Abstract
Vitamin A deficiency (VAD) is an important contributor to child morbidity and mortality. The prevalence of VAD, measured by retinol‐binding protein (RBP) or retinol, is overestimated in populations with a high prevalence of inflammation. We aimed to quantify and adjust for the effect of inflammation on VAD prevalence in a nationally representative survey of Liberian children 6 to 35 months of age. We compared five approaches to adjust RBP for inflammation and estimate VAD prevalence (defined as RBP < 0.7 µmol/L): (1) ignoring inflammation; (2) excluding individuals with inflammation (C‐reactive protein (CRP) >5 mg/L or alpha1‐acid glycoprotein (AGP) >1 g/)L; (3) multiplying each individual's RBP by an internal correction factor; (4) by an external correction factor; and (5) using regression (corrected RBP = exp(InRBP – β1(lnCRPobs‐lnCRPref) – β2(lnAGPobs‐lnAGPref)). Corrected RBP was based on a regression model where reference lnCRP and lnAGP were set to the maximum of the lowest decile. The unadjusted prevalence of VAD was 24.7%. Children with elevated CRP and/or AGP had significantly lower RBP concentrations than their apparently healthy peers (geometric mean RBP 0.79 µmol/L (95% CI: 0.76, 0.82) vs. 0.95 µmol/L (95% CI: 0.92, 0.97), P < 0.001). Using approaches 2–5 resulted in a prevalence of VAD of 11.6%, 14.3%, 13.5% and 7.3%, respectively. Depending on the approach, the VAD prevalence is reduced 10–17 percentage points when inflammation is taken into account. Further quantification of the influence of inflammation on biomarkers of vitamin A status from other national surveys is needed to compare and recommend the preferred adjustment approach across populations.
Keywords: nutrition, inflammation, retinol‐binding protein, vitamin A deficiency, C‐reactive protein, alpha1‐acid glycoprotein
Abbreviations
- VAD
vitamin A deficiency
- RBP
retinol‐binding protein
- CRP
C‐reactive protein
- AGP
alpha1‐acid glycoprotein
- APP
acute‐phase protein
- NMS
National Micronutrient Survey
Introduction
Vitamin A deficiency (VAD) is a major public health issue in many resource‐poor settings of the world (Taren 2012). It is estimated that 33%, or 190 million children, worldwide are vitamin A deficient (WHO 2009). The importance of VAD is well established with respect to child mortality and morbidity (Tanumihardjo 2012; Taren 2012). While there has been important progress globally with respect to the alleviation of overt signs of VAD, biochemical data suggest a persistent, elevated prevalence of low vitamin A status, with important impacts on growth, vision, immune function and reproduction (Ross et al. 2000). To assess the prevalence of VAD in a population, valid and reliable biomarkers for vitamin A status are necessary.
Nearly all vitamin A in the blood is associated with retinol‐binding protein (RBP). Therefore, serum RBP can be used as a surrogate measure for retinol content, the predominant circulating form of vitamin A in the blood, or vitamin A status (Erhardt et al. 2004). Some studies suggest that the molar ratio of retinol : RBP is not 1:1 (Gorstein et al. 2008; Engle‐Stone et al. 2011) and may change throughout the inflammation response (Willumsen et al. 1997); however, this 1:1 ratio is adopted by the World Health Organization (WHO 2011). RBP is affected by the acute‐phase response, resulting in interpretations of VAD prevalence, which are likely not reflective of actual vitamin A status in populations with high levels of inflammation and infection. Proteins whose concentrations change as part of the acute‐phase response are referred to as acute‐phase proteins (APP). RBP is itself a negative APP and its concentration temporarily decreases in response to inflammation, reflecting the decreased synthesis of RBP (Louw et al. 1992). Positive acute‐phase proteins, such as C‐reactive protein (CRP) and α‐1 acid glycoprotein (AGP), are synthesized by the liver. CRP can increase 20 to 1000‐fold and AGP can increase two to fivefold in response to inflammation (Koj 1985). The more severe the inflammation or infection, the more of an effect the acute‐phase response has on RBP (Duncan et al. 2012); therefore, the measurement of CRP and AGP can assist in the correct interpretation of this biochemical marker of vitamin A status.
A meta‐analysis of the effect of subclinical infection on plasma retinol concentrations found that the geometric mean retinol was reduced from anywhere between 11% to 24%, depending on the stage of inflammation (Thurnham et al. 2003). Despite the research demonstrating a definite temporary and concurrent response of retinol and RBP to commonly measured APPs, there is no current consensus on how to correct vitamin A biomarkers for the influence of inflammation (WHO 2011). Some have proposed using a new cutoff to determine vitamin A deficiency among individuals with elevated APP concentrations (Paracha et al. 2000). Others suggest using correction factors to adjust individuals' retinol and RBP concentrations based on their stage of inflammation, often categorized as the incubation, early and late convalescence stages (Thurnham et al. 2003; Baingana et al. 2013). The role of inflammation on micronutrient biomarker levels has been identified as a critical research gap (Raiten et al. 2015).
