Abstract
Advances in neurocritical care and interventional neuroradiology have led to a significant decrease in acute ischemic stroke (AIS) mortality. In contrast, due to the lack of an effective therapeutic strategy to promote neuronal recovery among AIS survivors, cerebral ischemia is still a leading cause of disability in the world. Ischemic stroke has a harmful impact on synaptic structure and function, and plasticity-mediated synaptic recovery is associated with neurological improvement following an AIS. Dendritic spines (DSs) are specialized dendritic protrusions that receive most of the excitatory input in the brain. The deleterious effect of cerebral ischemia on DSs morphology and function has been associated with impaired synaptic transmission and neurological deterioration. However, these changes are reversible if cerebral blood flow is restored on time, and this recovery has been associated with neurological improvement following an AIS. Tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) are two serine proteases that besides catalyzing the conversion of plasminogen into plasmin in the intravascular and pericellular environment, respectively, are also are efficient inductors of synaptic plasticity. Accordingly, recent evidence indicates that both, tPA and uPA, protect DSs from the metabolic stress associated with the ischemic injury, and promote their morphological and functional recovery during the recovery phase from an AIS. Here we will review data indicating that plasticity-induced changes in DSs and the associated post-synaptic density play a pivotal role in the recovery process from AIS, making special emphasis on the role of tPA and uPA in this process.
Keywords: Plasticity, Recovery, Plasminogen activation, Tissue-type plasminogen activator, Urokinase-type plasminogen activator, Ischemic stroke
Introduction
Despite the fact that the last 10 years have witnessed remarkable advances in the treatment of acute cerebral ischemia, we still lack an effective therapeutic strategy to promote neurological recovery in ischemic stroke (IS) survivors. In line with these observations, while the death rate from IS has decreased by ~35% in the last decade, cerebral ischemia is still a leading cause of disability in the world [1].
Five premises constitute the conceptual foundation for this review. First, that the occlusion of a large cerebral blood vessel causes the destruction of approximately 14 billion synapses every minute [2]. Second, that synaptic plasticity promotes synaptic repair in the ischemic brain. Third, that synaptic repair underlies the recovery of neurological function following an IS [3]. Fourth, that the plasminogen activation (PA) system is an effective inductor of synaptic plasticity [4]; and fifth, that although the definition of recovery most frequently used is the development of compensatory movement patterns that allows the performance of neurological functions lost after the IS [5], "true" recovery is defined by the repair of neuronal structures and brain circuits harmed by the ischemic injury [3].
Based on these considerations here we will review data supporting the idea that the neurological deficits observed after an IS are primarily due to the harmful effects of the ischemic injury on synaptic structure and function, particularly on the post-synaptic terminal assembled by dendritic spines (DSs) and the associated post-synaptic density (PSD). Thus, neurological recovery from an IS is largely dependent on the morphological, biochemical and electrophysiological recuperation of the post-synaptic terminal via the development of synaptic plasticity. Furthermore, we will review recent data indicating that two components of the PA system, namely tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), play a pivotal role in this process, and thus that they are potential therapeutic tools to promote neurological recovery among IS patients.
Taking into account these considerations, in the first part of this work we will discuss the most important morphological and functional features of the post-synaptic terminal, and will review recent data indicating that tPA and uPA have a direct impact on its morphology and function. In the second section we will use this knowledge to analyze the effects of cerebral ischemia on DSs and the PSD, and discuss what is currently known about the relation between plasticity-induced changes in DSs/PSD and functional recovery after an acute ischemic injury, making emphasis on the role of tPA and uPA in this process. Finally, in the last section we will review some data suggesting that modulation of different forms of synaptic plasticity may be a suitable target for the development of potential therapeutic strategies aimed to promote neurological recovery in patients that have suffered an acute IS.
The post-synaptic terminal
DSs and the associated PSD are capable to undergo structural, biochemical and functional modifications in response to changes in synaptic activity. As it will be discussed below, this property bestows on the post-synaptic terminal a pivotal role in the process of neurological recovery following an acute IS.
Dendritic spines
DSs are specialized dendritic protrusions that receive most of the excitatory input in the brain [6, 7]. In average, a dendrite harbors between 1 and 10 DS per µm of length [7] and each spine has a size and volume that ranges between 0.5 to 2 µm and 0.01 µm3 to 0.8 µm3 [7], respectively. The precursors of DS during development are thin and elongated structures known as filopodia [8, 9] that retract upon establishing synaptic contacts, adopting the appearance of a mature post-synaptic terminal in which is possible to recognize a head connected to the dendritic shaft through a 50 – 400 nm thin neck [10]. According to their shape, DSs can be classified as thin, stubby, or mushroom-shaped [11].
