Abstract
Background
Guidelines for treatment of primary hyperparathyroidism (PHPT) in young patients recommend surgery. Outcomes of minimally invasive parathyroidectomy (MIP) are well established in adults, but not in pediatric patients.
Objective
Determine effectiveness of pre-operative imaging and MIP aided by intraoperative PTH (ioPTH) measurement in children with primary hyperparathyroidism (PHPT).
Methods and Design
Retrospective chart review of diagnostic and follow-up data up to 12 months post-MIP of patients with PHPT who underwent parathyroidectomy at the Children’s Hospital of Philadelphia between Jan 1, 2009 and March 31, 2015.
Results
Data were available for 16 of 17 patients age 8 to 17 years (11 female, 6 male): 2 had ectopic intrathymic adenomas while 14 had eutopic adenomas Fifteen patients had ioPTH, including 14 who underwent MIP, defined as a 2 cm central neck incision. All patients with data at 6 months post parathyroidectomy (13/16) showed normal PTH and calcium. Ultrasound and Sestamibi scans had a combined sensitivity of 87.5%.
Conclusions
MIP is an appropriate alternative to standard neck exploration in pediatric patients with PHPT with a single parathyroid adenoma. IoPTH is especially useful to confirm cure and limit surgical exploration when imaging studies are negative. Sestamibi scans and ultrasound are complementary studies.
Keywords: hyperparathyroidism, minimally invasive parathyroidectomy, hypercalcemia, intra operative PTH
Introduction
Primary hyperparathyroidism (PHPT) is far less common in pediatric patients than in adults, with an estimated incidence of 1 per 200-300,000 and prevalence of 2-5 in 100,000 [1, 2]. The etiology and pathology of PHPT is very different in neonates and older children. Most neonatal cases are due to inactivating mutations of the calcium sensing receptor causing severe neonatal hyperparathyroidism, while in children and adolescents PHPT is principally (80-92%) due to a single parathyroid adenoma, and less commonly due to multiglandular disease (MGD) [3-22]. MGD may be due to the Multiple Endocrine Neoplasia syndromes MEN 1 and MEN 2A or familial isolated hyperparathyroidism syndromes. Guidelines for treatment of PHPT in young patients (i.e. age less than 50 years) is surgical parathyroidectomy but specific guidelines for pediatric patients are lacking[23]. The traditional surgical approach for PHPT has been bilateral neck exploration, with a success rate of 95% to 98 % in adults[24]. However, the improved sensitivity of imaging techniques, mainly Tc 99 sestamibi scan/SPECT CT and ultrasound (US), and evidence that the majority of cases are due to a single adenoma, have encouraged use of less invasive surgical procedures in adults. Unilateral neck exploration and minimally invasive parathyroidectomy (MIP) have shown favorable outcomes in adults, but there are a limited number of reports regarding the outcome of MIP in pediatric patients with PHPT [1, 5, 8, 10, 24]. Importantly, despite the growing popularity of MIP, there is no consensus on what constitutes a MIP procedure. Herein we use the common definition of MIP as a surgical procedure in which a 2 centimeter central transverse neck incision is employed, irrespective of the number of glands explored [25].
MIP has been supported by the use of intraoperative PTH (ioPTH) measurements, which has facilitated less invasive surgery with shorter procedure times[26, 27]. The short plasma half-life of intact PTH, and suppression of the uninvolved normal parathyroid glands by hypercalcemia induced by the adenoma, lead to a rapid decrease in circulating levels of intact PTH soon after excision of a solitary parathyroid adenoma. To our knowledge, only three prior reports have reported an extensive use of ioPTH during parathyroidectomy in children[1, 5, 10]. Our aim was to determine the short term and long term outcomes of MIP and conventional parathyroidectomy aided by ioPTH measurement in pediatric patients with PHPT at The Children’s Hospital of Philadelphia (CHOP).
Subjects and Methods
We conducted a retrospective chart review including all patients with a diagnosis of PHPT who underwent parathyroidectomy at CHOP between Jan 1, 2009 and March 31, 2015. We reviewed all the available diagnostic and follow-up data. Surgical cure was defined by normocalcemia at 2 to 12 months post -surgery. In addition, we assessed the diagnostic accuracy of imaging methods in our series. Lastly, we reviewed the clinical presentation in our patients. The study was approved by the local Institutional Review Board at CHOP.