The aim of this study was to present various approaches for adjusting for inflammation when measuring RBP and how they affect VAD prevalence in a high‐inflammation population of Liberian children aged between 6 to 35 months. We go beyond previous research to show the effect of adjustment using a continuous approach (‘the regression approach’), which accounts for inflammation in a continuous manner, rather than relying on previously identified categories of inflammation (Thurnham et al. 2003). Such a concept was deemed appropriate given the substantial variation observed in RBP concentrations above and below the CRP and AGP cutoffs that currently define inflammation (Suchdev et al. in press). We also examined the effect of malaria on VAD independently and in addition to the effect of CRP and AGP. This analysis is of particular interest given it examines VAD in a country that usually has wide coverage of their vitamin A capsule programme.
Key messages.
Ignoring inflammation in resource‐poor settings can lead to the incorrect diagnosis of vitamin A deficiency in individuals, as well as overestimation of the prevalence of vitamin A deficiency in a population.
The application of different adjustment techniques to RBP data in Liberian children 6–35 months of age reduces unadjusted vitamin A deficiency prevalence from 25% by 10 to 17 percentage points, influencing the interpretation of the magnitude of the public health problem.
Quantification of the influence of inflammation on biomarkers of vitamin A status from other national surveys is needed to compare and recommend the preferred adjustment approach across populations.
Methods
Study population
This secondary analysis is based on a nationally representative micronutrient survey, the National Micronutrient Survey (NMS), in Liberia. The NMS was conducted in a representative sample of 1434 Liberian children aged between 6 to 35 months from urban and rural areas between April and June 2011, with the primary objective of determining the national and rural/urban prevalence of key micronutrient deficiencies among children. Detailed descriptions of the study population and methods have been described elsewhere (LISGIS & UNICEF 2011). Briefly, this was a two‐stage cluster design that stratified the country into urban and rural areas. Probability proportional to size was used to select 57 communities from within each stratum. In each cluster, 15 households were systematically sampled to give a sample of 1710 households. The survey collected socio‐economic, health and nutrition information from the children. The survey also assessed infant and young child feeding practices, nutritional biomarkers as well as anthropometry, measured following standardized techniques. Signed informed consent was obtained from the parent or legal guardian of the child, and verbal assent was obtained from the child. Ethical approval was received from the Liberia Ethics Committee. A total of 1484 children under five completed the assessments, corresponding to a 98% eligible children response rate. Of these, 1434 had complete information that was used in the following analyses.
Biochemical assessments and laboratory methods
Hemoglobin was assessed with the Hemocue 201+, and serum samples were collected from all eligible children to measure RBP, CRP and AGP. A venous blood sample of two milliliters was drawn from each child in a vacutainer tube. Immediately after collection, whole blood samples were placed in self‐cooling containers containing frozen gel packs and outfitted with racks, additional foam insulation, and recording digital thermometers. Every day, following a minimum 2‐h period without movement, aliquots of serum were drawn off from the top of each whole blood sample collected that day and transferred to smaller serum vials. Serum samples were stored at −15°C and shipped to VitMin, Germany. RBP, CRP and AGP concentrations were measured with an optimized sandwich enzyme‐linked immunosorbent assay technique in duplicate in 15 μL serum (Erhardt et al. 2004). Clinical serum controls were used for calibration purposes and quality control samples from the Centers for Disease Control and Prevention in Atlanta were used to check the absolute values. A retinol : RBP ratio of 1:1 was assumed, and the cutoff value for RBP in children to define VAD was taken as <0.7 µmol/L (WHO 2011). Malaria was measured using a rapid diagnostic test, the Paracheck Pf test (Orchid Biomedical System, Goa, India).
