Summary
The insular cortex is injured in obstructive sleep apnea (OSA), and responds inappropriately to autonomic challenges, suggesting neural reorganization. The objective was to assess whether the neural changes result from γ-aminobutyric acid (GABA) and glutamate alterations. We studied 14 OSA patients (mean age±SD: 47.5±10.5 years; 9 male; AHI:29.5±15.6 events/hour) and 22 healthy participants (47.5±10.1 years;11 male), using magnetic resonance spectroscopy to detect GABA and glutamate levels in insular cortices. We localized the cortices with anatomical scans, and measured neurochemical levels from anterior-to-mid regions. Left and right anterior insular cortices showed lower GABA and higher glutamate in OSA vs. healthy subjects (GABA Left:OSA N=6: 0.36±0.10 [mean±std], healthy N=5:0.62±0.18 p<0.05), Right:OSA N=11:0.27±0.09, healthy N=14:0.45±0.16; p<0.05; glutamate Left:OSA N=6:1.61±0.32, healthy N=8:0.94±0.34; p<0.05, Right:OSA N=14:1.26±0.28, healthy N=19:1.02±0.28; p<0.05). GABA and glutamate levels were correlated only within the healthy group in the left insula (r=−0.9, p<0.05). The altered anterior insular levels of GABA and glutamate may modify integration and projections to autonomic areas, contributing to the impaired cardiovascular regulation in OSA.
Introduction
The insular cortex in obstructive sleep apnea (OSA) shows structural and functional alterations, which likely contribute to adverse autonomic and affective symptoms characteristic of the syndrome (Macey et al., 2008, Harper et al., 2003, Kumar et al., 2012). The altered functions include distorted insular functional magnetic resonance (fMRI) signals accompanying sympathetic nervous system challenges, and likely underlie the high sympathetic outflow in OSA, presumably contributing to the profuse sweating, cardiac arrhythmia, and hypertension in the disorder. The insular injury in OSA also likely facilitates development of depression and anxiety in the condition (Marin et al., 2012, Asghari et al., 2012, Kurth et al., 2010). Mechanisms underlying insular dysfunction in OSA are unclear, but understanding the nature of the brain alterations could provide insights into symptoms that persist even with treatment (Marin et al., 2012), as well as processes underlying partial or complete resolution with continuous positive airway pressure (CPAP) of other symptoms, such as high sympathetic tone (Fatouleh et al., 2015).
Structural brain alterations in OSA could arise from multiple pathologies. Reduced mean diffusivity, apparent in many OSA brain regions (Kumar et al., 2012), reflects an increase in barriers to inter-cellular water movement, which could arise from cell swelling due to inflammation, or increased cell size due to glial activation. Functional MRI (fMRI) differences appear in the insular cortex during autonomic challenges (Harper et al., 2003). The processes underlining those differences could be elucidated by determining the levels of the neurotransmitters glutamate and γ-aminobutyric acid (GABA), which can now be measured non-invasively. Levels of these chemicals are sensitive to neural reorganization in response to injury, and can reflect different functional states (Arckens et al., 2000). High levels of glutamate, in particular, are associated with excitotoxicity, a likely mechanism of injury in some brain areas in OSA.
The objective was to assess GABA and glutamate in the anterior insular cortex in OSA patients, relative to healthy subjects. The anterior-mid insula was selected, since that subregion preferentially serves autonomic roles (Macey et al., 2012). We hypothesized that, relative to healthy people, OSA patients would show elevated glutamate, based on the potential for hypoxia-induced excitotoxic conditions, and altered GABA levels, based on impaired insular action.