Although it was initially believed that DSs were fixed structures, it was soon evident that their morphology and biochemical composition change in response to variations in synaptic activity [12–18]. This property, known as spine motility [19], allows neurons to form and eliminate synapses [20] and is due to the presence of a highly dynamic network of actin filaments that in the DS head interacts with the plasma membrane and PSD, and in the neck assembles in long bundles. Actin filaments are polar structures with a barbed end to which actin monomers join, and a pointed end from which monomers dissociate [21]. The actin cytoskeleton in the DS is not fixed. Indeed, only ~ 5% of actin in the spine head is stable and treadmilling is assumed to occur, with continuous polymerization at the barbed ends and depolymerization at pointed ends [22]. This ability to change the structure of their cytoskeleton allows DSs to rapidly change their morphological features in response to variations in synaptic activity.
Calcium (Ca++) is the principal mediator of the effect of presynaptic activity on DSs. Accordingly, the simultaneous release of glutamate from the axonal bouton and depolarization of the postsynaptic terminal induces the influx of Ca++ into the DS via N-Methyl-D-Aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and voltage-gated Ca++ channels (VGCC), and stimulates the mobilization of Ca++ from internal sources such as the smooth endoplasmic reticulum. Here is important to keep in mind that DSs can regulate their Ca++ concentrations independently of the dendritic shaft, and although it was initially believed that the existence of a thin neck between the larger head of the DS and the dendritic shaft was the primary cause of this property, it has been recently proposed that this ability to compartmentalize Ca++ concentrations is independent of the presence of a physical barrier [23]. Regardless of its cause, this property allows each DS to maintain membrane potentials that differ from the parent dendrite and to act as an isolated signaling unit, able to operate independently of neighboring synapses [24]; and by doing so, each spine can participate in synapse-specific plasticity events important for functional modulation of individual synapses [25]. In summary, DSs are highly dynamic structures that have the ability to modify their morphology and biochemical composition in response to changes in synaptic activity [12–18]. This property enables them to participate in plasticity-dependent processes not only during physiological processes such as learning, but also during the recovery phase from an acute IS.
The post-synaptic density
The post-synaptic density (PSD) is a morphological and functional specialization of the postsynaptic terminal usually found near the tip of the DS, that contains a cytoskeleton that holds together a large number of scaffold proteins including NMDA, AMPA and metabotropic glutamate receptors. The morphology and biochemical composition of the PSD are not fixed, but instead undergo continuous modifications in response to variations in presynaptic activity. Accordingly, changes in synaptic strength have a direct effect not only on the size of the DS but also on the area of the corresponding PSD [24]. Hence, an increase in synaptic strength is followed by an enlargement of the DS and its PSD [7]. These variations have a direct effect on synaptic function, inasmuch as large spines not only are more stable but also have larger PSDs, express a higher number of AMPA receptors and form stronger synapses. In contrast, smaller spines change their shape more easily, have a larger number of NMDA receptors and the synaptic contacts that they establish are usually weaker [6]. More precisely, the population of NMDA and AMPA receptors in the PSD changes continuously in response to the electrical status of the synapse. Accordingly, the PSD of an inactive synapse contains NMDA but not AMPA receptors. However, when presynaptic activity increases, Ca++-induced activation of specific cell signaling pathways in DSs leads to the recruitment of AMPA receptors to their PSD, increasing its channel conductance and triggering a number of morphological, biochemical and electrophysiological changes that will be discussed below.
The plasminogen activation system and the post-synaptic terminal
The plasminogen activation (PA) system is an enzymatic cascade that was initially thought to be solely involved in the control of fibrin degradation by catalyzing the conversion of plasminogen into the broad-spectrum protease plasmin via two serine proteases: tPA and uPA. Hence, soon after their discovery it was proposed that uPA's and tPA's only role was the generation of plasmin in the pericellular and intravascular compartments, respectively [26]. However, subsequent work indicated that the control of fibrinolysis is not the only role of tPA and uPA, and that instead both PAs are also found in the synapse where they play a central role in the development of synaptic plasticity via mechanisms that not always require their ability to generate plasmin [27, 28]. Interestingly, it has been found that cerebral ischemia induces the secretion of both proteases into the synaptic cleft [29, 27], but that in contrast with an almost immediate release of tPA, uPA is secreted only during the recovery phase. Here it is important to keep in mind that neurons are not the only source of plasminogen activators (PAs), and that astrocytes and endothelial cells also release tPA in response to the ischemic injury [30]. Thus, not only neuronal tPA and uPA but also glial- and endothelial cell-derived PAs play a central role in the development of synaptic plasticity following an ischemic injury [31–33].
A growing body of experimental evidence indicates that once in the synaptic cleft both PAs are able to induce morphological and biochemical changes in DSs and the PSD [28]. More specifically, membrane depolarization induces the rapid release of tPA either from dendritic spines [34] or the axonal bouton [28], and this tPA increases DSs motility via plasmin-induced degradation of the extracellular matrix [35, 36], and induces Ca++ influx and miniature excitatory postsynaptic currents in the postsynaptic terminal by a mechanism that does not require plasmin generation.