Statistical analyses
The analyses were conducted in collaboration with the Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia working group, which has been working to identify an appropriate method to account for inflammation when measuring nutrition biomarkers (Suchdev et al. in press). Descriptive and biochemical status statistics were calculated for variables of interest. All analyses were weighted for the complex survey design. Five approaches were compared to adjust RBP for elevated APPs in order to account for the effect of subclinical inflammation on the assessment of vitamin A deficiency, based on a recent review (Raiten et al. 2015). Approach 1 ignored concurrent inflammation as defined by elevated CRP (>5 mg/L) or AGP (>1 g/L). Approach 2 excluded those with elevated APPs (CRP > 5 mg/L and/or AGP > 1 g/L). Approach 3 used internal correction factors based on a four‐level model of inflammation: the incubation period (CRP > 5 mg/L and AGP ≤ 1 g/L), early convalescence (CRP > 5 mg/L and AGP > 1 g/L), late convalescence (CRP ≤ 5 mg/L and AGP > 1 g/L) and apparently healthy (CRP ≤ 5 mg/L and AGP ≤ 1 g/L). Level‐specific adjustment factors were calculated as the ratios of geometric mean RBP for apparently healthy children to geometric mean RBP for those in the incubation period, the early convalescence period and the late convalescence period. Each individual's RBP value was then multiplied by their corresponding correction factor (based on their stage of inflammation) to estimate an adjusted RBP concentration. Approach 4 used correction factors established by the meta‐analysis of Thurnham et al. (2003), which are based on the same four‐level model of inflammation. This meta‐analysis used CRP, AGP and retinol data from 15 studies to calculate correction factors that could be applied to any dataset with retinol values. Because we assumed a retinol : RBP ratio of 1:1, we applied these correction factors to our RBP concentrations. Finally, Approach 5 employed a multiple linear regression model to obtain adjusted RBP concentrations: adjusted RBP = exp[unadjusted lnRBP – (regression coefficient for lnCRP)*(lnCRP‐(−1.47)) – (regression coefficient for lnAGP)*(lnAGP‐(−0.36)] (Suchdev et al. in press). RBP, CRP and AGP were ln transformed to reflect better regression diagnostics. The adjusted RBP was calculated by subtracting the influence of CRP and/or AGP. A reference concentration was subtracted from measured lnCRP and lnAGP values to mimic individuals with low levels of APPs and to avoid over‐adjusting RBP by excluding low levels of inflammation from the regression adjustment. There are not previously well‐defined cutoffs for CRP or AGP, specifically looking for the effects of inflammation on RBP concentrations, so the reference concentrations used were the maximum values of the lowest decile found in the sampled population, or lnCRP = −1.47 ln(mg/L) and lnAGP = −0.36 ln(g/L). These reference concentrations were chosen after running sensitivity analyses that demonstrated little change in the prevalence of VAD when using other low levels of APPs that were based on our literature review of low inflammation populations. This regression approach adjustment was only applied to individuals with lnCRP and lnAGP concentrations at or above the reference values, as those below are thought to be uninflamed individuals with unduly influenced RBP concentrations and therefore needing no inflammation correction. We also used this approach to control for the effect of malaria to examine its independent effect on RBP. In order to measure its effect in addition to what we observe from elevated CRP and AGP, we stratified adjusted RBP concentrations and VAD prevalence (based on the regression approach to adjust for CRP and AGP) by malaria status. VAD prevalence was calculated based on the adjusted RBP levels computed in each case.
Data analysis was done using sas 9.3 (SAS Institute, North Carolina) following complex survey analytic techniques. PROC SURVEYMEANS was used to calculate the mean of continuous demographic and biochemical characteristics. PROC SURVEYFREQ was used to calculate frequencies and to test for differences in proportions of categorical background characteristics, as well as prevalence of vitamin A deficiency resulting from each adjustment approach. Adjustments made using linear regression to subtract the effect of inflammation were done with PROC SURVEYREG. A P‐value of less than 0.05 was used to assess statistical significance.
Results
Basic demographic, nutritional and inflammation‐related characteristics are presented in Table 1. There was an equal proportion of women and men, with more living in rural areas. Prevalence of wasting, stunting and underweight was 9.2%, 35.1% and 23.2%, respectively. Almost a third of the children had positive malaria status based on a rapid diagnostic test, and among those with elevated CRP and AGP, most were in the early or late stages of convalescence. The prevalence of elevated CRP was significantly higher among children 12–35 months of age compared with children 6–11 months (31.0% vs. 24.9%, P = 0.04), whereas the prevalence of elevated AGP was significantly lower among older children (37.2% vs. 45.1%, P = 0.0004).
Table 1.
