRMS, these reported findings lend further support to the use of MR perfusion techniques to detect MS group differences in the absence of significant structural differences. In contrast to their findings, we did not detect CBF reduction between healthy controls and NI although the NI subgroup did demonstrate modest MTT prolongation relative to healthy controls. Whereas perfusion technique disparities could account for this disparity, there were other notable differences in the patient populations. While our NI patients demonstrated no significant cognitive test differences when compared with healthy controls, a borderline significant BVMT reduction compared to healthy controls was reported by Debernard et al. within his early RRMS cohort, suggesting a greater level of disease burden in their early RRMS cohort than our NI patients. Indeed, the upper EDSS range of their early RRMS patients was 4.5 compared to 3.5 in our NI patients supporting this assertion.
Multiple demographic, psychosocial, and structural factors exist that may contribute to cognitive impairment and confound analysis. Variables confounding our analysis that are well described in the literature include depression,20 anxiety,21 and level of education.22 Importantly, age and gender which alter perfusion were carefully matched, precluding further consideration. T2 hyperintense lesion volume, cortical lesion volume, cortical volume, and WM perfusion are variably implicated in cognitive impairment. Papadopoulou et al.23 found that T2 hyperintense lesions and GM volume, but not cortical lesions, significantly predicted SDMT. Other studies demonstrate only a modest relationship between WM T2 hyperintense lesion load, brain atrophy, and cognitive impairment or that cortical lesions and cortical atrophy, but not T2 hyperintense lesions, were independent predictors of global cognitive impairment.6 Several studies have evaluated the link between WM, basal ganglia, or thalamic perfusion and cognition, but few have studied cortical perfusion. Using DSC perfusion, Inglese et al.24 demonstrated an association between normal-appearing white matter (NAWM) and deep GM hypoperfusion and cognitive impairment, specifically visuospatial skills. After Bonferroni correction, deep GM CBV and CBF correlated with the color-word interference inhibition switching test and with the Rey Complex Figure Test, respectively.24 In a separate study, Inglese et al.25 reported deep GM CBF reduction in the caudate in RRMS patients compared to healthy controls although no correlation with cognition was attempted. Rashid et al. demonstrated CBF reduction in cortical and deep GM and WM in primary and SPMS, but not RRMS, patients compared to healthy controls. They found no association between CBF and the MS functional composite score.26 More recently, Aviv et al.14 showed cortical CBV reduction in SPMS patients which was significantly associated with cognitive impairment after controlling for brain atrophy. CBV reduction was localized to bilateral (left greater than right) frontal cortex and also correlated highly with SDMT, COWAT, and BVMT.27 Our findings extend these prior observations by showing cortical CBF and CBV reduction in cognitive impairment independent of more traditional parameters such as brain volume, WM T2 hyperintense lesion volume, cortical lesion volume, and WM perfusion. We also show that the addition of cortical CBF and CBV to these markers accounts for a greater degree of variance for cognitive impairment. The greater variance of cognitive impairment explained by cortical compared to WM CBF and CBV reinforces the increasingly recognized role of the cortex in both physical and cognitive disability28 while maintaining the well-established association between functional correlates of WM integrity and cognitive impairment.11,29 The findings are also in agreement with a 5-year prospective study reporting an independent contribution of cortical lesions and WM T2 hyperintense lesion volume to both EDSS and cognitive impairment with a stronger cortical lesions contribution.2
Several different potential etiologies for perfusion reduction in MS are described which are not mutually exclusive and may potentially coexist. Both the present and prior studies showed no association between WM volume and cortical CBF or CBV, suggesting an independent mechanism for cortical hypoperfusion arguing against either an anterograde or retrograde axonal degeneration with resultant secondary cortical hypoperfusion. The absence of structural differences between RRMS patients without and with cognitive impairment despite significant CBF and CBV abnormalities also argues against a primary neuronal loss leading to cortical hypoperfusion. In contradistinction to cortex, WM hypoperfusion was associated with WM volume loss suggesting that neuronal loss in WM is associated with secondary hypoperfusion similar to that previously described.30 Primary cortical metabolic or mitochondrial dysfunction31 and primary cerebral vascular etiologies could also be considered. Hypoperfusion secondary to a vascular etiology is potentially multifactorial; endothelin-1, a potent vasoconstrictor, is increased in MS and implicated in widespread cerebral hypoperfusion.32 Astrocytes play an important role in vessel tone regulation. β2-adrenergic receptor deficiency is described within astrocytes preventing inward-rectifying K+ reuptake after synaptic activity and reducing intracellular K+ availability at the astrocytic endfeet. Lower K+ recovery leads to reduced Ca2+-activated K+ channel function and reduced vasodilatory capacity.33 More recently, McMahon et al.34 demonstrated increased endoplasmic reticular stress molecules (C/enhancer-binding protein (EBP) homologous protein) and hypoxia-associated D110 in NAGM positing their role in microglial activation and cortical lesion development. Irrespective of the exact mechanism, the strong association between WM and cortical CBF and CBV reduction suggests a potential overlap in the underlying etiology.
