Abstract
Successful navigation in the world requires effective visuospatial processing. Unfortunately, older adults have many visuospatial deficits, which can have severe real-world consequences. While some of these age effects are well documented, some others, such as the perception of depth from motion parallax, are poorly understood. Depth perception from motion parallax requires intact retinal image motion and pursuit eye movement processing. Decades of research have shown that both motion processing and pursuit eye movements are affected by age; it follows that older adults may also be less sensitive to depth from motion parallax. The goals of the current study were to characterize motion parallax depth thresholds in older adults, and to explain older adults’ sensitivity to depth from motion parallax in terms of motion and pursuit deficits. Younger and older adults’ motion thresholds and pursuit accuracy were measured. Observers’ depth thresholds across several different stimulus conditions were measured as well. Older adults had higher motion thresholds and less accurate pursuit, compared to younger adults. They were also less sensitive to depth from motion parallax, at slow and moderate pursuit speeds. Although older adults had higher motion thresholds than younger adults, they used available motion signals optimally, and age differences in motion processing could not account for older adults’ increased depth thresholds. Rather, these age effects can be explained by changes in older adults’ pursuit signals.
Keywords: depth perception, motion parallax, aging, depth thresholds, smooth pursuit eye movements
Visuospatial deficits in older adults have many adverse effects on health and on social and physical functioning (Crews & Campbell, 2004). Self-reported visual impairment contributes to the incidence of falls in older adults (Lopez et al., 2011) and is a significant predictor of mortality, especially in women (Lee, Gomez-Marin, Lam, & Zhang, 2002). Beyond visual impairment at the ocular level, research on sensory processing in older adults has revealed that perceptual deficits are associated with many negative outcomes, including difficulties with balance (Choy, Brauer, & Nitz, 2008), reading speed (Yu, Cheung, Legge, & Chung, 2010), and driving abilities (Owsley et al., 1998). Given that the population of older adults in the US is projected to nearly double by 2050 (Ortman, Velkoff, & Hogan, 2014), research on understanding age-related visuospatial deficits is especially timely and important. Some aspects of age effects on visuospatial abilities have been relatively well studied (for reviews, see Andersen, 2012; Owsley, 2011). However, very little is known about the effects of age on one important cue to relative depth, motion parallax.
Motion parallax (MP) is produced during observer or scene translation. As an observer moves through the environment, stationary objects appear to move relative to one another, creating relative image motion on the retina. Concomitantly, the translating observer maintains fixation on particular objects in the scene, generating smooth pursuit eye movements (Miles & Busettini, 1992). This combination of retinal image motion and pursuit are used by the visual system to generate a depth percept (Nawrot & Joyce, 2006). The geometric relationship of retinal image motion velocity (change in the angular position of an object’s retinal image, dθ), the observer’s pursuit eye movement velocity (change in gaze angle, dα), viewing distance to the AGING AND point of fixation (f), and object distance from fixation (dMP), is described by the Motion/Pursuit Ratio (M/PR) (Nawrot & Stroyan, 2009; Stroyan & Nawrot, 2012):
| (1) |
The M/PR provides a more useful quantification of depth from MP than “equivalent disparity” (ED) because the M/PR takes into account the role of pursuit eye movements in generating the depth percept. Moreover, the proximal stimulus variables used by the M/PR are dynamic (velocity), thereby better reflecting the dynamic characteristics of MP than the static spatial displacements used in quantification of MP with ED. Similarly, these dynamic variables used with the M/PR appear to better describe the underlying perceptual mechanisms of depth from MP. Although ED is useful and intuitive when directly comparing depth percepts from binocular disparity and MP, M/PR is generally a more precise approximation of depth (Stroyan, 2010).
Recent research (Holmin & Nawrot, 2015) has characterized the roles of motion (dθ) and pursuit (dα) signals in limiting depth thresholds in younger adults. However, research on the perception of depth from MP in older adults is scarce. Older adults have a well-documented deficit in motion perception, with higher thresholds for motion detection (Atchley & Andersen, 1998; Bennett, Sekuler, & Sekuler, 2007), motion direction discrimination (Ball & Sekuler, 1986; Snowden & Kavanagh, 2006), and speed discrimination (Norman, Ross, Hawkes, & Long, 2003). The recovery of structure from motion (a perceptual process related to that of depth from MP) is likewise affected by age (Norman, Bartholomew, & Burton, 2008; Norman et al., 2013; Norman, Dawson, & Butler, 2000; Norman et al., 2012), though it is unknown to what degree the neural mechanisms are shared (Nawrot & Joyce, 2006). Aging also affects pursuit eye movements—older adults have poorer pursuit accuracy than younger adults (measured as gains: eye velocity/target velocity) (Sharpe & Sylvester, 1978; Sprenger et al., 2011). Given these deficits in motion and pursuit (dθ and dα signals), it follows that older adults might have deficits in the perception of depth from MP as well.
In view of the logic just outlined, it is surprising that studies of aging and MP have not found any differences in depth perception between younger and older adults. Norman, Clayton, Shular, & Thompson (2004) presented an MP wave stimulus that varied in depth and had older and younger observers adjust the length of a response bar to indicate the magnitude of depth that was shown. Across three different stimulus depth magnitudes (2.0, 4.0, and 6.0 cm), both younger and older observers overestimated the amount of depth presented, but there was no age difference. In another study, observers adjusted a palm board to indicate the perceived amount of slant presented in MP-defined stimuli (Norman, Crabtree, Bartholomew, & Ferrell, 2009). Again, there was no difference between younger and older observers in the magnitude of adjusted slant, at any of the four degrees of stimulus slant.