Demographic and biochemical characteristics of a nationally representative population of Liberian children 6 to 35 months of age (n = 1434)a
| Characteristics | Weighted % (n) or mean | 95% CI |
|---|---|---|
| Age (months) | 19.9 | 19.4, 20.4 |
| Men | 50.4 (720) | 47.6, 53.2 |
| Rural | 62.5 (590) | 59.0, 66.0 |
| Positive malaria status | 29.4 (348) | 26.2, 32.6 |
| Recent diarrhoea (in the past 2 weeks) | 46.6 (656) | 43.3, 50.0 |
| Recent fever (in the past 2 weeks) | 71.8 (1023) | 68.7, 74.9 |
| Anaemia (hemoglobin < 110 g/L) | 59.5 (833) | 55.6, 63.4 |
| Wasting (WHZ < −2 SD) | 9.2 (150) | 7.4, 11.0 |
| Stunting (HAZ < −2 SD) | 35.1 (841) | 32.0, 38.2 |
| Underweight (WAZ < −2 SD) | 23.2 (349) | 20.9, 25.5 |
| Received vitamin A supplementation in the past 6 months | 91.2 (1239) | 88.9, 93.4 |
| RBP < 0.70 µmol/L | 24.7 (351) | 21.2, 28.1 |
| CRP > 5 mg/L | 29.5 (375) | 26.5, 32.5 |
| AGP > 1 g/L | 56.2 (762) | 52.5, 60.0 |
| Any inflammation (CRP > 5 mg/L or AGP > 1 g/L) | 59.1 (801) | 55.6, 62.7 |
| Stage of inflammation | ||
| Apparently healthy (CRP ≤5 mg/L and AGP ≤1 g/L) | 40.9 (633) | 37.3, 44.4 |
| Incubation (CRP >5 mg/L and AGP ≤1 g/L) | 2.9 (39) | 2.0, 3.9 |
| Early convalescence (CRP >5 mg/L and AGP >1 g/L) | 26.6 (336) | 23.7, 29.6 |
| Late convalescence (CRP ≤5 mg/L and AGP >1 g/L) | 29.6 (426) | 26.4, 32.8 |
CI, confidence interval; SD, standard deviation; WHZ, weight‐for‐height Z‐score; HAZ, height‐for‐age Z‐score; WAZ, weight‐for‐age Z‐score; CRP, C‐Reactive Protein; AGP, α1‐acid‐glycoprotein.
The prevalence of VAD was significantly higher among children with malaria compared with those without (37.7% vs. 19.3%, P < 0.0001). The weighted means of RBP, CRP and AGP significantly differed by stage of inflammation. Geometric means for RBP in children in the apparently healthy, incubation, early and late convalescence stages of inflammation were 0.95 µmol/L, 0.86 µmol/L, 0.70 µmol/L and 0.87 µmol/L, respectively (P < 0.0001). The weighted means for CRP in each of the stages were 1.0 mg/L, 11.3 mg/L, 19.3 mg/L and 2.1 mg/L, respectively (P < 0.0001), and for AGP, they were 0.8 g/L, 0.9 g/L, 1.3 g/L and 1.2 g/L, respectively (P < 0.0001). RBP significantly decreased with increasing deciles of CRP and AGP while the prevalence of VAD significantly increased (Figs 1, 2). This trend was observed irrespective of the inflammation cutoffs of CRP = 5 mg/L and AGP = 1 g/L, reinforcing the need for a continuous approach to adjust for inflammation, such as the ‘regression approach’.
Figure 1.
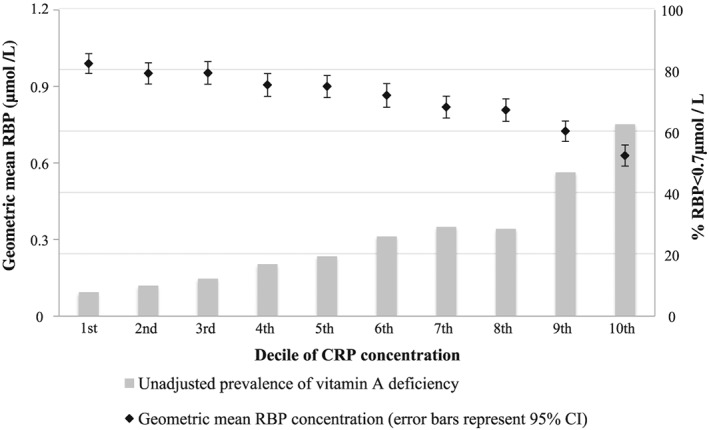
Geometric mean retinol‐binding protein (RBP) and proportion of children with unadjusted RBP < 0.7 µmol/L by C‐reactive protein (CRP) concentration decile.
Figure 2.
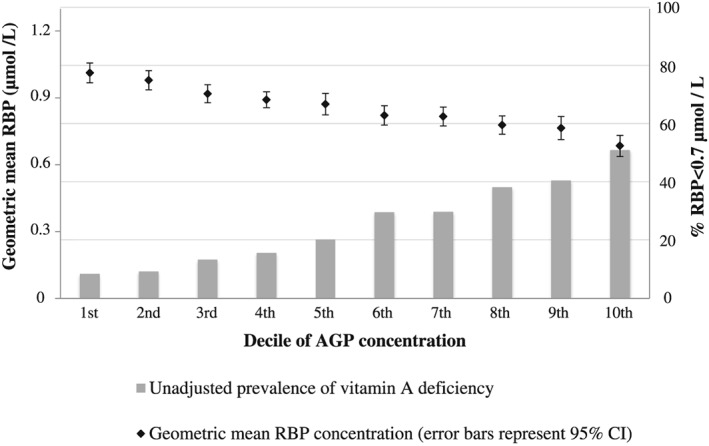
Geometric mean retinol‐binding protein (RBP) and proportion of children with unadjusted RBP < 0.7 µmol/L by α1‐acid‐glycoprotein (AGP) concentration decile.