Methods
We studied 14 OSA patients (mean age=47.5±10.5 years; 9 male; mean AHI:29.5±15.6 events/hour; SAO2 min:83±9%) and 22 healthy subjects (mean age=47.5±10.1 years; 11 male). Patients were diagnosed at the UCLA Sleep Disorders Center. No participants had a history of head trauma or disease, current mental illness, use of psychoactive medications, cancer, or cardiac disease. Patients were untreated, apart from two who had a prior history of CPAP use. Healthy subjects were screened to exclude sleep disorders. Procedures were approved by the UCLA Institutional Review Board, and participants provided written, informed consent.
Neurotransmitters were measured with a magnetic resonance spectroscopy (MRS) method based on “two-dimensional” MRS (Sarma et al., 2014; see supporting information). Neurochemicals changes were measured using compressed sensing (CS)-based 4D echo-planar J-resolved spectroscopic imaging (EP-JRESI). High resolution T1-weighted scans were acquired for localization of insular cortex. Group differences in GABA and glutamate concentrations (ratios with respect to creatine) in the anterior-to-mid insular cortex and adjacent tissue were assessed with t-tests. The relative concentrations of “Glx,” a combination of glutamate and glutamine indicative of glutamate levels, were also assessed, since Glx is a more robust MRS measure than glutamate.
Results
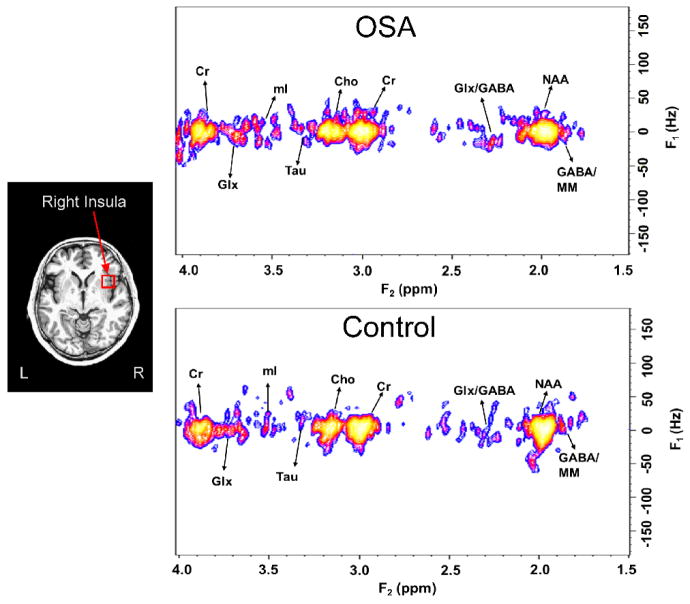
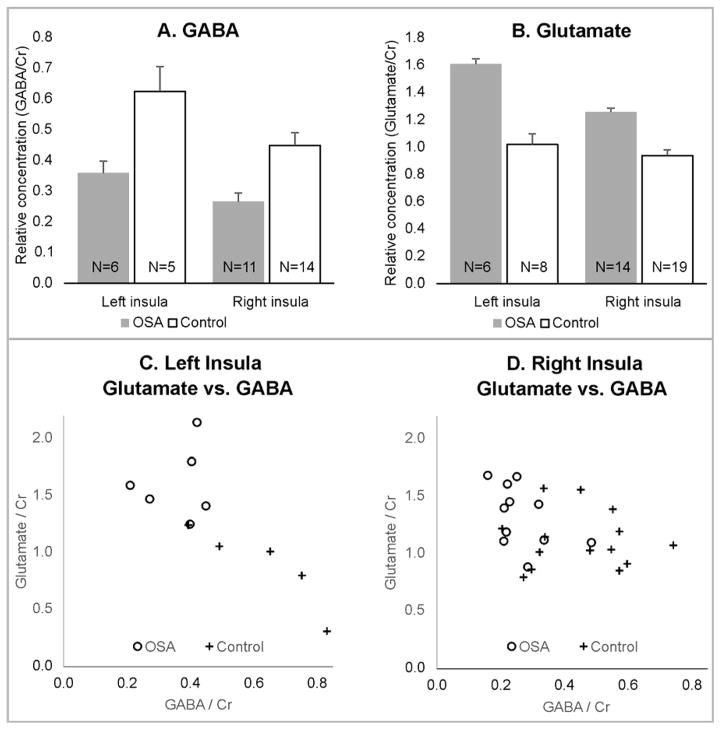
Neurotransmitter concentrations were successfully recorded from subsets of the 22 healthy and 14 OSA subjects (Table 1; example spectra Fig. 1). Differences between OSA and healthy subjects emerged for GABA (Fig. 2A) and glutamate (Fig. 2B) in right and left insulae. The left insula showed poorer data quality in more subjects than the right (right successful measures: 69% GABA, 92% glutamate; left successful measures: 31% GABA, 39% glutamate). Success rates were similar for OSA and healthy groups. When the two OSA patients with a prior history of CPAP were excluded, the