Results
Seventeen patients age 8 to 17 years (median age 14y, 11 female, 6 male) underwent parathyroidectomy at CHOP between January 1, 2009 and March 31, 2015 (Table 1). No patient had a family history or genetic diagnosis of MEN1 or MEN2a. Follow up data were not available for one international patient, who could not be reached, and hence was not included this series. Preoperative levels of serum calcium (12.1 ± 0.9, mean ± SD) and intact PTH (177.3 pg/mL ± 121.8, 18.7± 12.8 pmol/L) were elevated in all patients. Serum levels of phosphate were mildly reduced in most patients (3.4 ± 0.7 mg/dL). The Ca/creat ratio in random urine samples was mildly elevated in 12 of 15 patients (mean 0.27 mg/mg ±0.11). The FeCa was also elevated on random urine samples in 9 patients with a mean of 1.5% (0.75-3.2), being below 1% in 2 patients, one of whom had normal sequencing of the CASR gene encoding the calcium sensing receptor. Most patients (75%) were symptomatic, and commonly presented with gastrointestinal symptoms (41%) often in combination with fatigue/depression (29%), renal calculi (12%) or headaches, while 25% were asymptomatic. One patient had a family history of PHPT with no other features of MEN.
Table 1. Patient characteristics and surgical outcomes.
| Patient # |
Age at diagnosis (yr)/ gender |
Symptoms | Pre-op Ca (mg/dL) |
Pre-op PTH (pg/mL) |
Imaging study |
Procedure | Adenoma location /weight (mg) |
|
|---|---|---|---|---|---|---|---|---|
| US | MIBI | |||||||
| 1 | 11/F | Anxiety, depression, HA, fracture |
12.1 | 203 | + | + | MIP | LI/143 |
| 2 | 13/F | Fatigue, abdominal pain |
11.1 | 106 | − | − | MIP | R intrathymic /130 |
| 3 | 8/M | Gastroenteritis Dehydration |
12 | 69 | + | − | MIP | RS/151 |
| 4 | 15/M | Asymptomatic | 12.3 | 159 | + | + | MIP | RS/236 |
| 5 | 10/F | Asymptomatic | 12.3 | 283 | + | + | MIP | RI/755 |
| 6 | 15/M | Abdominal pain, fatigue |
10.7 | 133 | − | − | MIP | LS/270 |
| 7 | 13/F | Renal calculus, abdominal pain |
11.6 | 105 | − | + | Cervical approach to mediastinum |
Intrathymic mediastinum /520 |
| 8 | 12/F | Renal calculus | 10.5 | 82 | + | − | MIP | RS/130 |
| 9 | 17/F | Fatigue, depression Abdominal pain |
12.4 | 237 | + | + | MIP | LI/379 |
| 10 | 13/M | Asymptomatic | 12.2 | 170 | + | + | MIP | RI/147 |
| 11 | 13/M | Nausea, vomiting abdominal pain |
13.5 | 168 | + | + | MIP | RS/600 |
| 12 | 16/F | Fatigue, anxiety difficulty with concentration |
11.7 | 174 | − | + | Conventional PTX |
LI redo/380 |
| 13 | 17/F | Abdominal pain, HA, mood instability |
13.3 | 582 | ND | + | MIP | LI/2440 |
| 14 | 17/F | Headache | 13 | 119 | − | + | MIP | LI/1420 |
| 15 | 15/M | Asymptomatic | 11.6 | 133 | + | + | MIP | RS/ ND |
| 16 | 17/F | Abdominal pain, vomiting, HA |
12.8 | 113 | + | − | MIP | LS/480 |
US ultrasound, SS/SPECT CT Sestamibi scan with SPECT/CT, RI right inferior, LI left inferior, RS right superior, RI right inferior, ND not done HA headache
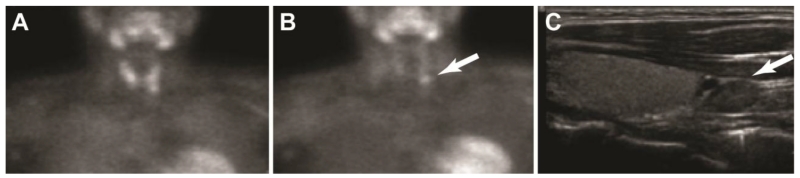
Fourteen patients had the postoperative diagnosis of a solitary parathyroid adenoma in the neck while 2 patients were found to have a single ectopic inthrathymic parathyroid adenoma. Sestamibi scan supplemented with SPECT/CT identified a single adenoma in 11/16 patients (69%), including an intrathymic adenoma in 1 case (Table 2). The negative studies were in 4 patients with eutopic adenomas that were smaller (130-480 mg) and 1 patient with an intrathymic adenoma. Preoperative neck ultrasound (US) was performed in 15 patients, and was positive in 9 (60%); both patients with intrathymic adenomas had negative US. Overall, US or sestamibi scan localized an adenoma in 87.5 %, and co-localized an adenoma in 44%. Representative images on US and Tc-99m Sestamibi scan with SPECT/CT are shown in Figure 1.