In contrast with tPA, membrane depolarization does not induce the immediate release of uPA. Instead, neuronal uPA is released during the recovery phase from an ischemic injury, and binding of uPA to its receptor uPAR promotes the assemblage of F-actin bundles in DSs and the formation of filopodia by a mechanism that does not require the generation of plasmin (Figure 1). More precisely, uPA-uPAR binding on the surface of DSs induces the expression of the actin polymerizing protein profilin and inactivates the actin-depolymerizing protein cofilin by triggering its phosphorylation at serine 3 [27]. The net effect of this sequence of events is the addition of G-actin monomers to the barbed end, thus promoting the formation of stable F-actin filaments. Together these data indicate that the release of tPA and uPA in the synaptic cleft has a direct effect on the morphology and function of DSs and their PSDs and that as will be discussed below, this plays a central role in the process of neurological recovery following an ischemic injury.
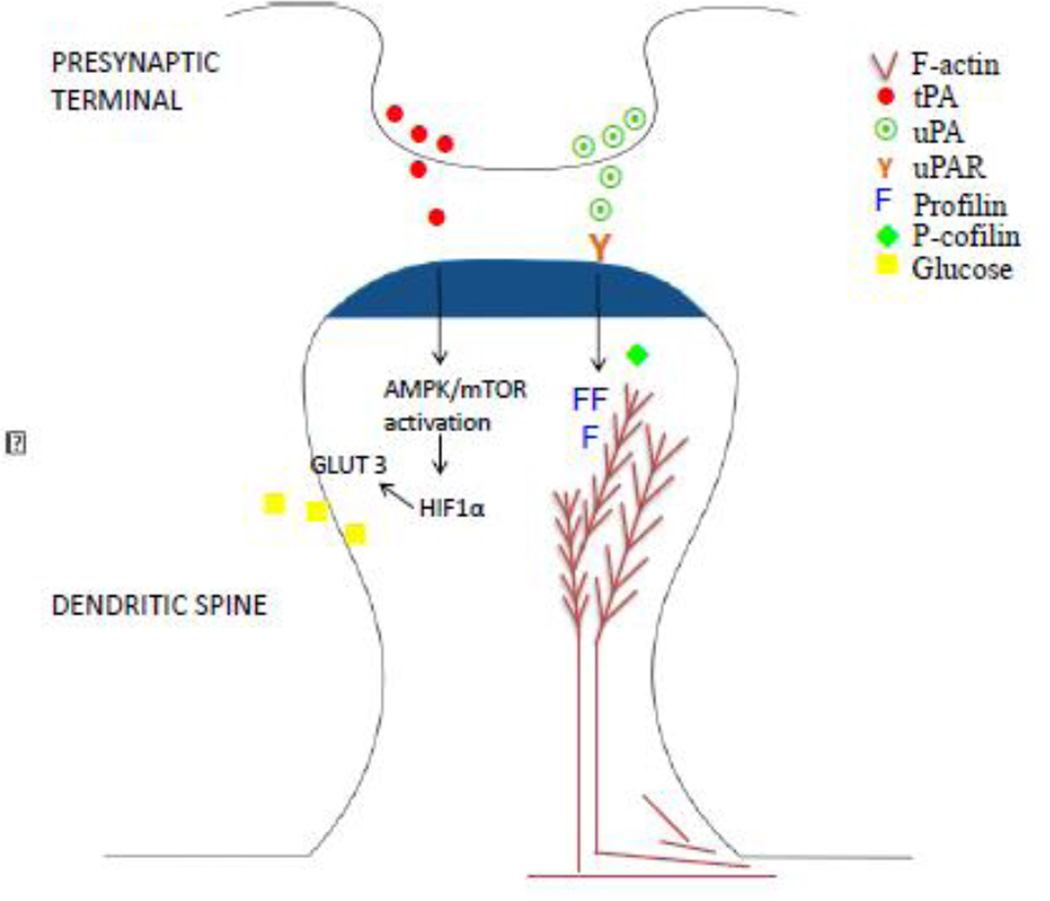
Figure 1. Schematic representation of tPA and uPA on the post-synaptic density.
TPA (red dots) is rapidly released from the presynaptic terminal following membrane depolarization. The interaction of this tPA with NMDA receptors on the post-synaptic density (dark blue shadow) induces activation of the AMPK/mTOR pathway, leading to HIF-1α accumulation, and subsequent membrane recruitment of the neuronal transporter of glucose GLUT3. This sequence of events lead to increase uptake of glucose by the post-synaptic terminal. In contrast with tPA, uPA (green dots) is released from the presynaptic terminal during the recovery phase from a hypoxic/ischemic injury. This uPA interacts with its receptor uPAR leading to the assembly of F-actin bundles and the recovery of the dendritic spine.
Effect of cerebral ischemia on dendritic spines and the postsynaptic density
Cerebral ischemia has a direct impact on synaptic structure. Indeed, it has been estimated that one hour of cerebral ischemia is enough to destroy 830 billion synapses and 714 km of myelin [2] with the ensuing bearing on synaptic function. Of special relevance for this review is a substantial body of experimental data indicating that ischemia-induced morphological and biochemical changes in DSs and associated PSDs not only underlie the development of functional deficits but also the recovery of neurological function following an acute IS.