mean differences were similar, and the significant differences between OSA and healthy subjects remained, with the exception of glutamate in the right insula. However, Glx showed significantly higher levels in OSA versus healthy subjects in the right and left insulae, with and without excluding the two previously-treated subjects (Table 1). Correlations between GABA and glutamate were only significant in healthy subjects in the left insula (r = −0.90, P=0.04; Table 2; Fig. 2 C, D).
Table 1.
Levels of GABA and glutamate in subsets of 14 OSA (12 never treated) and 22 healthy subjects (p: t-test for group differences); Glx = glutamate + glutamine.
| Left insula | Right insula | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Healthy | p | OSA | Healthy | p | |||||
| N | mean ± std | N | mean ± std | N | mean ± std | N | mean ± std | |||
| GABA | 6 | 0.36±0.10 | 5 | 0.62±0.18 | 0.03 | 11 | 0.27±0.09 | 14 | 0.45±0.16 | <0.01 |
| Glutamate | 6 | 1.61±0.32 | 8 | 0.94±0.34 | <0.01 | 14 | 1.26±0.28 | 19 | 1.02±0.28 | 0.02 |
| Glx | 6 | 1.92±0.38 | 6 | 1.22±0.44 | 0.01 | 14 | 1.75±0.42 | 19 | 1.34±0.42 | 0.01 |
| Never treated OSA | Never treated OSA | |||||||||
| GABA | 5 | 0.35±0.10 | (as above) | 0.03 | 9 | 0.27±0.10 | (as above) | <0.01 | ||
| Glutamate | 5 | 1.50±0.21 | (as above) | <0.01 | 12 | 1.20±0.25 | (as above) | 0.08 | ||
| Glx | 5 | 1.79±0.18 | (as above) | 0.01 | 12 | 1.69±0.43 | (as above) | 0.04 | ||
Figure 1.
Left: Example insula voxel is shown in red (15 mm3) overlaid on average anatomical scan (mean over N=36). Right: Example 2D-MRS spectra from insula in OSA (56 year female) and control (53 year male) subjects, with resonances of various neurochemicals indicated (Cho=choline, Cr=creatine, mI=myo-inisotol, MM=other macromolecules, NAA= N-acetylaspartate, Tau=taurine).
Figure 2.
Insular (A) GABA and (B) glutamate levels in subsets of 14 OSA and 22 healthy subjects (mean±SEM). Scatterplots of glutamate vs. GABA for subjects with both measures in the left (C) and right (D) insular cortices.
Table 2.