There are several possible reasons why no age differences were found in either of these studies (Norman et al., 2004; Norman et al., 2009). One possibility, of course, is that there are simply no effects of age on depth perception from motion parallax. Although older adults show deficits in the two components of depth perception from MP (i.e., motion processing and pursuit), it could be that these deficits are compensated for by the visual system when generating a depth percept, or perhaps that the deficits are too small to have an effect on depth from MP. Another possibility is that the magnitudes of stimulus depth presented to observers by Norman and colleagues were suprathreshold; that is, it is possible that older adults may have higher thresholds for the perception of depth from MP, compared to younger adults. To illustrate, consider an observer with a deficit in motion processing. This observer might be able to perform a suprathreshold task, such as discriminating 5 deg/sec from 2 deg/sec motion, but show a deficit AGING AND in motion detection at 1 deg/sec. In a similar vein, older adults might be able to make reliable judgments about the magnitude of suprathreshold MP depth stimuli, but have higher thresholds for detecting or discriminating depth from MP compared to younger adults. That is, older adults may be less sensitive to depth magnitudes near threshold stimulus values. One last possibility is that the effects of age on MP may only be apparent under some stimulus conditions. For example, in Norman et al.’s (2009) study, the velocity at which the MP stimulus translated (generating pursuit eye movements, or dα), was 12 deg/sec. In their 2004 study, observers’ head movements were not strictly controlled, so the speed at which observers moved their heads (and generated the concomitant pursuit signal in the direction opposite that of head movement; Nawrot & Joyce, 2006) is unknown. It is conceivable that older adults may have lower depth thresholds at some pursuit velocities, such as those used by Norman and colleagues, but might have higher thresholds at other velocities.
In the current study, we sought to characterize depth from MP in younger and older adults across a range of stimulus conditions by measuring depth thresholds at slow, medium, and fast pursuit velocities, as well as measure motion thresholds and pursuit accuracy. We quantified depth thresholds using the simple M/PR (dθ/dα). We also examined how changes (or lack thereof) in older adults’ depth thresholds might be explained using the M/PR model, by asking if age differences in motion (dθ) and pursuit (dα) could explain older adults’ performance in our depth threshold task.
Method
Observers
Thirty-two younger (M = 25.3 years, SD = 3.8) and 32 older adults (M = 66.3 years, SD = 4.7) participated in the experiment. In the older age group, 26 observers were female and six were male. In the younger age group, 17 observers were female and 15 were male. The younger observers were student volunteers from North Dakota State University, who were either paid or given course credit for their participation. Older observers were recruited from the surrounding community, and were paid for their participation. All observers gave informed consent to participate.
After giving informed consent, but prior to beginning the study, observers underwent screening for a variety of exclusionary criteria. Far visual acuity was assessed using a Snellen visual acuity chart (Graham-Field, Atlanta, GA). Contrast sensitivity was assessed using a Pelli-Robson Contrast Acuity chart (Haag-Streit, Essex, UK). Observers’ neurological health status was assessed using a revised form of Christensen, Armson, Moye, and Kern’s (1992) health questionnaire. Observers who scored a 20/40 or worse on the (best corrected) acuity test or scored 1.65 or worse on contrast sensitivity test, or indicated any neurological problems on the questionnaire, were not included in the study. During the study, observers adjusted their corrective lenses for viewing distance if necessary. The procedures were overseen by the local Institutional Review Board and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Apparatus
For depth and motion tasks, stimuli were generated using Matlab PsychToolbox (Brainard, 1997; Kleiner, 2007) and presented on an Apple 5K iMac computer with a 27” retina display and a resolution of 5120 × 2880 pixels × 60 Hz. At 200 cm viewing distance, the monitor subtended 15.4 degrees of visual angle and each pixel subtended 10.8 arcsec. For eye tracking, stimuli were generated on a Macintosh computer and presented on a 20” flat screen NEC CRT monitor with a resolution of 1600 × 1200 pixels × 85 Hz. At 57 cm viewing distance, the monitor AGING AND subtended 34.8 degrees of visual angle and each pixel subtended 90 arcsec. An ASL Eye-trac 6000 (Applied Science Laboratory, Bedford, MA) with D6 remote optics and a sampling rate of 120 Hz was used to measure eye position. The eye tracking system is accurate within 0.5 deg, and has a precision of 0.25 deg. The system communicated eye position information to the stimulus computer through a 16-bit analog connection with a National Instruments multifunction I/O board.
The experiment was conducted in a dimly-lit (~ 1 lux) room. During all three tasks, observer movement was restricted by a chinrest and an eye patch occluded the observers’ left eyes for monocular viewing.
Stimuli
These stimuli and methods were modeled after Holmin and Nawrot (2015). Depth stimuli were composed of 6,400 43 × 43 arcsec black dots positioned randomly on a white background, contained within a 6.3 × 6.3 deg stimulus window. A small black square (6 arc min) in the center of the stimulus window served as a fixation point. In separate conditions, the stimulus window translated leftward or rightward at one of three velocities (2.3, 10.1, and 25 deg/sec) to generate pursuit eye movements (dα) as the observer maintained fixation on the center point of the translating stimulus. Stimulus window translations began with a positional offset opposite the direction of translation so that the fixation point at the center of the stimulus window would traverse the vertical midline midway through the window’s translation. To generate smooth stimulus window translation, an updated window position was calculated and redrawn every 16.7 ms.