Bookend perfusion is limited by a relatively large voxel size (1.7 mm × 1.7 mm × 4 mm) compared to structural and high field strength approaches; however, this is similar to other functional techniques like DTI with the advantage of whole head coverage performed in less than 2 minutes. Additionally, the DSC sequence is ubiquitous and often performed in clinical practice facilitating a relatively simple translation of the bookend technique to multiple centers. We found no significant structural differences for GM (cortex, basal ganglia, and thalamus) or WM between the CI and NI patient groups. However, non-significant reductions were present, reflecting the expectation that volume loss is associated with disease progression albeit not significantly in our patient cohort. The cognitive impairment rate of ~50% in our patient cohort is not reflective of population prevalence in this study. Rather this number of CI patients was prospectively selected based on sample size calculations and then matched by age and gender with NI RRMS patients and healthy controls. The study is limited by relatively small sample size, yet demonstrates reduced cortical CBF and CBV in CI patients. While reduced CBF and CBV in RRMS and reduced CBV in SPMS are previously demonstrated in cognitive impairment, longitudinal studies are required to determine whether CBF and CBV measurements are sensitive to disease progression.
In conclusion, cortical CBF and CBV reduction occurs in CI RRMS patients in the absence of significant structural differences, suggesting that perfusion imaging might be a useful biomarker of cortical disease severity and cognitive impairment.
Acknowledgments
Funding
Dr Aviv was supported by Canadian institute of Health Research operating grant. Dr Aviv and Dr Hojjat are supported by a Biogen fellowship funding award. Charles Cantrell is supported by the American Heart Association (14PRE20380310). Dr Carroll is supported by the US NIH (1R21EB017928-01A1).
Footnotes
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Seyed-Parsa Hojjat, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
Charles Grady Cantrell, Department of Biomedical Engineering, Northwestern University, Chicago, IL, USA.
Timothy J Carroll, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Department of Radiology, Northwestern University, Chicago, IL, USA; Department of Biomedical Engineering, Northwestern University, Chicago, IL, USA.
Rita Vitorino, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Department of Radiology, Northwestern University, Chicago, IL, USA.
Anthony Feinstein, Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
Lying Zhang, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
Sean P Symons, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
Sarah A Morrow, Department of Clinical Neurological Sciences, Western University, London, ON, Canada.
Liesly Lee, Department of Neurology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
Paul O’Connor, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
Richard I Aviv, Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; University of Toronto, Toronto, ON, Canada.
References
- 1.Damasceno A, Damasceno BP, Cendes F. Subclinical MRI disease activity influences cognitive performance in ms patients. Mult Scler Relat Disord. 2015;4:137–143. doi: 10.1016/j.msard.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–2961. doi: 10.1093/brain/aws246. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese M, Rocca MA, Atzori M, et al. Cortical lesions in primary progressive multiple sclerosis: A 2-year longitudinal MR study. Neurology. 2009;72:1330–1336. doi: 10.1212/WNL.0b013e3181a0fee5. [DOI] [PubMed] [Google Scholar]
- 4.Geurts JJ, Bo L, Pouwels PJ, et al. Cortical lesions in multiple sclerosis: Combined postmortem MR imaging and histopathology. Am J Neuroradiol. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 5.Amato MP, Portaccio E, Goretti B, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol. 2007;64:1157–1161. doi: 10.1001/archneur.64.8.1157. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese M, Gallo P. Magnetic resonance evidence of cortical onset of multiple sclerosis. Mult Scler. 2009;15:933–941. doi: 10.1177/1352458509106510. [DOI] [PubMed] [Google Scholar]
- 7.Yao B, Hametner S, van Gelderen P, et al. 7 Tesla magnetic resonance imaging to detect cortical pathology in multiple sclerosis. PLoS ONE. 2014;9:e108863. doi: 10.1371/journal.pone.0108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mainero C, Louapre C, Govindarajan ST, et al. A gradient in cortical pathology in multiple sclerosis by in vivo quantitative 7 T imaging. Brain. 2015;138:932–945. doi: 10.1093/brain/awv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardif CL, Bedell BJ, Eskildsen SF, et al. Quantitative magnetic resonance imaging of cortical multiple sclerosis pathology. Mult Scler Int. 2012;2012:742018. doi: 10.1155/2012/742018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seewann A, Vrenken H, Kooi EJ, et al. Imaging the tip of the iceberg: Visualization of cortical lesions in multiple sclerosis. Mult Scler. 2011;17:1202–1210. doi: 10.1177/1352458511406575. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese M, Rinaldi F, Seppi D, et al. Cortical diffusion-tensor imaging abnormalities in multiple sclerosis: A 3-year longitudinal study. Radiology. 2011;261:891–898. doi: 10.1148/radiol.11110195. [DOI] [PubMed] [Google Scholar]