Cerebral ischemia and the dendritic spine
Several post-mortem studies have demonstrated that the ischemic injury induces changes in dendritic branch complexity, DS density, and the number of synapses in the peri-infarct regions [37]. In line with these observations, in vivo work using two-photon confocal microscopy has shown that the induction of focal cerebral ischemia is followed by substantial morphological changes in DSs (Figure 2) [38, 39]. More specifically, early after the onset of the ischemic injury DSs are replaced by focal areas of swelling known as varicosities [39, 40, 27], that despite not having an as yet clear function, it is believed that constitute a protective mechanism that allows the post-synaptic terminal to compartmentalize abnormally high Ca++ concentrations without compromising neighboring synapses [41, 42]. Furthermore, in addition to the formation of varicosities, some spines elongate purportedly because a long neck also allows them to isolate from neighboring synapses the higher concentrations of Ca++ produced by the excitotoxic injury [43, 44]. Remarkably, if cerebral blood flow is restored within 20 – 60 minutes of the onset of the ischemic injury, DSs in the periinfarct cortex re-emerge again from these varicosities and filopodia, and in some cases form de novo from the parent dendrite [39, 27]. These observations are of paramount importance because it has been postulated that morphological and functional changes in the periinfarct cortex are associated with recovery of neurological function after an IS [45, 46]. However, it should be noted that despite the importance of these observations, the establishment of an experimentally demonstrable cause-effect relationship between dendritic spines recovery and improvement of neurological function after an ischemic stroke is still lacking.
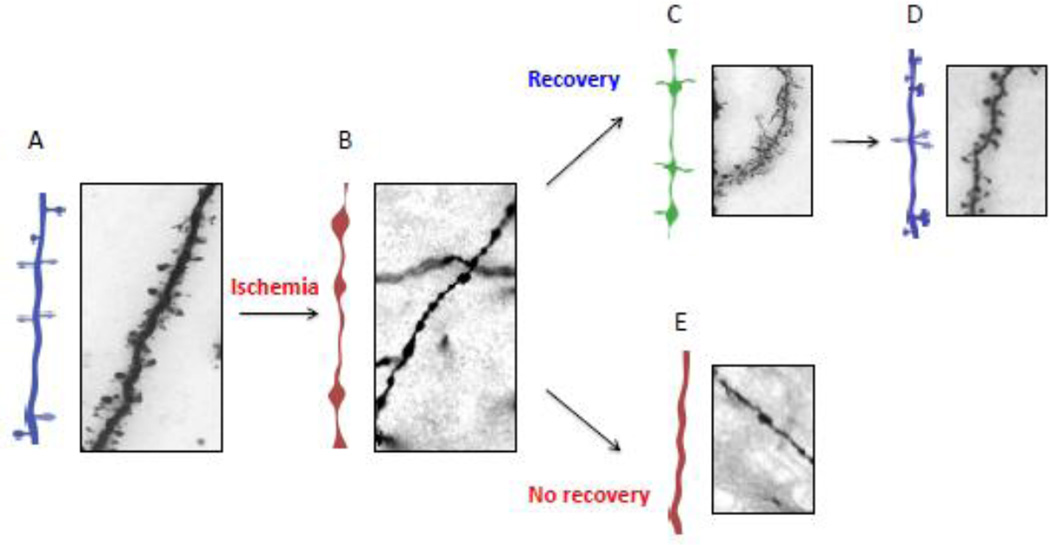
Figure 2. Effect of cerebral ischemia on dendritic spines.
A & B. Diagram and representative micrograph of a Golgi staining of a distal dendrite from the fronto-temporal cortex of a wild-type mice either under physiological conditions (A), or six hours after the onset of cerebral ischemia (B), or 24 hours after reperfusion (C – E). Note that dendritic spines in A are replaced by varicosities in B. However, during in the early stages of reperfusion (recovery phase), filopodia (C) and then recovered dendritic spines (D) re-emerge from some of these dendrites, in contrast with other dendrites (E) in which dendritic spines do not recover (E).
Cerebral ischemia and the post-synaptic density
A direct effect of the ischemic injury on the PSD was suggested by early in vitro work with neuronal cultures showing that excitotoxic exposure to glutamate increases its thickness and changes its appearance [47]. These observations were corroborated by in vivo studies indicating that a brief period of cerebral ischemia transiently increases the size of the PSD by modifying its protein composition. More exactly, this and other studies demonstrated that the ischemic injury induces the recruitment of several proteins to the PSD [48]. One of them is the Ca++ /Calmodulin (CaM)-dependent protein kinase II (CaMKII), a serine/threonine kinase highly abundant in DSs that, as will be discussed below, activates the molecular mechanism that lead to the recruitment of AMPA receptors to the PSD with the subsequent induction of a form of synaptic plasticity known as long-term potentiation (LTP). This event is of central importance in the ischemic brain. Indeed, in the non-ischemic brain a large number of excitatory synapses harbor NMDA but not AMPA receptors in their PSDs rendering them non-functional or “silent”. However, in the early phases of the ischemic injury the binding of glutamate to NMDA receptors leads to Ca++-induced CaMKII activation, and CaMKII-mediated recruitment of AMPA receptors to the PSD, converting them from “silent” into active synapses. Furthermore, the PSD becomes discontinuous soon after the onset of the ischemic injury, originating multiple spines from a single post-synaptic terminal (perforated synapses) with a high density of AMPARs and therefore enhanced electrical activity [7, 49]. These changes lead to a transient but significant increase in synaptic transmission in the earlier phases of the ischemic injury [3].