Stimulus parallax was achieved through relative motion of the dots, translating in opposite directions (shearing), within two different regions of the stimulus window during translation of the entire stimulus window (see Figure 1). Dots within the bounds of one region of the stimulus moved in the same direction as the stimulus window, thereby generating dθ in the same direction as dα. This region should appear nearer than the fixation point. Dots within the bounds of the other region moved (dθ) in the direction opposite the direction of stimulus window movement (dα), and should appear farther than the fixation point. The two regions corresponded to the upper and lower halves of the stimulus window, and the direction of dot movement within the two regions varied randomly between trials.
Figure 1.
Schematic of the stimulus used in the depth threshold task. In this example, background motion is leftward. A rectangle, positioned flush with the horizontal meridian, contains dot motion in the rightward direction. This rectangle appears to be nearer in depth to the observer. Note that in the stimulus, there is no shadow visible behind the rectangle in depth; the shadow in the schematic is meant to represent the fact that this rectangle is nearer in depth compared to the background.
To generate this stimulus, on every frame (every 16.7 ms) the dots were shifted laterally, at the same rate as the stimulus window and fixation dot (dα: 2.3, 10.1, or 25.0 deg/sec). That is, the stimulus window, and the dots appearing within the window, were translated every 16.7 ms. To add retinal image motion (dθ) to these dots (which were moving with the fixation spot and should otherwise remain stationary on the retina), dots within the stimulus were further translated seven times (every 100 ms) throughout the 800 ms stimulus presentation. The speed of dot movement relative to the window movement (dθ: 0.013–0.92 deg/sec) was the same for both directions of dot motion, and was determined for each trial using a staircase procedure (see below). The overall perception of the motion parallax stimulus was approximately that of a square-wave grating composed of smoothly moving dots.
In our depth threshold stimulus, parallax was defined by the relative (shearing) motion of dots within the stimulus. To allow for a direct comparison of dθ values between motion thresholds and depth thresholds, we designed a motion stimulus that was likewise defined by shearing motion (modeled after Snowden, 1992). The motion stimulus was identical to the depth threshold stimulus (i.e., same dot density, contrast, and retinal motion), but was stationary in the center of the screen. Similar to the depth threshold stimulus, the dots in the region above fixation moved to the left or right, and the dots in the region below fixation moved in the direction opposite that of the dots in the upper half of the stimulus. In the depth threshold stimulus, there was no retinal image motion when the dots within the stimulus moved at the same speed as the fixation point; retinal image motion was added every 100 ms. Similarly, in the motion stimulus, the dots moved once, halfway during the stimulus presentation, at 100 ms; the minimum magnitude of displacement that could be presented on a given trial was 10.8 arcsec. It is important to note that although the motion threshold stimuli and depth threshold stimuli were as similar as possible (excepting the presence of pursuit and stimulus duration) in no case did observers report a depth percept in viewing the motion stimulus, nor did either of the authors. The motion stimulus contained only shearing motion, with the dots in the lower and upper regions of the stimulus moving at the same speed; this lack of a relative velocity gradient coupled with the absence of a pursuit signal diminished any cues to depth.
In the pursuit condition, the pursuit target was a single white dot presented on a black background. At 57 cm viewing distance the target subtended 0.23 × 0.23 deg. The target was translated leftward or rightward every 11.76 ms (85 Hz) at one of three velocities (2.1, 10.6, and 25.5 deg/sec).
Procedure
Depth thresholds
A trial began when the fixation spot was presented in the center of the screen. Following a button press by the experimenter to initiate a trial, the fixation spot jumped to the left or to the right to indicate the starting position of the stimulus translation. The magnitude of the fixation point jump was calculated from the pending stimulus velocity such that the stimulus, when it appeared, would cross the vertical midline halfway through its translation. Following a variable interval from 0.5–3.5 sec, the stimulus window appeared and began to move. Observers maintained fixation in the center of the translating stimulus window throughout the 800 ms duration trial. To simplify data collection, we did not enforce fixation using an eye tracker in this task, as Nawrot and Stroyan (2009) did not find a difference in depth judgments between conditions in which fixation was objectively enforced and those in which observers were given verbal instructions to maintain fixation. Note that observers remained stationary, and MP was generated with stimulus dot movement tied to stimulus window translation, rather than stimulus dot movement yoked to observer head movement. The same mechanisms of pursuit that are crucial for recovering depth from MP are operating under both head-stationary and head-translating conditions; however, other somatosensory and vestibular signals that arise during head movements, including the translational vestibulo-ocular reflexive (TVOR) eye movement, are not necessary for recovering unambiguous MP depth information (Nadler, Nawrot, Angelaki, & DeAngelis, 2009; Nawrot & Joyce, 2006; Nawrot, Ratzlaff, Leonard, & Stroyan, 2014). Keeping the observer’s head stationary eliminates these unnecessary signals. The direction of stimulus window movement (left or right) was randomly determined on each trial. The order of condition presentation was determined using a Latin Square.