The adjusted VAD prevalence using each of the described approaches is displayed in Table 2. Ignoring inflammation, the VAD prevalence in this population of children was 24.7%. Excluding individuals with inflammation reduced the prevalence of VAD to 11.6%. Internal correction factors were calculated as 1.10 for children in the incubation phase, 1.34 for those in the early convalescence phase, and 1.09 for children in the late convalescence phase of inflammation. Correction factors established by Thurnham et al. (2003) in their meta‐analysis are 1.14 for those in the incubation phase, 1.31 for those in the early convalescence phase and 1.12 for those in the late convalescence phase. Using the internal and Thurnham et al. (2003) correction factors on individuals' RBP concentrations resulted in prevalence estimates of VAD of 14.3% and 13.5%, respectively. Using linear regression to adjust for malaria status decreased the prevalence of VAD to 18.8%, and using linear regression to adjust for AGP and CRP decreased the prevalence to 7.3%. Even after adjusting RBP for CRP and AGP using the regression approach, there was a significant difference in RBP concentrations and VAD prevalence among children with and without malaria (10.7% (95% CI: 7.3, 14.1) vs. 5.9% (95% CI: 4.4, 7.5), P = 0.023).
Table 2.
Summary of vitamin A deficiency by approach (weighted analysis)†
| Approach | Prevalence of vitamin A deficiency (95% CI) |
|---|---|
| Approach 1: Ignoring APPs | 24.7 (21.2, 28.1) |
| Approach 2: Excluding those with elevated APP (CRP and/or AGP) | 11.6 (8.4, 14.8) |
| Approach 3: Adjusting RBP using internal correction factors†† | 14.3 (11.9, 16.8) |
| Approach 4: Adjusting RBP using Thurnham et al.'s (2003) correction factors††† | 13.5 (11.1, 15.9) |
| Approach 5: Adjusting RBP using regression approach†††† | 7.3 (5.7, 9.0) |
APP, acute‐phase protein; CRP, C‐reactive protein; AGP, α1‐acid‐glycoprotein; RBP, retinol‐binding protein; VAD prevalence defined as defined as RBP < 0.7 µmol/L; elevated CRP defined as CRP > 5 mg/L; elevated AGP defined as AGP > 1 g/L.
Internal correction factors: CRP = 1.10, CRP + AGP = 1.34, AGP = 1.09.
Thurnham correction factors: CRP = 1.14, CRP + AGP = 1.31, AGP = 1.12.
Adjusted RBP = exp[unadjusted lnRBP – (regression coefficient for lnCRP)*(lnCRP‐(−1.47)) – (regression coefficient for lnAGP)*(lnAGP‐(−0.36))].
The strong correlation (r = 0.6, P < 0.0001) between CRP and AGP demonstrated that they partially share variance in their quantification of inflammation. RBP was significantly correlated with both CRP (r = −0.3, P < 0.0001) and AGP (r = −0.3, P < 0.0001). The multiple linear regression model indicated that lnCRP and lnAGP were not collinear and that 16% of the variance in lnRBP was explained by lnCRP and lnAGP.
Discussion
The results of this study indicate that approximately 60% of the children in this national survey had elevated APPs. The children in the incubation, early and late convalescence stages of inflammation had significantly lower RBP levels than those who were apparently healthy, with a corresponding higher prevalence of VAD. The findings demonstrate that the statistical adjustment of RBP levels for inflammation results in substantially lower estimates of VAD compared with not adjusting for it in a resource‐poor setting with high rates of inflammation. During an acute‐phase response, RBP levels may be temporarily reduced because of decreased synthesis of RBP (Thurnham & Singkamani 1991); however, this may be reflective of the underlying infection, not the individual's actual vitamin A status. The effect of inflammation on decreasing concentrations of vitamin A biomarkers, occurring independently of actual vitamin A status, has been previously reported (Filteau et al. 1993, 1995; Paracha et al. 2000; Wieringa et al. 2002; Thurnham et al. 2003; Baingana et al. 2013; Schulze et al. 2014; Wessells et al. 2014). Reductions in RBP during the acute‐phase response are transitory and its concentration is likely to return to normal once the infection or trauma has subsided (Filteau et al. 1993). Therefore, the estimation of VAD prevalence in the population is likely overestimated if no adjustment is made for APPs.