Taken all together, the experimental evidence available to this date indicates that DSs and the associated PSDs are dynamic structures that undergo morphological and biochemical modifications in response to cerebral ischemia, and that these changes are reversible if the ischemic insult is relieved on time. These variations, that are similar to those observed during development and activity-dependent forms of synaptic plasticity [50], seem to underlie the recovery of neurological function after an acute IS [27].
The plasminogen activation (PA) system protects dendritic spines and promotes their recovery following an acute ischemic injury
Several in vitro and in vivo studies indicate that in the ischemic brain tPA and uPA have distinct effects on DSs and PSD morphology, biochemistry and function (Figure 1). More specifically while tPA protects DSs from the metabolic stress induced by the ischemic injury [51, 52], uPA promotes the re-emergence of DSs from dendritic varicosities during the recovery phase from an acute IS [27].
Tissue-type plasminogen activator triggers cell-signaling pathways that allow DSs to detect and adapt to metabolic stress
It has been recognized that cerebral ischemia [29, 53] and pharmacologically-induced membrane depolarization [34] induce the release of tPA from axonal boutons and DSs; and several groups have reported that once in the synaptic cleft this tPA induces morphological and biochemical changes in the presynaptic and postsynaptic terminals. In fact, recent studies have found that the release of tPA in the early phases of an ischemic injury activates the synaptic vesicle cycle in glutamatergic neurons [28]. More specifically, tPA induces the recruitment of the cytoskeletal protein βII-Spectrin and voltage gated calcium channels (VGCC) to the active zone, leading to Ca++-mediated phosphorylation of synapsin I, a protein that when non-phosphorylated clusters synaptic vesicles in the reserve pool of the presynaptic terminal. However, upon phosphorylation synapsin I detaches from the surface of synaptic vesicles, freeing them to translocate to the synaptic release site. Furthermore, these studies showed that tPA not only promotes Ca++-mediated synapsin I phosphorylation, but also the binding of βII-Spectrin to these synaptic vesicles, bringing them to the active zone where they release their load of neurotransmitters [28].
Besides its action on the presynaptic terminal, tPA also has two important morphological and biochemical effects on DSs and their PSDs (Figure 1). First, it increases the size of the PSD [28] and interacts with NMDA receptors promoting the entrance of Ca++ into the DS [54]. And second, activates the mammalian target of rapamycin (mTOR) [51] and the adenosine monophosphate (AMP)-activated protein kinase (AMPK) [55] pathways that, as will be discussed below, protect DSs from the harmful effects of metabolic stress.
Tissue-type plasminogen activator and the mTOR/AMPK pathways
The mTOR pathway is assembled by a complex of protein kinases that regulates cell growth and survival [56]. Several studies have described a central role for mTOR in the ischemic brain. Accordingly, while mTOR activation has been linked to the development of synaptic plasticity and cell survival [57], inactivation of mTOR has been associated to neuronal death in the early phases of the ischemic injury [58, 59]. Recent in vitro and in vivo studies with neuronal cultures and an animal model of cerebral ischemia have shown that either the release of neuronal tPA or the intravenous administration of recombinant tPA (rtPA) promotes neuronal survival in the ischemic brain via its ability to induce mTOR activation in DSs. These investigators identified that this neuroprotective effect is mediated by mTOR’s ability to induce the neuronal expression and accumulation of hypoxia-inducible factor 1α (HIF1α), a transcription factor that mediates the detection and adaptation to metabolic stress [60]. Furthermore, it was found that HIF1α induces the expression of GLUT3, a member of the family of membrane transporter proteins that regulates the uptake of glucose by neurons [61, 62] (Figure 1).
AMPK is an evolutionary conserved kinase that acts as a sensor of cellular energy status. Thus, energy depletion induces the phosphorylation of AMPK’s α sub-unit at Thr172, triggering a number of biochemical events that inactivate ATP-consuming events such as protein synthesis and activate ATP-generating processes such as glycolysis and glucose uptake via membrane recruitment of GLUT3 receptors [63]. Strikingly, tPA is a very efficient activator of AMPK in DSs, and this leads to the recruitment to the neuronal membrane of GLUT3 receptors synthesized via the tPA-induced mTOR/ HIF1α pathway described above. In line with these observations, in vivo work with an animal model of cerebral ischemia and positron emission tomography (PET) studies indicate that either the release of neuronal tPA or treatment with rtPA promotes the uptake of glucose by dendritic spines located in the ischemic tissue [51].