Observers performed a depth-phase judgment task in which they were asked to indicate verbally the location of the half-cycle of stimulus depth (above or below fixation; see Figure 1) that was closer in depth, relative to the other half-cycle. The observers’ responses were recorded on the computer by the experimenter. No feedback was given. The threshold M/PR (dθ/dα) for unambiguous depth perception was found for each direction of stimulus translation (leftward and rightward) at each of the stimulus speed conditions using a staircase procedure with a three-down, one-up decision rule (Wetherill and Levitt, 1965). The initial dot motion (dθ) was set to 0.24 deg/sec, and each step in the staircase increased or decreased dot motion by 0.026 deg/sec. Each staircase ended either when observers had had six reversals, which would track to the 79% threshold, or when the ceiling (0.92 deg/sec) had been reached. For the block to end at ceiling, the participant had to reach the ceiling, and have no reversals for the following five trials. If a block ended at ceiling, and the observer had made fewer than six reversals, the data from that block were not included in the data analysis. Observers completed two blocks of trials for each pursuit direction, for each pursuit speed. Each block typically contained 20–40 trials. At the slow and moderate pursuit speeds, all observers were able to generate six reversals in at least one of the two blocks for each pursuit direction. Two older observers were not able to perform the task at 25.0 deg/sec, and were not included in the data analysis.
Motion thresholds
To begin each trial a fixation spot was presented in the middle of the screen. Following a button press by the experimenter to initiate a trial, the stimulus window was presented, centered on the fixation spot. Observers were to maintain fixation throughout the duration of the stimulus presentation (200 ms). The observers reported which direction (left or right) the dots in the top region (i.e., the region of the stimulus above the fixation point) were moving. The experimenter recorded observer responses on the computer. No feedback was given. As in the depth threshold task, a three-down, one-up procedure (Wetherill & Levitt, 1965) was used to determine dmin, or the minimum amount of displacement observers needed to reliably discriminate motion direction. Staircases started with a dot displacement of 97.2 arcsec, moved in steps of 10.8 arcsec, and ended with nine reversals. Observers completed two blocks of trials in the motion threshold task.
Pursuit gains
The eye tracker was calibrated for each observer using a 9-point calibration of the ASL system, followed by a 2-point calibration of the experimental computer’s recording of the eye position signal, and a final 5-point calibration along the horizontal axis of the pursuit target’s movement. On each trial, the target was presented in the middle of the screen, and a button press by the experimenter initiated target movement. The experimenter did not initiate a trial until the observer was fixating on the centered target. Once the trial was initiated, the target “stepped” either to the left or to the right (Rashbass, 1961), before translating in the direction opposite that of the step. The magnitude of the step varied depending on the pending stimulus velocity, such that for each trial the target would pass through the vertical midline of the screen 100 ms after onset of translation. The target was erased from the display at 870 ms, and eye position was recorded for an additional 306 ms, giving a trial duration of 1176 ms. The observer’s task was to maintain fixation on the target as it translated. The target translated twice leftward and twice rightward at one of three different speeds (2.1, 10.6, and 25.5 deg/sec), for a total of 12 trials per block. The order of pursuit speed presentation was randomly determined at the beginning of each block for each observer. Observers completed three blocks at each speed, for a total of 36 trials per observer.
Results
Analyses were conducted in Microsoft Excel and SPSS 21 (SPSS II, New York, NY). Depth thresholds were quantified using the M/PR (dθ/dα; Nawrot & Stroyan, 2009). For each of the two blocks, in each of the three conditions, the threshold dθ (measured in deg/sec) for each direction of stimulus translation (left and right) was determined from the last four reversals. For each observer, threshold dθ for each stimulus direction was averaged across the two blocks to give two mean threshold dθs (one for each stimulus direction). A 3 (pursuit speed (dα): 2.3, 10.1, and 25.0 deg/sec) x× 2 (age group: young and old) × 2 (stimulus translation direction: left and right) ANOVA revealed that there was no significant main effect of direction of stimulus movement, and no interaction with any other variables, in the depth task (all Fs < 1). Directional differences were therefore dropped from all further analyses.
Results from the depth task are shown in Figures 2 and 3. In Figure 2, threshold dθs found in the depth task are plotted for older and younger observers. Threshold dθs were converted to depth measures using the M/PR: in each condition, the ratio of threshold dθs to pursuit speed (dα) that defined that particular condition was calculated (Figure 3). These M/PR values can be converted to expected relative depth magnitudes (dMP) using Equation 1 and an expected viewing distance. Similar to binocular disparity, a particular M/PR corresponds to different physical depth magnitudes depending upon viewing distance. For example, a threshold M/PR of 0.1 would correspond to a dMP = 20 cm at a viewing distance of 200 cm, but a dMP = 5 cm at a distance of 50 cm.
Figure 2.
Threshold dθs for younger and older observers. The minimum within-stimulus motion (dθ) in deg/sec that observers required to perceive depth is plotted against pursuit speed condition (2.3, 10.1, and 25.0 deg/sec) on the x-axis. Black squares represent younger adults (YA), and white squares represent older adults (OA).
Figure 3.
Depth thresholds (dθ/dα) for older and younger observers. Depth thresholds are plotted against pursuit speed (dα: 2.3, 10.1, and 25 deg/sec) on the x-axis. The data plotted on solid lines represent obtained thresholds for younger (YA) and older adults (OA). The data plotted on the dashed line represent the depth threshold values predicted for older adults (see text).