Table 2.
Sensitivity of imaging studies
| Adenoma location | Ultrasound | Tc-99m Sestamibi scan with SPECT/CT |
||||
|---|---|---|---|---|---|---|
| Positive | Negative | Sensitivity | Positive | Negative | Sensitivity | |
| Parathyroid | 9 | 4 | 69% | 10 | 4 | 71% |
| Intrathymic | 0 | 2 | 0 | 1 | 1 | 50% |
| Total | 9 | 6 | 60% | 11 | 5 | 69% |
Fig 1.
Images of parathyroid adenoma on US and Tc-99 Sestamibi Scan with SPECT A. Anterior initial images post Tc-99m sestamibi B. Anterior delay 2hs post Tc-99m sestamibi C. Thyroid US showing a 1.2 cm hypoechoic nodule inferior to the left thyroid lobe
Fourteen of the 16 patients underwent MIP. A standard parathyroidectomy was performed in one patient who was a reoperative case following a likely ruptured adenoma at initial resection 3 years earlier at another institution. The second case operated by a standard cervical approach corresponded to an intrathymic adenoma localized to the anterior mediastinum. The other intrathymic adenoma was localized to the upper thymus and resected by MIP. No side effects were described except for mild, transient hypocalcemia on the first post-operative day in 75% of cases, which resolved with calcium and cacitriol supplementation. There were no surgical complications of recurrent laryngeal nerve injury, bleeding, or infection. Table 1 summarizes the preoperative and postoperative data on all the patients. The median weight of the adenomas was 380 mg (130-2440 mg).
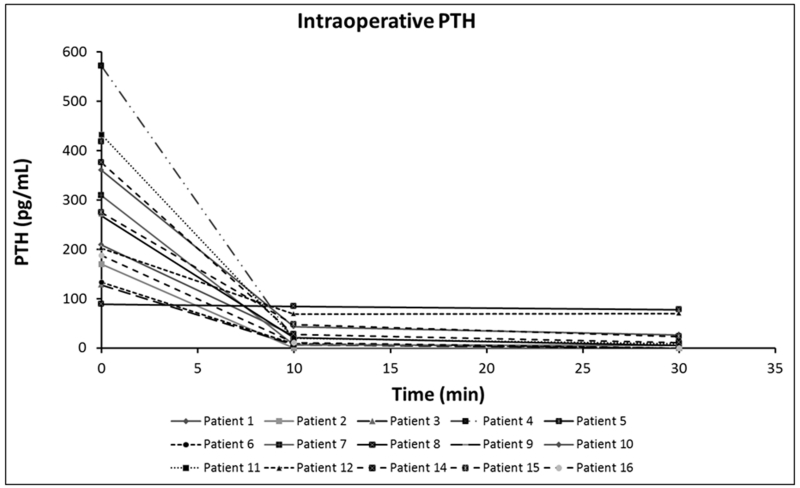
IoPTH was measured in 15 of 16 patients, and 14/15 patients showed > 50% decrease in PTH levels 10 minutes post resection of a single parathyroid adenoma, suggesting cure (Figure 2). Most patients had another level measured 30 min post resection and it remained low in all except the one patient that did not show an initial decrease. IoPTH facilitated the use of MIP in 2 patients who had negative pre-operative imaging studies, one was an intrathymic adenoma localized to the right upper thymus, and the second was one of the smaller adenomas (270 mg) in the neck. The patient that showed no decrease in ioPTH was found to have a solitary adenoma that corresponded to the preoperative US findings. A bilateral neck exploration through a minimal incision was performed in her case and no additional adenomas were identified.
Fig.2.
IoPTH was performed in 15 of 16 patients, 14/15 patients showed > 50% decrease in PTH levels 10-30 min post resection of a PA. PTH decreased from 170 to <5 pg/mL after resection in one patient with an intrathymic adenoma that had not been localized by pre-op imaging.