Urokinase-type plasminogen activator promotes the recovery of dendritic spines in the periinfarct cortex
UPA is a serine protease that upon binding to its receptor uPAR is cleaved by membrane-bound plasmin to generate an active two-chain form that catalyzes the conversion of plasminogen into plasmin on the cell surface [64]. Additionally, uPAR is a glycosylphosphatidylinositol-anchored glycoprotein that promotes tissue remodeling, cell proliferation, adhesion and migration via its interaction with a large number of proteins on the cell surface and extracellular matrix [65]. Although uPA is expressed in cerebral cortical and hippocampal neurons its role in the adult CNS remained unclear for a long time until a study showed two very important findings [27]: first, that neurons release uPA during the recovery phase from a hypoxic/ischemic injury; and second, that genetic deficiency of either uPA (uPA−/−) or uPAR (uPAR−/−) does not have an effect on the volume of the necrotic core but impairs the recovery of neurological function after an acute IS. Together, these two observations suggested that uPA binding to uPAR promotes neurological recovery in the ischemic brain. Soon thereafter it was identified that cerebral ischemia has a comparable effect on the formation of dendritic varicosities in the distal dendrites of wild-type (Wt), uPA−/− and uPAR−/− mice. However, while Wt neurons developed filopodia and DSs from these varicosities within the first 24 hours of recovery from the ischemic injury, DSs of uPA−/− and uPAR−/− mice failed to re-emerge from these varicosities, and instead became smooth and without protrusions. Later studies found that during the early phase of an ischemic stroke uPAR clusters on the surface of dendritic varicosities from which DS will re-emerge during the recovery phase. More specifically, it was shown that the binding of uPA to uPAR induces profilin-mediated F-actin assembly in dendritic varicosities leading to the formation of filopodia that later become DSs. Importantly, it was also reported that treatment with recombinant uPA (ruPA) had a rescue effect on uPA−/− but not uPAR−/− mice. In summary, the evidence available to this date suggests that uPA-uPAR binding plays a pivotal role in the recovery process from an acute IS via its ability to promote the re-emergence of DSs from dendritic varicosities via reorganization of their actin cytoskeleton.
Synaptic plasticity, dendritic spines and neurological recovery after stroke
Taking into consideration the data discussed above is easy to understand that neurological recovery is inherently associated with the development of synaptic plasticity [66–68], defined as the mechanism whereby a synapse modifies its strength, efficacy and excitability in response to specific patterns of neural activity [69]. Here we will consider two types of synaptic plasticity of significant importance in the ischemic brain. The first form is governed by the Hebb's rule stating that the strength of a synapse increases when there is a simultaneous increase in presynaptic activity and a critical level of post-synaptic depolarization. Accordingly, its main electrophysiological correlate is an enhancement in post-synaptic firing rates in response to presynaptic activity [70], and its two best known forms are long-term potentiation (LTP) and long- term depression (LTD). The second one is known as homeostatic plasticity and its main role is to act as a compensatory feedback mechanism aimed to maintain network stability [71].
Long-term potentiation in the ischemic brain
LTP is a form of Hebbian plasticity that causes a rapid increase in synaptic strength only in those inputs where the presynaptic and postsynaptic terminals fire together. Accordingly, the induction of LTP involves the influx of Ca++ into the DS via NMDA receptors that open only if there is simultaneous presynaptic release of glutamate and substantial postsynaptic depolarization. Although the increase of Ca++ in the DS lasts only 2–3 seconds [72] it is enough to activate a complex cascade of intracellular signaling pathways that cause the morphological and functional changes required to increase synaptic strength via the induction of LTP. CaMKII is the primary effector of the Ca++-activated signals that mediate the development of LTP [73], and among a vast variety of functions, activated CaMKII phosphorylates the GluR1 sub-unit of AMPA receptors at Serine 381 [73] and promotes F-actin bundling in the spine head [74], leading to the recruitment of GluR1-containing AMPA receptors to the PSD with increase in AMPA receptor conductance, and DS enlargement.
From this description is easy to recognize that many of the molecular events that are involved in the development of LTP are also activated during excitotoxicity. Hence, LTP can also be induced by anoxia [75], cerebral ischemia, and inhibition of glycolysis [76]. The induction of LTP by cerebral ischemia also causes morphological changes in the synapse, namely increase in the number of perforated synapses and multiple synapse boutons, filopodia growth, PSDs thickening, and enlargement of existing spines and formation of new ones [77]. Noteworthy, although some studies indicate that excitotoxic activation of NMDA receptors inhibits CaMKII activity [78], others have shown that glutamate treatment- and cerebral ischemia-induced Ca++ influx into the DS induce CaMKII translocation to the PSD and the formation of extrasynaptic CaMKII clusters [48, 79].
The implications of these findings are yet unclear. Indeed, while some studies have linked the development of LTP after an ischemic injury to delayed neuronal death [80], others have found that an increase in activity-dependent LTP in the areas surrounding the necrotic core underlies the development of synaptic plasticity required for the recovery of neurological function [81]. Independently of these considerations, the clinical relevance of these data is supported by studies with IS patients indicating that functional recovery is associated with increased neuronal excitability not only in the peri-infarct cortex but also in non-ischemic tissues [82].