A 2 × 3 mixed factorial ANOVA was conducted on depth thresholds, with age group (young and old) as the between-subjects factor, and pursuit speed (2.3, 10.1, and 25.5 deg/sec) as the within-subjects factor. Older adults had significantly higher thresholds than younger adults overall (F(1, 59) = 10.58, p < 0.01, η2 = 0.03; all η2 are complete η2). There was also a significant main effect of speed: as pursuit speed increased, depth thresholds decreased (F(1.04, 61.42) = 121.63, p < 0.01, η2 = 0.66). There was also an interaction between age and speed (F(1.04, 61.42) = 7.21, p < 0.01, η2 = 0.04). At the slowest speed, older adults had significantly higher thresholds than younger adults (t(62) = 3.04, p < 0.01). Older adults also had higher thresholds than younger adults at 10.1 deg/sec (t(61) = 4.54, p < 0.01). There was no difference in thresholds between younger and older adults at the fastest speed (t(60) = −1.58, p = 0.12).
Motion thresholds (or dmin) were found for each observer using the last six reversals in each of the two blocks, and then averaging across the blocks. Older adults’ motion thresholds were more than six times higher than younger adults’ motion thresholds (t(59) = 8.72, p < 0.01), as seen in Figure 4. In a simple ideal observer analysis, it is possible to use these older observers’ motion threshold values (M = 144.70 arcsec, SE = 13.41), found during a 1 step/200 ms stimulus duration, to extrapolate the minimum dθ that older adults would need during a 7 step/800 ms stimulus duration, as in the depth threshold task. (This extrapolation method is reasonable, given that previous research has shown that when the stimulus duration between displacements is 100 ms or more, adding steps [i.e., more displacements] does not affect motion processing [Snowden & Braddick, 1989; 1990]). The older adults’ extrapolated dθ value is 0.35 deg/sec, and represents the “best” dθ we might expect older observers to achieve during the depth threshold task, in order to reliably report depth-sign. We can also estimate the magnitude of the pursuit signal (dα) generated by each observer in response to the stimulus translation in the depth task. Actual dα was estimated for each observer by multiplying the obtained gains in each pursuit task condition (i.e., gains for 2.1, 10.6, and 25.5 deg/sec) by the stimulus speed in each depth task condition, for each observer. This extrapolated dθ value and actual dα can be used to generate an ideal observer model for older adults—that is, the results we would expect if observers were performing using all of the information available in the motion and pursuit signals, and combining these two signals optimally. Ideal M/PRs are plotted on the dashed lines in Figure 3.
Figure 4.
Motion thresholds (dmin) for younger and older observers. Thresholds are plotted as magnitudes of displacement, in arcsecs. YA = younger adults; OA = older adults.
For eye tracking analysis, eye velocity was derived from eye position using a two-point central difference algorithm. Data were then low-pass filtered at 40 hz using a 3-term moving average filter. Saccades were identified as velocities greater than 40 deg/sec (Burke & Barnes, 2006) and those sections were removed from further analysis. The first 223 ms and the last 306 ms were discarded, to exclude open-loop pursuit and pursuit after the target had disappeared from the screen. Gains were then analyzed by averaging velocity over the remaining data points (647 ms) and computing the average eye velocity/target velocity.
Figure 5 shows the relationships between the two components of depth from MP (dθ and dα) and depth thresholds, for older adults. The left panel shows each older adult’s M/PR plotted as a function of their motion threshold. Each observer generated three data point: one M/PR per pursuit velocity condition in the depth condition (see legend). Because motion threshold (dmin) was measured once for each observer, their three data points are stacked vertically. Overall, as pursuit speed increased, depth thresholds decreased, and at the lowest speed (black squares) there seems to be much more variability in M/PRs than in the moderate (white circles) and high (black diamonds) velocities. The right panel shows older adults’ M/PRs plotted as a function of pursuit gains (recall that gains were measured for three different pursuit velocities). Again, each observer generated three data points. As in the left panel, the right panel makes apparent that as pursuit speed in the depth task increased, depth thresholds decreased, and there is more variability in depth thresholds at the slowest pursuit speed condition. Relationships between pursuit gains, motion thresholds, and M/PRs will be explored in subsequent analyses.
Figure 5.
Relationship of two MP components and depth thresholds, for older adults. In both panels, M/PRs are plotted on the Y-axis. The left panel plots M/PRs as a function of motion thresholds (dmin), and in the right panel, M/PRs are plotted as a function of gain. The legend on the right gives the symbols for each of the three pursuit velocity conditions in the depth task.