The objective of this analysis was to estimate and correct for the influence of inflammation (as measured by elevated CRP and AGP) on RBP levels and VAD prevalence in children 6 to 35 months of age in Liberia using five different approaches. To our knowledge, this is the first study to present a comparison of approaches to adjust for inflammation on VAD prevalence and the first to use a linear regression model to account for the transient effect of APPs on RBP concentrations. All the adjustment approaches significantly reduced the prevalence of VAD compared with ignoring inflammation. We also confirm previous findings that excluding children with raised APPs may not be the most appropriate approach for adjusting for inflammation in the estimation of VAD because it results in a much reduced sample size, especially in younger children with high rates of morbidity. Others have also found potential sample bias with this approach (Maqsood et al. 2004; Baingana et al. 2013). Internal correction factors were similar in magnitude to those found in the meta‐analysis by Thurnham et al. (2003), which supports the latter's applicability to survey data that do not have the capacity or sample size to calculate internal correction factors. Using internal or external (Thurnham et al. 2003) corrections factors significantly reduces the prevalence of VAD; however, it categorizes individuals into stages of inflammation in which there is still considerable variability in RBP concentrations. The regression approach may offer some advantage and precision when controlling for these APPs because it retains the entire sample size and it better reflects the association between RBP and the entire range of APPs, not needing to rely on predefined cutoffs of CRP = 5 mg/L and AGP = 1 g/L. Our results show that using the regression approach to control for the effect of CRP and AGP leads to a much lower adjusted prevalence of VAD compared with ignoring inflammation. However, in certain populations, the inflammatory role of APPs may exhibit curvilinear or plateau relationships with RBP, and a linear regression coefficient alone may not fully quantify their full inflammatory effect. Nevertheless, it is also possible that residual confounding or mediation from covariates not considered in this study, or interactions among those modelled, might alter the type of relationship between RBP and APPs via mechanisms that could themselves, be either synergistic or antagonistic (bidirectional) (Szklo & Nieto 2012), and less likely to discount the observed main effects of APPs on RBP concentration. A larger study, as planned by Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia, with more diverse populations could further elucidate these mechanisms and determine whether the findings are consistent across countries with high and low inflammation prevalence.
Prior to any adjustment, the levels of VAD suggested a severe public health problem (WHO 1996). In the present study, adjusting for inflammation using internal and external correction factors resulted in a prevalence of VAD that is indicative of a moderate public health problem. Using linear regression to adjust for CRP and AGP reduced the prevalence to a mild public health problem. Although this study was conducted in children who are vulnerable to nutritional deficiencies and infection, almost all children (91.2%) had received vitamin A supplementation in the past 6 months through the country's semi‐annual vitamin A supplementation campaign, which is integrated with immunization services through the Ministry of Health of Liberia. This may explain the low levels of VAD after adjustment for inflammation. However, similar to what has been previously found in response to universal vitamin A supplementation strategies (Palmer et al. 2012), mean RBP concentration was not significantly different among children who had and had not received vitamin A supplementation in the past 6 months, as was the absolute risk of vitamin A deficiency. This observation was held before and after adjustment for APPs.
In the present study, there was a significant added effect of malaria status on RBP concentrations and VAD prevalence estimates even after adjusting for CRP and AGP using the regression approach, a finding that is consistent with a recent study, which examined the association of malaria and inflammation‐corrected RBP (Wessells et al. 2014). Wessells et al. (2014) reported that in young children in Burkina Faso there was a significant association between elevated plasma histidine‐rich protein II and RBP even after adjusting for APPs using correction factors. In our findings, using multiple linear regression to account for the effects of CRP and AGP may not have fully captured the effect of malaria status on RBP concentrations. The effects of malaria infection on reducing RBP and retinol concentrations have been shown to be transitory (Filteau et al. 1993); thus, statistical adjustments to correct for both elevated APPs and malaria infection need to be explored.
Because of its cross‐sectional nature, the results of the current study are not evidence for a causal effect of the inflammatory response on RBP concentrations. Physiologic processes that alter both nutritional status and inflammation could exist, therefore confounding the association between the two. For instance, a child with malaria could have higher inflammation and a reduced appetite resulting in reduced nutrient intakes, such as vitamin A. Therefore, the reduced RBP levels would be a reflection of the child's nutrient status and not only a response to the inflammation itself (Duncan et al. 2012). A further limitation of this study is that information on the causes of inflammation in this population was not concurrently measured in this survey. Causes of inflammation other than malaria, such as intestinal parasites, respiratory infections and HIV, could affect vitamin A metabolism in addition to or independent of what is observed from elevated CRP and AGP. A final limitation of our study is the lack of calibration between RBP and serum retinol, and if the molar ratio of 1:1 does not hold in this population, then the cutoff point of <0.7 µmol/L should be adjusted. However, this would not change our main conclusion. In fact, using a cutoff of <0.84 µmol/L (Gorstein et al. 2008; Engle‐Stone et al. 2011) with the regression adjustment approach resulted in a prevalence that was 24 percentage points lower than the VAD prevalence without adjustment. Serum retinol is the recommended biomarker for determining population level VAD; however, because of the high cost for this analysis, the measurement of RBP may be preferred in low‐income and middle‐income countries where high levels of inflammation are often seen (Erhardt et al. 2004).