Homeostatic plasticity
Although the induction of LTP plays an important role in physiological processes such as learning and memory formation [83, 84] and in recovery of neurological function after an ischemic lesion [3], it is clear that once LTP is induced, potentiated synapses can be excited to undergo further potentiation reaching an unstable state of hyperexcitation and circuit failure. To prevent this from happening, neurons have the ability to develop a form of homeostatic plasticity whereby they stabilize their activity in the face of perturbations [84]. As discussed above, in Hebbian forms of plasticity such as LTP, changes in synaptic strength are confined to active synapses, a property known as "input specificity". In contrast, although in some cases may also operate at a local level, homeostatic plasticity has been considered to be predominantly a global process that involves all the inputs received by a neuron [71]. However, independently of its local or global nature, the development of homeostatic plasticity requires the induction of morphological and biochemical modifications in the post-synaptic terminal, most notably changes in DS size and in the population of AMPA receptors in the PSD [85]. There is a growing awareness of the importance of the concept of homeostatic plasticity as a mechanism of neurological recovery after an ischemic injury. Indeed, the interruption of synaptic activity days to weeks after the onset of cerebral ischemia may have a deleterious effect on neurological function. However, this phase of impaired synaptic activity is followed days later by neuronal hyperexcitability arguably by the successful development of homeostatic plasticity that generates a permissive environment for axonal sprouting, dendritic spine recovery and formation of new synaptic contacts.
Conclusions and future perspectives
The data reviewed here indicate that cerebral ischemia has a direct impact on the structure and function of the post-synaptic terminal, and that plasticity-induced functional and morphological reorganization of dendritic spines in the periinfarct tissue is associated with improvement in neurological function following an ischemic injury. We evaluated experimental evidence indicating that key components of the PA system, namely tPA and uPA, play a central role in the process of neurological recovery via their ability to induce synaptic plasticity. We revised some of the most important biochemical pathways underlying the development of Hebbian and non-Hebbian synaptic plasticity and analyzed their importance in the recovery process from an acute ischemic stroke. However, despite the importance of these findings, it is clear that an experimentally demonstrable cause-effect relationship between dendritic spine recovery and improvement in neurological function is lacking. Likewise, it remains to be understood whether the development of long-term potentiation and other forms of synaptic plasticity have a harmful effect on neuronal survival in the ischemic brain, or if instead it they underlie the recovery of synaptic function in the periinfarct cortex. Finally, the data analyzed here indicate that the PA system is a potential target for the development of therapeutic strategies aimed at promoting neurological recovery among AIS survivors.
Acknowledgments
Sources of Support
This work has been supported in part by National Institutes of Health Grants NS-079331 (to MY) and NS-091201 (to MY).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Time is brain--quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature reviews Neuroscience. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 4.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 5.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39(5):788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 6.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends in neurosciences. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 7.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nature reviews Neuroscience. 2001;2(12):880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 8.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(21):8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17(1):91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 10.Adrian M, Kusters R, Wierenga CJ, Storm C, Hoogenraad CC, Kapitein LC. Barriers in the brain: resolving dendritic spine morphology and compartmentalization. Frontiers in neuroanatomy. 2014;8:142. doi: 10.3389/fnana.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual review of neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420(6917):812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 13.Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20(5):847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 16.Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(13):3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 18.Tailby C, Wright LL, Metha AB, Calford MB. Activity-dependent maintenance and growth of dendrites in adult cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4631–4636. doi: 10.1073/pnas.0402747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasbani MJ, Schlief ML, Fisher DA, Goldberg MP. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(7):2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandel E. Principles of Neural Science. Fifth. McGraw-Hill Education; 2013. [Google Scholar]
- 21.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. The Journal of cell biology. 2010;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35(6):1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 23.Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nature neuroscience. 2000;3(7):653–659. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]
- 24.Yuste R, Majewska A. On the function of dendritic spines. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2001;7(5):387–395. doi: 10.1177/107385840100700508. [DOI] [PubMed] [Google Scholar]
- 25.Yuste R. Electrical compartmentalization in dendritic spines. Annual review of neuroscience. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- 26.Pittman RN, Ivins JK, Buettner HM. Neuronal plasminogen activators: cell surface binding sites and involvement in neurite outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9(12):4269–4286. doi: 10.1523/JNEUROSCI.09-12-04269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu F, Catano M, Echeverry R, Torre E, Haile WB, An J, et al. Urokinase-type plasminogen activator promotes dendritic spine recovery and improves neurological outcome following ischemic stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(43):14219–14232. doi: 10.1523/JNEUROSCI.5309-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F, Torre E, Cuellar-Giraldo D, Cheng L, Yi H, Bichler EK, Garcia PS, Yepes M. Tissue-Type Plasminogen Activator Triggers the Synaptic Vesicle Cycle in Cerebral Cortical Neurons. Journal of Cerebral Blood Flow and Metabolism. 2015 Dec;35(12):1966–1976. doi: 10.1038/jcbfm.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. JClinInvest. 2010;120(6):2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Frontiers in cellular neuroscience. 2015;9:304. doi: 10.3389/fncel.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin H, Li Y, Shen LH, Liu X, Hozeska-Solgot A, Zhang RL, et al. Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study) Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(11):2181–2188. doi: 10.1038/jcbfm.