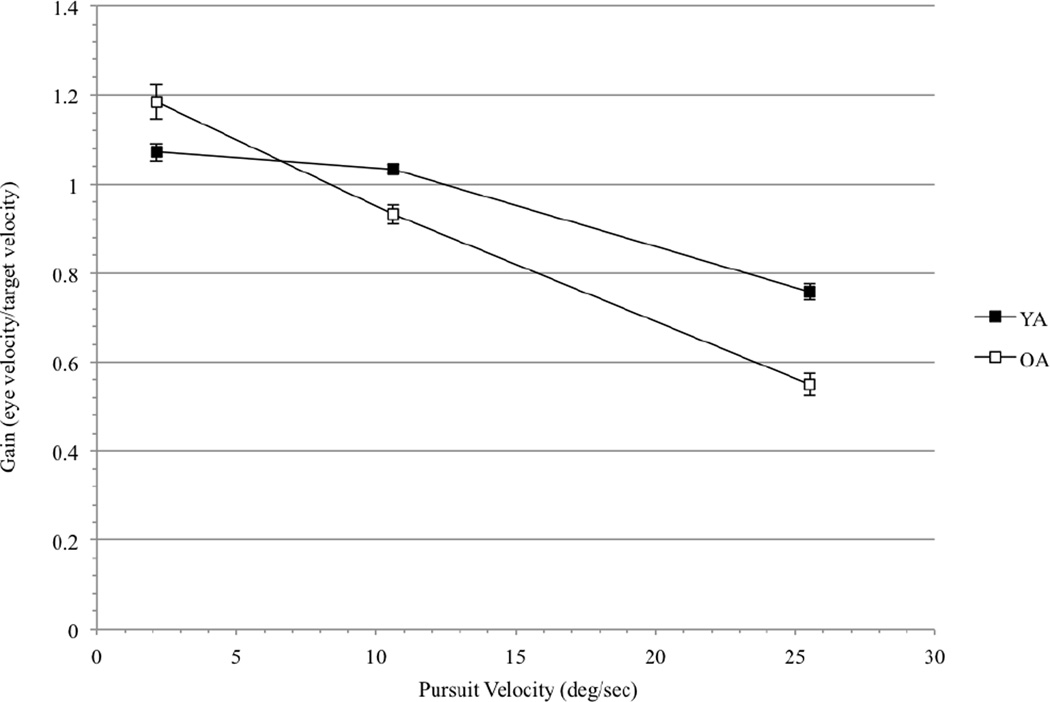
A 2 × 2 × 3 mixed factorial ANOVA, with age group (young and old) as a between-subjects factor, pursuit direction (left and right) as a within-subjects factor, and pursuit speed (2.1, 10.6, and 25.5 deg/sec) as a within-subjects factor, revealed a significant interaction between pursuit speed and age (F(1.31, 83.13) = 24.45, p < 0.01, η2 = 0.09) in the pursuit task. At a pursuit speed of 2.1 deg/sec, older adults had higher gains than younger adults; at 10.6 and 25.5 deg/sec, older adults had lower gains than younger adults (Figure 6). There was no effect of pursuit direction or, and pursuit direction did not interact with any other variables (all F’s < 1).
Figure 6.
Pursuit gains for younger and older observers. Gains (eye velocity/target velocity) are plotted against the target speed (2.1, 10.6, and 25.5 deg/sec) on the x-axis. Black squares represent younger adults (YA), and white squares represent older adults (OA). Note that a gain of 1.0 represents perfect performance (i.e., eye velocity = target velocity).
While the ideal observer model (Figure 3) predicts observers’ results if they are combining the two independent sources of information (motion and pursuit) optimally as described by the M/PR, a regression analysis provides another way to assess how changes in motion, pursuit, and the mechanism that combines them are affected by normal aging. To investigate the effects of age on dθ and dα on thresholds, difference scores for each older observer were generated for each of the three tasks. Difference scores were calculated by subtracting the mean of the younger adults’ scores from each individual older adult’s score. For example, in the depth task, for each older adult, the younger adults’ mean M/PR in the 2.3 deg/sec condition was subtracted from that older adult’s M/PR, giving a difference score for each older adult in that condition. The process was repeated for each of the other two conditions in the depth task (10.1 and 25 deg/sec), for the actual dαs estimated for each observer (as detailed above), and for the motion task. The absolute values of the difference scores were found, and these values underwent a natural log transformation, generating logΔmpr, logΔdθ, and logΔdα scores for each older observer (the relationship of dθ, dα, and depth thresholds is not linear in the M/PR model; transforming these difference scores enabled us to treat this relationship as linear and perform a regression analysis; Nawrot et al., 2014). A regression analysis was used to assess how well age related differences in motion perception (logΔdθ) and pursuit (logΔdα) accounted for changes in depth perception from MP (logΔmpr). The full model was significant (R2 = 0.22; F(2, 86) = 11.52, p < 0.01):
| (2) |
Change in pursuit (logΔdα) was a significant independent predictor of change in depth perception (logΔmpr) (p < 0.01), but change in motion perception (logΔdθ) was not (p > 0.05). A comparison of the age related differences in pursuit (Figure 6) and depth thresholds (Figure 3) illustrate that both varied together with pursuit speed (r = −0.45, p < 0.01) thereby resulting in the predictive power of logΔdα. In contrast, while there was a large effect of age on motion perception (Figure 4), there was no correlation of logΔdθ with logΔmpr (r = 0.006) meaning that some older adults who had larger changes in motion thresholds had smaller changes in depth thresholds, and vice-versa. This finding is not unprecedented—Andersen and Atchley (1995) similarly found that older adults’ performance on a 2-dimensional motion task was not related to their performance on a 3-dimensional motion task.
Discussion
Older adults had higher depth thresholds than younger adults in the slow (2.3 deg/sec) and moderate (10.1 deg/sec) MP pursuit speed conditions. When the stimulus was moving at a fast speed (25.0 deg/sec), older and younger adults had similar depth thresholds. Age had an effect on pursuit and motion processing, as expected. At the three speeds tested in the pursuit task (2.1, 10.6, and 25.5 deg/sec), older adults had less accurate pursuit than younger adults. Older adults also had higher motion thresholds than younger adults, by a factor of six.