We monitored biochemical data for six months or longer after parathyroidectomy for 13 of the 16 patients; we were able to obtain post-operative biochemical data at five months in one patient and at two months for an additional two patients, all of whom were subsequently lost to follow up. Serum levels of calcium and phosphate were normal in all patients (range for Ca 8.8-10.4). PTH levels were available for 10 patients at 6 to 12 months post-parathyroidectomy and were similarly normal. The higher calcium level was observed in the reoperative case of likely parathyroidomatosis that will require extended follow-up to assure permanent cure. The calcium/creatinine ratio in a random urine was measured in 11 patients post-operatively and has remained normal in all. The single patient who had no decrease in ioPTH remained normocalcemic with a normal PTH level 28 months post-surgery.
Discussion
We reviewed the surgical outcome in sixteen patients who underwent parathyroidectomy for PHPT at CHOP from 2009 to 2015. Fourteen of 16 patients had solitary parathyroid adenomas that were localized in the neck and 2 were intrathymic. Fourteen of the 16 patients were cured by MIP and 2 patients required more extensive surgery. IoPTH was used in 15 of 16 cases and predicted cure in 14 of them, with one false negative result.
Our series differs from other pediatric series in that all of our patients had a solitary adenoma. This may be explained by the lack of infants with Neonatal Severe Hyperparathyroidism (NSHPT) and only one possible familial case who was found to have a solitary adenoma. The bias of our cohort is related to improved options for medical treatment for NSHPT [28], and earlier recognition of genetic syndromes in which PHPT is due to multiglandular disease, with deferred surgical management of these patients. During this time period one patient with MGD was evaluated by our service but due to age was referred to the adult endocrinology service. The presentation in our cases is similar to that described in the literature, with predominance in adolescents, 50% of our patients were 15 to 17 years old and 43% 10 to 13 years old at the time of surgery. Unlike previous pediatric reviews, we found a higher incidence in females, similar to the experience in adult PHPT. The proportion of asymptomatic patients was higher in our series than that described in 2 recent pediatric reviews (24% vs. 15%) but it was lower than that reported in adults [3, 4]. This is consistent with recent reviews that indicate that PHPT at a younger age is usually more symptomatic at diagnosis [5, 29].
In our experience US or sestamibi scan localized an adenoma in 87.5 %, with an individual sensitivity for each test of 60 and 69% respectively. These results are less favorable than the typical experience described in the pediatric and adult literature; US was found to have an accuracy of 79% in a recent review of all studies to date in children, and for sestamibi scans the accuracy was 86% in the same review, while the largest review of hyperparathyroidism in adults found a sensitivity of 88% for sestamibi scan and 75.5% for US[3, 24]. This difference may in part be explained by the smaller size of some adenomas and a high proportion of ectopic adenomas (12%) in our series. Ectopic adenomas, mediastinal, intrathyroidal and inthrathymic, accounted for 9.5% in one of the larger pediatric series [15].
The improved sensitivity of imaging techniques and evidence that the majority of cases of PHPT are due to a single adenoma, have led to less invasive surgical procedures. In adults, prospective and retrospective studies have shown no significant difference in cure rates or complications between standard parathyroidectomy, unilateral neck exploration and MIP [24, 30, 31]. However, there are only a limited number of reports describing the outcome of less invasive surgical procedures in children. A radioguided minimal approach proved to be curative in 19 patients in a recent series [8]. Durkin showed an 89% cure rate (8/9) using MIP and ioPTH in children <19 years of age without a family history of PHPT. Algaratnam showed 100% cure rate using MIP in 9 children, with MIP defined as a small lateral neck incision to remove a single parathyroid gland without visualization of other glands [1, 5, 8, 10]. To our knowledge, our series is the largest pediatric series reported to date using MIP guided by preoperative imaging and ioPTH.
IoPTH predicted postoperative normocalcemia in 93% of our cases with one false negative result. We used the Miami criteria whereby ioPTH measured 10 min post complete resection drops ≥ 50% of the highest pre incision or pre excision PTH level, predicting postoperative levels in 96% of cases [32]. These rapid PTH assays have been used more routinely in adults; Nussbaum first described the use of ioPTH using sensitive immunoradiometric assay (IRMA) and demonstrated that a decrease of PTH levels to less than 40% of baseline values 15 minutes after parathyroidectomy confirmed cure[26]. Mandell et al showed that this measurement led to successful surgery in 96 % of patients in whom sestamibi scans were not conclusive in localizing adenomas[27]. They showed that a decrease in PTH level of 46% or more at 10 min and 59% or more at 20 min after excision of the adenoma predicted cure. Two of our cases had negative imaging studies but were cured using a small central neck incision, with resection of a visualized adenoma and confirmation of cure with ioPTH, eliminating the need for a more extensive neck exploration. Thus, our data confirm the role of ioPTH in limiting the extent of surgery in pediatric cases of primary hyperparathyroidism with negative imaging studies. To our knowledge, this is the largest pediatric series reported to date using ioPTH, confirming the high success rate in minimal procedures aided by this assay [1, 5, 10].