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casse F, Bardou I, Danglot L, Briens A, Montagne A, Parcq J, et al. Glutamate controls tPA recycling by astrocytes, which in turn influences glutamatergic signals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(15):5186–5199. doi: 10.1523/JNEUROSCI.5296-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Liu S, Wang SJ, Yu C, Paganini-Hill A, Fisher MJ. Tissue plasminogen activator expression and barrier properties of human brain microvascular endothelial cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28(4):631–638. doi: 10.1159/000335785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. JNeurobiol. 2006;66(6):564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- 35.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44(6):1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. ProcNatlAcadSciUSA. 2002;99(11):7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez CL, Kolb B. A comparison of different models of stroke on behaviour and brain morphology. The European journal of neuroscience. 2003;18(7):1950–1962. doi: 10.1046/j.1460-9568.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(46):11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(22):5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(7):1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasbani MJ, Schlief ML, Fisher DA, Goldberg MP. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. JNeurosci. 2001;21(7):2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. NeurobiolDis. 1996;3(3):215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 43.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39(4):1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- 44.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annual review of neuroscience. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 45.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128(Pt 5):1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- 46.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68(3):793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 47.Dosemeci A, Tao-Cheng JH, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(2):625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402(6760):421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 50.Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nature neuroscience. 2000;3(6):545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 51.Wu F, Nicholson AD, Haile WB, Torre E, An J, Chen C, et al. Tissue-type plasminogen activator mediates neuronal detection and adaptation to metabolic stress. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33(11):1761–1769. doi: 10.1038/jcbfm.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(29):9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96(2):569–576. [PubMed] [Google Scholar]
- 54.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32(1):48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 55.An J, Haile WB, Wu F, Torre E, Yepes M. Tissue-type plasminogen activator mediates neuroglial coupling in the central nervous system. Neuroscience. 2014;257:41–48. doi: 10.1016/j.neuroscience.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 57.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. BiochemBiophysResCommun. 2011;404(4):941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 58.Magagnin MG, van den BT, Sergeant K, Lambin P, Koritzinsky M, Devreese B, et al. The mTOR target 4E-BP1 contributes to differential protein expression during normoxia and hypoxia through changes in mRNA translation efficiency. Proteomics. 2008;8(5):1019–1028. doi: 10.1002/pmic.200700551. [DOI] [PubMed] [Google Scholar]
- 59.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. NatRevCancer. 2008;8(11):851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 60.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. AnnNeurol. 2000;48(3):285–296. [PubMed] [Google Scholar]
- 61.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17(21):2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 62.Semenza G. Signal transduction to hypoxia-inducible factor 1. BiochemPharmacol. 2002;64(5–6):993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 63.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature reviews Molecular cell biology. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 64.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nature reviews Molecular cell biology. 2010;11(1):23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 65.Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A, et al. The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thrombosis and haemostasis. 2005;93(2):205–211. doi: 10.1160/TH04-09-0592. [DOI] [PubMed] [Google Scholar]
- 66.Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain research Brain research reviews. 2001;36(2–3):169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 67.Rijntjes M, Weiller C. Recovery of motor and language abilities after stroke: the contribution of functional imaging. Progress in neurobiology. 2002;66(2):109–122. doi: 10.1016/s0301-0082(01)00027-2. [DOI] [PubMed] [Google Scholar]
- 68.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke; a journal of cerebral circulation. 2007;38(2 Suppl):840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 69.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annual review of neuroscience. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 70.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 71.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10(3):358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 72.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 73.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature reviews Neuroscience. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Filippo M, Tozzi A, Costa C, Belcastro V, Tantucci M, Picconi B, et al. Plasticity and repair in the post-ischemic brain. Neuropharmacology. 2008;55(3):353–362. doi: 10.1016/j.neuropharm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Calabresi P, Centonze D, Pisani A, Cupini L, Bernardi G. Synaptic plasticity in the ischaemic brain. Lancet Neurol. 2003;2(10):622–629. doi: 10.1016/s1474-4422(03)00532-5. [DOI] [PubMed] [Google Scholar]
- 77.Jourdain P, Nikonenko I, Alberi S, Muller D. Remodeling of hippocampal synaptic networks by a brief anoxia-hypoglycemia. JNeurosci. 2002;22(8):3108–3116. doi: 10.1523/JNEUROSCI.22-08-03108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 79.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 80.Hsu KS, Huang CC. Characterization of the anoxia-induced long-term synaptic potentiation in area CA1 of the rat hippocampus. British journal of pharmacology. 1997;122(4):671–681. doi: 10.1038/sj.bjp.0701409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schiene K, Bruehl C, Zilles K, Qu M, Hagemann G, Kraemer M, et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16(5):906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 82.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2(8):493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 83.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor perspectives in biology. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. Journal of neurophysiology. 2006;96(4):2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]