- 12.Faiss JH, Dahne D, Baum K, et al. Reduced magnetisation transfer ratio in cognitively impaired patients at the very early stage of multiple sclerosis: A prospective, multicenter, cross-sectional study. BMJ Open. 2014;4:e004409. doi: 10.1136/bmjopen-2013-004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debernard L, Melzer TR, Van Stockum S, et al. Reduced grey matter perfusion without volume loss in early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:544–551. doi: 10.1136/jnnp-2013-305612. [DOI] [PubMed] [Google Scholar]
- 14.Aviv RI, Francis PL, Tenenbein R, et al. Decreased frontal lobe gray matter perfusion in cognitively impaired patients with secondary-progressive multiple sclerosis detected by the bookend technique. Am J Neuroradiol. 2012;33:1779–1785. doi: 10.3174/ajnr.A3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peruzzo D, Castellaro M, Calabrese M, et al. Heterogeneity of cortical lesions in multiple sclerosis: An MRI perfusion study. J Cereb Blood Flow Metab. 2013;33:457–463. doi: 10.1038/jcbfm.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vakil P, Lee JJ, Mouannes-Srour JJ, et al. Cerebrovascular occlusive disease: Quantitative cerebral blood flow using dynamic susceptibility contrast MR imaging correlates with quantitative h2[15o] pet. Radiology. 2013;266:879–886. doi: 10.1148/radiol.12120756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin W, Horowitz S, Ragin A, et al. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: Evaluation of reproducibility and age-and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58:1232–1241. doi: 10.1002/mrm.21420. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Demaree HA, Gaudino E, DeLuca J. The relationship between depressive symptoms and cognitive dysfunction in multiple sclerosis. Cogn Neuropsychiatry. 2003;8:161–171. doi: 10.1080/13546800244000265. [DOI] [PubMed] [Google Scholar]
- 21.Morrow SA, Rosehart H, Pantazopoulos K. Anxiety and depressive symptoms are associated with worse performance on objective cognitive tests in MS. J Neuropsychiatry Clin Neurosci. 2015 doi: 10.1176/appi.neuropsych.15070167. http://www.ncbi.nlm.nih.gov/pubmed/26569152. [DOI] [PubMed]
- 22.Scarpazza C, Braghittoni D, Casale B, et al. Education protects against cognitive changes associated with multiple sclerosis. Restor Neurol Neurosci. 2013;31:619–631. doi: 10.3233/RNN-120261. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulou A, Muller-Lenke N, Naegelin Y, et al. Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult Scler. 2013;19:1290–1296. doi: 10.1177/1352458513475490. [DOI] [PubMed] [Google Scholar]
- 24.Inglese M, Adhya S, Johnson G, et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab. 2008;28:164–171. doi: 10.1038/sj.jcbfm.9600504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inglese M, Park SJ, Johnson G, et al. Deep gray matter perfusion in multiple sclerosis: Dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol. 2007;64:196–202. doi: 10.1001/archneur.64.2.196. [DOI] [PubMed] [Google Scholar]
- 26.Rashid W, Parkes LM, Ingle GT, et al. Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:1288–1293. doi: 10.1136/jnnp.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis PL, Jakubovic R, O’Connor P, et al. Robust perfusion deficits in cognitively impaired patients with secondary-progressive multiple sclerosis. Am J Neuroradiol. 2013;34:62–67. doi: 10.3174/ajnr.A3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Politis M, Giannetti P, Su P, et al. Increased PK11195 pet binding in the cortex of patients with MS correlates with disability. Neurology. 2012;79:523–530. doi: 10.1212/WNL.0b013e3182635645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: Impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–1032. doi: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]
- 30.Varga AW, Johnson G, Babb JS, et al. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. J Neurol Sci. 2009;282:28–33. doi: 10.1016/j.jns.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 32.D’Haeseleer M, Cambron M, Vanopdenbosch L, et al. Vascular aspects of multiple sclerosis. Lancet Neurol. 2011;10:657–666. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- 33.De Keyser J, Steen C, Mostert JP, et al. Hypoperfusion of the cerebral white matter in multiple sclerosis: Possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab. 2008;28:1645–1651. doi: 10.1038/jcbfm.2008.72. [DOI] [PubMed] [Google Scholar]
- 34.McMahon JM, McQuaid S, Reynolds R, et al. Increased expression of ER stress- and hypoxia-associated molecules in grey matter lesions in multiple sclerosis. Mult Scler. 2012;18:1437–1447. doi: 10.1177/1352458512438455. [DOI] [PubMed] [Google Scholar]