We conclude that MIP, defined as a 2 cm transverse central neck incision, is an appropriate alternative operation to standard neck exploration for primary hyperparathyroidism in children. Preoperative sestamibi scans and US are complementary studies. IoPTH predicts post -operative normocalcemia and is especially useful to confirm cure and limit surgical exploration when imaging studies are negative.
Acknowledgements
We thank Norma Rendon and Kelsey Berger for valuable assistance with data collection.
Funding sources: Sadikoglu Family Foundation and CHOP Research Institute
Footnotes
Disclosure statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mallet E, Working Group on Calcium M Primary hyperparathyroidism in neonates and childhood. The French experience (1984-2004) Hormone research. 2008;69:180–188. doi: 10.1159/000112592. [DOI] [PubMed] [Google Scholar]
- 2.Lawson ML, Miller SF, Ellis G, et al. Primary hyperparathyroidism in a paediatric hospital. QJM: monthly journal of the Association of Physicians. 1996;89:921–932. doi: 10.1093/qjmed/89.12.921. [DOI] [PubMed] [Google Scholar]
- 3.Belcher R, Metrailer AM, Bodenner DL, et al. Characterization of hyperparathyroidism in youth and adolescents: a literature review. International journal of pediatric otorhinolaryngology. 2013;77:318–322. doi: 10.1016/j.ijporl.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Roizen J, Levine MA. Primary hyperparathyroidism in children and adolescents. Journal of the Chinese Medical Association: JCMA. 2012;75:425–434. doi: 10.1016/j.jcma.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alagaratnam S, Brain C, Spoudeas H, et al. Surgical treatment of children with hyperparathyroidism: single centre experience. Journal of pediatric surgery. 2014;49:1539–1543. doi: 10.1016/j.jpedsurg.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Allo M, Thompson NW, Harness JK, et al. Primary hyperparathyroidism in children, adolescents, and young adults. World journal of surgery. 1982;6:771–776. doi: 10.1007/BF01655371. [DOI] [PubMed] [Google Scholar]
- 7.Bhadada SK, Bhansali A, Dutta P, et al. Characteristics of primary hyperparathyroidism in adolescents. Journal of pediatric endocrinology & metabolism: JPEM. 2008;21:1147–1153. doi: 10.1515/jpem.2008.21.12.1147. [DOI] [PubMed] [Google Scholar]
- 8.Burke JF, Jacobson K, Gosain A, et al. Radioguided parathyroidectomy effective in pediatric patients. The Journal of surgical research. 2013;184:312–317. doi: 10.1016/j.jss.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronin CS, Reeve TS, Robinson B, et al. Primary hyperparathyroidism in childhood and adolescence. Journal of paediatrics and child health. 1996;32:397–399. doi: 10.1111/j.1440-1754.1996.tb00937.x. [DOI] [PubMed] [Google Scholar]
- 10.Durkin ET, Nichol PF, Lund DP, et al. What is the optimal treatment for children with primary hyperparathyroidism? Journal of pediatric surgery. 2010;45:1142–1146. doi: 10.1016/j.jpedsurg.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 11.George J, Acharya SV, Bandgar TR, et al. Primary hyperparathyroidism in children and adolescents. Indian journal of pediatrics. 2010;77:175–178. doi: 10.1007/s12098-009-0289-5. [DOI] [PubMed] [Google Scholar]
- 12.Girard RM, Belanger A, Hazel B. Primary hyperparathyroidism in children. Canadian journal of surgery Journal canadien de chirurgie. 1982;25:11–13. 32. [PubMed] [Google Scholar]
- 13.Harman CR, van Heerden JA, Farley DR, et al. Sporadic primary hyperparathyroidism in young patients: a separate disease entity? Archives of surgery. 1999;134:651–655. doi: 10.1001/archsurg.134.6.651. discussion 655-656. [DOI] [PubMed] [Google Scholar]
- 14.Hsu SC, Levine MA. Primary hyperparathyroidism in children and adolescents: the Johns Hopkins Children’s Center experience 1984-2001. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17(Suppl 2):N44–50. [PubMed] [Google Scholar]
- 15.Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115:974–980. doi: 10.1542/peds.2004-0804. [DOI] [PubMed] [Google Scholar]