It is interesting to note that, despite age differences in dθ and dα, older observers’ obtained thresholds were very similar to the depth thresholds generated by the ideal observer model (see dashed line, Figure 3). Indeed, although their motion thresholds were higher than younger adults’, and their pursuit eye movements were considerably less accurate, older adults were apparently using the available motion and pursuit signals in an optimal way. The threshold dθs obtained in the slow and moderate conditions of the depth task (and used along with pursuit speed to find the M/PR) were 0.37 and 0.39 deg/sec, respectively (see Figure 2). These obtained threshold dθs are very similar to the optimal motion threshold, 0.35 deg/sec, which was extrapolated from dmin and added as a constraint to the ideal observer model. Older observers’ threshold dθs were higher in the fastest depth condition than in the slow and moderate depth conditions (Figure 2), perhaps because older adults’ eye velocities were too slow to be able to accurately track the stimulus when it moved at 25.0 deg/sec. Gain at 25.5 deg/sec was 0.55 for older adults, meaning that older adults’ average eye velocity was only 55% of the target velocity. It is not surprising that older adults could not optimally use motion signals at the fast stimulus velocity; they could not move their eyes quickly enough to “keep up” with the stimulus, making motion processing in this stimulus condition difficult (Stone & Krauzlis, 2003). However, although older observers needed stronger motion signals to accurately recover depth sign at 25.0 deg/sec, they still had relatively low depth thresholds at this speed.
The similarity in depth thresholds between younger and older adults at 25.0 deg/sec is due to the fact that younger adults’ depth thresholds did not continue to decrease with increasing stimulus velocity, as older observers’ did (Figure 3). That is, at 25.0 deg/sec, younger adults required higher threshold dθs in order to recover depth from MP—at this speed, older and younger adults were performing similarly (Figure 2). This increase in threshold dθ in younger adults is not entirely unexpected, however; recent research has shown that younger adults require higher dθs for depth perception at fast velocities compared to moderate velocities (Holmin & Nawrot, 2015), and that younger observers’ depth thresholds are stable at moderate and fast pursuit velocities.
At all velocities tested, older adults had less accurate pursuit eye movements than younger adults (Figure 5). An inaccurate dα signal will contribute to higher thresholds, as may be seen in the difference between age groups at 2.3 and 10.1 deg/sec. The similarity between younger and older adults’ thresholds at 25.0 deg/sec is not due to an improvement in pursuit performance in older adults; rather, as described in the paragraph above, younger adults’ performance at this speed becomes more like older adults’.
Although age differences in depth from MP have only been investigated in a few studies, the effects of age on the recovery of shape or structure from motion has received more attention. In the first experiment of Norman et al. (2004) discussed above, older and younger adults discriminated the shape depicted in a random-dot stimulus in a MP display. Here the dot lifetimes, that is, the duration for which individual dots in the stimuli survived before being erased and redrawn in a new position, were varied. Older adults required longer dot lifetimes than younger adults in order to reliably discriminate the surface shape, and this age difference remained even when dot lifetime was unlimited. The results of the current study and the first experiment in Norman et al. are not directly comparable, as the recovery of surface depth and shape information are different perceptual processes (see Norman et al., 2004, for a discussion); this difference is illustrated by the fact that Norman and colleagues found, in the same study, an effect of age for MP-defined shapes, but not for MP-defined suprathreshold surface depth. Older adults also have deficits in recovering structure from motion, compared to younger adults (Andersen & Atchley, 1998; Norman et al., 2008; Norman et al., 2013; Norman et al., 2000; Norman et al., 2012), though, again, structure from motion and depth from MP are different processes. However, when one considers that both processes require the use of motion information to recover form or surface depth information, and that one of these processes (structure from motion) is well-known to be affected by age, the age effects found in the current study are not unexpected.
Recall that and Norman and colleagues (2004; 2009) found no effect of age on MP depth magnitude judgments, whereas the current study finds an effect of age on MP depth thresholds. The chief difference in methodology among these studies is in the manner in which MP was generated—in Norman et al.’s studies observers generated lateral head translations, while stationary observers generated lateral pursuit eye movements in the current study. In order to maintain fixation during head translation, both tVOR and pursuit eye movements are generated (Freeman & Fowler, 2000). However, it is only the pursuit component that is necessary—tVOR does not contribute to the perception of depth from MP (Nawrot & Joyce, 2006).1 In the current study, we have eliminated the unnecessary tVOR signal, leaving the pursuit signal, which generates a vivid and robust depth percept. It is important to note, though, that pursuit eye movements are still being generated in Norman et al.’s studies. Given that the necessary pursuit signals are operating under both head-moving and head-stationary conditions, it is unlikely that the significant age differences in the current study are a result of the method used to produce the MP stimuli. Instead, the differences in the results of Norman and colleagues’ and the current study are likely due to differences in our measures of interest: the current study determined factors affecting minimum detectable depth (thresholds) whereas Norman and colleagues studied the perceptual magnitude of suprathreshold stimuli. That is, it would be unusual to study perceived depth magnitudes for stimuli that older observers could not even detect. Therefore, our results are not incompatible with those of Norman and colleagues’, but rather, extend our understanding of different aspects of age effects on depth from MP.
It is possible to demonstrate the limits of depth from MP in real-world situations, based on results from the current study. Imagine an observer walking at a rate of 5 km/h. This observer is fixating on an object that is to the right, orthogonal to the direction in which the observer is walking. If this fixated object is 35 m away (less than length of a average suburban lawn in the United States), the observer’s gaze angle (dα) changes at a rate of 2.3 deg/sec, the slowest speed in the current study. A younger observer could reliably discriminate between the fixated object and a relatively nearer or farther distractor object given at least 3.8 m of depth between these objects. Under the same conditions, an older observer could reliably discriminate which object was nearer if the objects were at least 5.6 m apart. Now imagine if the observer were driving at a rate of 50 km/h. If the observer is fixating an object 77 m away (almost the length of a city block), dα = 10.1 deg/sec, the middle speed in the current study. In this case, a younger observer could make reliable judgments about the relative depths of a fixated and distractor object if the objects were at least 1.54 m apart. In contrast, an older observer could make a reliable depth judgment at 3.8 m, or double the amount of depth required by the younger observer.