Nonetheless, our results highlight the importance of inflammation on the interpretation of VAD in a population of young children, independent of actual vitamin A status. Accounting for inflammation may have important implications for evaluating vitamin A supplementation and fortification programmes. The findings demonstrate that taking into account APPs in a population survey reduces the prevalence of VAD. We present five approaches to adjust for inflammation when evaluating levels of RBP with the notion that this could help estimate VAD prevalence more accurately. The influence of adjusting for APPs on biomarkers of vitamin A status using these five approaches needs to be further investigated, so as to recommend a preferred adjustment approach across populations with high levels of inflammation. As such, we recommend reporting unadjusted findings in addition to any adjusted results.
Source of funding
The 2011 NMS in Liberia was conducted by the Liberia Ministry of Health and Social Welfare (MOHSW), Liberia Institute of Statistics and Geo‐Informational Services (LISGIS), with support from UNICEF, World Bank, World Food Program, World Health Organization and Project Healthy Children.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
LML carried out the data analysis, interpretation and drafted the manuscript. OYA assisted with the data management. PS and RF oversaw the analysis and interpretation of the findings. FS and KF assisted with country context interpretation. RK helped with the design of the survey, interpretation of findings and drafting of the manuscript. All authors read and approved the final manuscript as submitted.
Acknowledgements
Laboratory analyses, equipment and technical assistance was provided by VitMin Lab, Germany. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. R.K., F.S. and K.F. are UNICEF staff members. The opinions and statements in this article are those of the authors and may not reflect official UNICEF policies. We would like to acknowledge Emory University's Nutrition and Health Sciences Program and the Laney Graduate School for L.M.L.'s support in pursuing her PhD.
Larson, L. M. , Addo, O. Y. , Sandalinas, F. , Faigao, K. , Kupka, R. , Flores‐Ayala, R. , and Suchdev, P. S. (2017) Accounting for the influence of inflammation on retinol‐binding protein in a population survey of Liberian preschool‐age children. Maternal & Child Nutrition, 13: e12298. doi: 10.1111/mcn.12298.
References
- Baingana R., Matovu‐Kasozi D. & Garrett D. (2013) The importance of controlling for the acute‐phase response in the population‐based assessment of vitamin A status: a study in children of pre‐school age in Uganda. Public Health Nutrition 16, 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A., Talwar D., McMillan D.C., Stefanowicz F. & O'Reilly D.S. (2012) Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. American Journal of Clinical Nutrition 95, 64–71. [DOI] [PubMed] [Google Scholar]
- Engle‐Stone R., Haskell M.J., Ndjebayi A.O., Nankap M., Erhardt J.G., Gimou M.M. et al. (2011) Plasma retinol‐binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. Journal of Nutrition 141, 2233–2241. [DOI] [PubMed] [Google Scholar]
- Erhardt J.G., Estes J.E., Pfeiffer C.M., Biesalski H.K. & Craft N.E. (2004) Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. Journal of Nutrition 134, 3127–3132. [DOI] [PubMed] [Google Scholar]
- Filteau S.M., Morris S.S., Abbott R.A., Tomkins A.M., Kirkwood B.R., Arthur P. et al. (1993) Influence of morbidity on serum retinol of children in a community‐based study in northern Ghana. American Journal of Clinical Nutrition 58, 192–197. [DOI] [PubMed] [Google Scholar]
- Filteau S.M., Morris S.S., Raynes J.G., Arthur P., Ross D.A., Kirkwood B.R. et al. (1995) Vitamin A supplementation, morbidity, and serum acute‐phase proteins in young Ghanaian children. American Journal of Clinical Nutrition 62, 434–438. [DOI] [PubMed] [Google Scholar]
- Gorstein J.L., Dary O., Pongtorn, Shell‐Duncan B., Quick T. & Wasanwisut E. (2008) Feasibility of using retinol‐binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low‐resource settings. Public Health Nutrition 11, 513–520. [DOI] [PubMed] [Google Scholar]
- Koj A. (1985) Definition and classification of acute‐phase proteins. The Acute‐Phase Response to Injury and Infection, 139–144.
- LISGIS & UNICEF (2011) Liberia National Micronutrient Survey 2011: Summary of Key Findings. UNICEF, LISGIS.