Correlation table in subjects with GABA and glutamate measures (p: correlation between GABA and glutamate).
| Left insula | Right insula | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Healthy | OSA | Healthy | |||||||||
| N | r | p | N | p | N | r | p | N | r | p | ||
| glutamate vs. GABA | 6 | 0.16 | 0.76 | 5 | −0.90 | 0.04 | 11 | −0.47 | 0.14 | 14 | −0.04 | 0.90 |
Discussion
The anterior insular cortex in OSA shows low GABA and high glutamate, conditions which would modify neural patterns within the structure. GABA normally acts as an inhibitory neurotransmitter, while glutamate normally is excitatory to neural processes. The influences of the insular cortices are complex, with both inhibitory and excitatory influences on hypothalamic, cingulate, and frontal cortical structures, among others. On a simplistic level, the combination of a reduction in the inhibitory GABA and increase in the excitatory glutamate could reflect a more active state overall; however, the complexity of the insular projections to multiple structures precludes such an exclusive generalization (Kurth et al., 2010). For excitatory projections, enhanced glutamate could enhance insular influences, and the normal inhibition over hypothalamic structures would be reduced by the lower GABA levels, potentially leading to the exaggerated sympathetic tone in the condition. The altered neurotransmitter levels could also modify cingulate and hippocampal projections influencing mood, such as depression and anxiety, both of which are common in OSA. Low GABA in the insula has been associated with high levels of anxiety (Rosso et al., 2014), and levels appear to be associated with processing of interoceptive stimuli, a function linked with depressive mechanisms (Wiebking et al., 2014). The structures involved in anxiety and depression include the anterior cingulate, recipient of fibers from the cingulum and insular cortices. High glutamate has been found in these limbic areas in bipolar disorder (Soeiro-de-Souza et al., 2015). The hypoxia exposure during apneic events likely leads to excessive excitation of the insular connecting fibers, and such excitotoxic processes are facilitated by glutamate. It is unknown whether the altered neurotransmitter levels revert to normal with CPAP treatment, but such knowledge would assist selection of interventions, and determination of whether regional GABA and glutamate levels could be useful biomarkers of local neural tissue state in OSA.
The findings of reduced GABA and higher glutamate build on earlier demonstration of alterations in the anterior insular cortex in OSA, including changes in structure, function, and metabolite levels. The insula shows impaired responses to autonomic challenges in OSA, especially during periods of sympathetic activity increases (Harper et al., 2003). Resting state functional patterns also differ from heathy subjects when those patterns are correlated with other regions (Zhang et al., 2015). Changes in water diffusivity reflect structural changes likely arising from glial activation or inflammation due to hypoxia, and damage to neurons or axons and myelin (Kumar et al., 2012, Macey et al., 2008). Increased myo-inositol in the left insula of OSA patients is also consistent with glial alterations (Yadav et al., 2014). Increased glutamate levels may arise directly from astrocyte activation, since astrocytic glutamate is an energy source for neurons (Pellerin and Magistretti, 1994); if metabolism is altered by OSA in the insular cortex, glutamate from astrocytes may increase to compensate. The combined chemical, functional and structural findings confirm injury and reorganization of the insula in OSA, which likely contribute to clinical symptoms in the condition (Marin et al., 2012, Asghari et al., 2012, Kurth et al., 2010, Macey et al., 2013).
The high glutamate may reflect damaging excitotoxic processes arising from intermittent hypoxia (Jagadapillai et al., 2014). Only 5 hours of intermittent hypoxia simulating OSA effects can lead to cellular death (Pae et al., 2005); thus, similar processes are likely operating in people with OSA. However, the high glutamate measured here was in whole tissue, and does not necessarily correspond to concentrations in extracellular fluid, where the neurochemical is key for excitotoxicity.
Interventions complimentary to CPAP that influence neurotransmitter levels may help normalize brain function in OSA. Both GABA and glutamate can be manipulated directly with pharmacological approaches, and indirectly with behavioral changes. The measurements presented here could serve as a biomarker of any intervention targeting these brain chemicals.
Measurements were successful in 58% of instances, with the remainder not meeting quality criteria, especially in the left insula. Since OSA and healthy measurements were similarly affected, the low data quality is likely due to technical, rather than biological reasons (e.g., poor shimming). The resolution of the MRS voxels was limited (example in Fig. 1), so the measures did not cover the entire anterior-to-mid portion of the structure, and included adjacent tissue; the sub-region studied will vary slightly between subjects.