- 16.Li CC, Yang C, Wang S, et al. A 10-year retrospective study of primary hyperparathyroidism in children. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2012;120:229–233. doi: 10.1055/s-0032-1301895. [DOI] [PubMed] [Google Scholar]
- 17.Libansky P, Astl J, Adamek S, et al. Surgical treatment of primary hyperparathyroidism in children: report of 10 cases. International journal of pediatric otorhinolaryngology. 2008;72:1177–1182. doi: 10.1016/j.ijporl.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Loh KC, Duh QY, Shoback D, et al. Clinical profile of primary hyperparathyroidism in adolescents and young adults. Clinical endocrinology. 1998;48:435–443. doi: 10.1046/j.1365-2265.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 19.Pashtan I, Grogan RH, Kaplan SP, et al. Primary hyperparathyroidism in adolescents: the same but different. Pediatric surgery international. 2013;29:275–279. doi: 10.1007/s00383-012-3222-3. [DOI] [PubMed] [Google Scholar]
- 20.Paunovic I, Zivaljevic V, Stojanic R, et al. Primary hyperparathyroidism in children and young adults:--a single institution experience. Acta chirurgica Belgica. 2013;113:35–39. doi: 10.1080/00015458.2013.11680882. [DOI] [PubMed] [Google Scholar]
- 21.Rapaport D, Ziv Y, Rubin M, et al. Primary hyperparathyroidism in children. Journal of pediatric surgery. 1986;21:395–397. doi: 10.1016/s0022-3468(86)80505-x. [DOI] [PubMed] [Google Scholar]
- 22.Shah VN, Bhadada SK, Bhansali A, et al. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. Journal of postgraduate medicine. 2012;58:107–111. doi: 10.4103/0022-3859.97171. [DOI] [PubMed] [Google Scholar]
- 23.Udelsman R, Akerstrom G, Biagini C, et al. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. The Journal of clinical endocrinology and metabolism. 2014;99:3595–3606. doi: 10.1210/jc.2014-2000. [DOI] [PubMed] [Google Scholar]
- 24.Ruda JM, Hollenbeak CS, Stack BC., Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2005;132:359–372. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.James BC, Kaplan EL, Grogan RH, et al. What’s in a Name?: Providing Clarity in the Definition of Minimally Invasive Parathyroidectomy. World journal of surgery. 2014 doi: 10.1007/s00268-014-2902-7. [DOI] [PubMed] [Google Scholar]
- 26.Nussbaum SR, Thompson AR, Hutcheson KA, et al. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery. 1988;104:1121–1127. [PubMed] [Google Scholar]
- 27.Mandell DL, Genden EM, Mechanick JI, et al. The influence of intraoperative parathyroid hormone monitoring on the surgical management of hyperparathyroidism. Archives of otolaryngology--head & neck surgery. 2001;127:821–827. [PubMed] [Google Scholar]
- 28.Gannon AW, Monk HM, Levine MA. Cinacalcet monotherapy in neonatal severe hyperparathyroidism: a case study and review. The Journal of clinical endocrinology and metabolism. 2014;99:7–11. doi: 10.1210/jc.2013-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roizen J, Levine MA. A meta-analysis comparing the biochemistry of primary hyperparathyroidism in youths to the biochemistry of primary hyperparathyroidism in adults. The Journal of clinical endocrinology and metabolism. 2014;99:4555–4564. doi: 10.1210/jc.2014-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerdahl J, Bergenfelz A. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: five-year follow-up of a randomized controlled trial. Ann Surg. 2007;246:976–980. doi: 10.1097/SLA.0b013e31815c3ffd. discussion 980-971. [DOI] [PubMed] [Google Scholar]
- 31.Russell CF, Dolan SJ, Laird JD. Randomized clinical trial comparing scan-directed unilateral versus bilateral cervical exploration for primary hyperparathyroidism due to solitary adenoma. Br J Surg. 2006;93:418–421. doi: 10.1002/bjs.5250. [DOI] [PubMed] [Google Scholar]
- 32.Carneiro DM, Solorzano CC, Nader MC, et al. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery. 2003;134:973–979. doi: 10.1016/j.surg.2003.06.001. discussion 979-981. [DOI] [PubMed] [Google Scholar]