- Louw J.A., Werbeck A., Louw M.E., Kotze T.J., Cooper R. & Labadarios D. (1992) Blood vitamin concentrations during the acute‐phase response. Critical Care Medicine 20, 934–941. [DOI] [PubMed] [Google Scholar]
- Maqsood M., Dancheck B., Gamble M.V., Palafox N.A., Ricks M.O., Briand K. et al. (2004) Vitamin A deficiency and inflammatory markers among preschool children in the Republic of the Marshall Islands. Nutrition Journal 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A.C., West K.P. Jr., Dalmiya N. & Schultink W. (2012) The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutrition 15, 1201–1215. [DOI] [PubMed] [Google Scholar]
- Paracha P.I., Jamil A., Northrop‐Clewes C.A. & Thurnham D.I. (2000) Interpretation of vitamin A status in apparently healthy Pakistani children by using markers of subclinical infection. American Journal of Clinical Nutrition 72, 1164–1169. [DOI] [PubMed] [Google Scholar]
- Raiten D.J., Ashour F.A., Ross A.C., Meydani S.N., Dawson H.D., Stephensen C.B. et al. (2015) Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). Journal of Nutrition 145, 1039S–1108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.A., McCaffery P.J., Drager U.C. & De Luca L.M. (2000) Retinoids in embryonal development. Physiological Reviews 80, 1021–1054. [DOI] [PubMed] [Google Scholar]
- Schulze K.J., Christian P., Wu L.S., Arguello M., Cui H., Nanayakkara‐Bind A. et al. (2014) Micronutrient deficiencies are common in 6‐ to 8‐year‐old children of rural Nepal, with prevalence estimates modestly affected by inflammation. Journal of Nutrition 144, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev P.S., Namaste S., Aaron G., Raiten D.J., Brown K.H. & Flores‐Ayala R. (in press) Overview of the biomarkers reflecting inflammation and determinants of anemia project. Advances in Nutrition. [Google Scholar]
- Szklo M. & Nieto F.J. (2012) Epidemiology: Beyond the Basics. Jones & Bartlett Publishers: Burlington, MA. [Google Scholar]
- Tanumihardjo S.A. (2012) Biomarkers of vitamin A status: what do they mean? In: World Health Organization. Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010. World Health Organization: Geneva. [Google Scholar]
- Taren D. (2012) Historical and practical uses of assessing night blindness as an indicator for vitamin A deficiency. In: World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. World Health Organization: Geneva. [Google Scholar]
- Thurnham D.I. & Singkamani R. (1991) The acute phase response and vitamin A status in malaria. Transactions of the Royal Society of Tropical Medicine & Hygiene 85, 194–199. [DOI] [PubMed] [Google Scholar]
- Thurnham D.I., McCabe G.P., Northrop‐Clewes C.A. & Nestel P. (2003) Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta‐analysis. Lancet 362, 2052–2058. [DOI] [PubMed] [Google Scholar]
- Wessells K.R., Hess S.Y., Ouedraogo Z.P., Rouamba N., Ouedraogo J.B. & Brown K.H. (2014) Asymptomatic malaria infection affects the interpretation of biomarkers of iron and vitamin A status, even after adjusting for systemic inflammation, but does not affect plasma zinc concentrations among young children in Burkina Faso. Journal of Nutrition 144, 2050–2058. [DOI] [PubMed] [Google Scholar]
- WHO (1996) Indicators for Assessing Vitamin A Deficiency and their Application in Monitoring and Evaluation Intervention Programmes. World Health Organization: Geneva: Available at: http://www.who.int/nutrition/publications/micronutrients/vitamin_a_deficiency/WHONUT96.10.pdf. (accessed 10 March 2015) [Google Scholar]
- WHO (2009) Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. World Health Organization: Geneva: Available at: http://apps.who.int/iris/bitstream/10665/44110/1/9789241598019_eng.pdf. (accessed 10 March 2015) [Google Scholar]
- WHO (2011) Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. World Health Organization: Geneva: http://www.who.int/vmnis/indicators/retinol.pdf. (accessed 10 March 2015) [Google Scholar]
- Wieringa F.T., Dijkhuizen M.A., West C.E., Northrop‐Clewes C.A. & Muhilal (2002) Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. Journal of Nutrition 132, 3061–3066. [DOI] [PubMed] [Google Scholar]
- Willumsen J.F., Simmank K., Filteau S.M., Wagstaff L.A. & Tomkins A.M. (1997) Toxic damage to the respiratory epithelium induces acute phase changes in vitamin A metabolism without depleting retinol stores of South African children. Journal of Nutrition 127, 1339–1343. [DOI] [PubMed] [Google Scholar]