In conclusion, the anterior-to-mid insular cortex shows lower GABA and higher glutamate levels in OSA, compared with healthy subjects. These alterations likely reflect a functional neural reorganization that contributes to the autonomic and psychological symptoms in OSA, such as high sympathetic tone and refractory hypertension, and anxiety, depression and cognitive difficulties. High glutamate would facilitate excitotoxic processes. The altered neurotransmitter levels provide a biomarker of regional functional status of the OSA brain, and will allow insights into the neurological effects of treatment with CPAP or other interventions.
Supplementary Material
Acknowledgments
Support: NIH-NR-013693.
Footnotes
Contributions
- Study design
- Data collection
- Analysis
- Interpretation and Manuscript preparation
Paul Macey: 1, 2, 3, 4
Manoj Sarma: 2, 3, 4
Rajakumar Nagarajan: 2, 3, 4
Ravi Aysola: 2, 4
Jerome M. Siegel: 4
Ronald M. Harper: 4
M. Albert Thomas: 1 2, 3, 4
References
- Arckens L, Schweigart G, Qu Y, et al. Cooperative changes in GABA, glutamate and activity levels: the missing link in cortical plasticity. Eur J Neurosci. 2000;12:4222–32. doi: 10.1046/j.0953-816x.2000.01328.x. [DOI] [PubMed] [Google Scholar]
- Asghari A, Mohammadi F, Kamrava SK, Tavakoli S, Farhadi M. Severity of depression and anxiety in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2012;269:2549–53. doi: 10.1007/s00405-012-1942-6. [DOI] [PubMed] [Google Scholar]
- Fatouleh RH, Lundblad LC, Macey PM, Mckenzie DK, Henderson LA, Macefield VG. Reversal of functional changes in the brain associated with obstructive sleep apnoea following 6 months of CPAP. NeuroImage Clinical. 2015;7:799–806. doi: 10.1016/j.nicl.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Henderson LA, et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–95. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- Jagadapillai R, Mellen NM, Sachleben LR, Jr, Gozal E. Ceftriaxone preserves glutamate transporters and prevents intermittent hypoxia-induced vulnerability to brain excitotoxic injury. PLoS One. 2014;9:e100230. doi: 10.1371/journal.pone.0100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Yan-Go FL, Harper RM. Heart rate responses to autonomic challenges in obstructive sleep apnea. PLoS One. 2013;8:e76631. doi: 10.1371/journal.pone.0076631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Wu P, Kumar R, et al. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci. 2012;168:72–81. doi: 10.1016/j.autneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–8. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Weiner MR, Crowley DJ, Silveri MM, Rauch SL, Jensen JE. Insula and anterior cingulate GABA levels in posttraumatic stress disorder: preliminary findings using magnetic resonance spectroscopy. Depress Anxiety. 2014;31:115–23. doi: 10.1002/da.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Macey PM, et al. Accelerated echo-planar j-resolved spectroscopic imaging in the human brain using compressed sensing: a pilot validation in obstructive sleep apnea. AJNR Am J Neuroradiol. 2014;35:S81–9. doi: 10.3174/ajnr.A3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-De-Souza MG, Henning A, Machado-Vieira R, et al. Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol. 2015;25:2221–9. doi: 10.1016/j.euroneuro.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, et al. GABA in the insula - a predictor of the neural response to interoceptive awareness. NeuroImage. 2014;86:10–8. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Kumar R, Macey PM, Woo MA, Yan-Go FL, Harper RM. Insular cortex metabolite changes in obstructive sleep apnea. Sleep. 2014;37:951–8. doi: 10.5665/sleep.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Qin W, He X, et al. Functional disconnection of the right anterior insula in obstructive sleep apnea. Sleep Med. 2015;16:1062–70. doi: 10.1016/j.sleep.2015.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.