To put this result into context, consider the following situation: looking down from one end of a city block to the other end, an older adult who is fixating on a car that is stopped less than three meters from a road sign would not be able to tell if this car is stopped in front of or behind the road sign. This inability to discriminate the relative depth of objects affects a driver’s planning and maneuvering. Now imagine an older adult driving past a full parking lot, searching for an empty parking space (the average parking space is approximately three meters wide). In this situation, older adults will not perceive the space between two cars parked on either side of an empty parking space, and will therefore miss the parking space.
In real-world situations such as the ones described above, the successful recovery of relative depth between objects enables us to act on these objects and plan our movements in the world (Foster, Fantoni, Caudek & Domini, 2011). Aging affects motion parallax depth thresholds, with potentially substantial consequences for the detection of depth differences in the real world. As the population of older adults continues to grow (Ortman et al., 2014), it will be important to better understand these functional deficits and address how the perception of depth from MP in older adults might be improved.
Acknowledgments
This work was supported by a Centers of Biomedical Research Excellence (COBRE) grant: NIH P20 GM103505; and by a North Dakota State University Doctoral Dissertation Fellowship. Special thanks to Dan Gu for his help in programming this study.
Footnotes
Readers unfamiliar with pursuit’s crucial role in the perception of depth from MP are referred to Nawrot and Joyce (2006) and Nadler et al (2009), which show a pursuit eye movement signal, not a head movement signal or a tVOR signal, provides the extra-retinal signal to disambiguate depth from MP. That is, perceived depth from MP changes with changes in pursuit direction, even when head movement and tVOR remain constant. While Rogers and Graham (1979, Figure 5, p. 132) is widely taken to have shown the superiority of translational head movement, more recently Nawrot et al. (2014, Figure 8, p. 10) demonstrate that perceived MP depth magnitude is reduced in head-translating, compared to head-stationary, conditions because the involuntary tVOR signal, generated in the head-translating condition, reduces the magnitude of pursuit necessary to maintain fixation.
References
- Andersen GJ. Aging and vision: Changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3:403–410. doi: 10.1002/wcs.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GJ, Atchley P. Age-related differences in the detection of three-dimensional surfaces from optic flow. Psychology and Aging. 1995;10:650–658. doi: 10.1037//0882-7974.10.4.650. [DOI] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13:297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Improving visual perception in older observers. Journal of Gerontology. 1986;41:176–182. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision Research. 2007;47:799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Burke MR, Barnes GR. Quantitative differences in smooth pursuit and saccadic eye movements. Experimental Brain Research. 2006;175:596–608. doi: 10.1007/s00221-006-0576-6. [DOI] [PubMed] [Google Scholar]
- Choy NL, Brauer S, Nitz J. Linking stability to demographics, strength and sensory system function in women over 40 to support pre-emptive preventive intervention. Climacteric. 2008;11:144–154. doi: 10.1080/13697130801931821. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Armson RR, Moye J, Kern TM. Health screening and random recruitment for cognitive aging research. Psychology and Aging. 1992;7:204–208. doi: 10.1037//0882-7974.7.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. American Journal of Public Health. 2004;94:823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Fantoni C, Caudek C, Domini F. Integration of disparity and velocity information for haptic and perceptual judgments of object depth. Acta Psychologica. 2011;136:300–310. doi: 10.1016/j.actpsy.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Freeman TCA, Fowler TA. Unequal retinal and extra-retinal motion signals produce different perceived slants of moving surfaces. Vision Research. 2000;40:1857–1868. doi: 10.1016/s0042-6989(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Holmin J, Nawrot M. Motion parallax thresholds for unambiguous depth perception. Vision Research. 2015;115:40–47. doi: 10.1016/j.visres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3. Perception. 2007;36:1. [Google Scholar]
- Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and morality in US adults. Archives of Ophthalmology. 2002;120:1544–1550. doi: 10.1001/archopht.120.11.1544. [DOI] [PubMed] [Google Scholar]
- Liao K, Walker MF, Joshi A, Millard R, Leigh RJ. Vestibulo-ocular responses to vertical translation in normal human subjects. Experimental Brain Research. 2008;185:553–563. doi: 10.1007/s00221-007-1181-z. [DOI] [PubMed] [Google Scholar]
- Lopez D, McCaul KA, Hankey GJ, Norman PE, Almeida OP, Dobson AJ, Flicker L. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment—Is there a gender difference? Mauritas. 2011;69:359–364. doi: 10.1016/j.maturitas.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Miles FA, Busettini C. Ocular compensation for self-motion: Visual mechanisms. Annals of the New York Academy of Sciences. 1992;656:220–232. doi: 10.1111/j.1749-6632.1992.tb25211.x. [DOI] [PubMed] [Google Scholar]
- Nadler JW, Nawrot M, Angelaki DE, DeAngelis GC. MT neurons combine visual motion with a smooth pursuit eye movement signal to code depth-sign from motion parallax. Neuron. 2009;63:523–532. doi: 10.1016/j.neuron.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot M, Joyce L. The pursuit theory of motion parallax. Vision Research. 2006;46:4709–4725. doi: 10.1016/j.visres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Ratzlaff M, Leonard Z, Stroyan K. Modeling depth from motion parallax with the motion/pursuit ratio. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot M, Stroyan K. The motion/pursuit law for visual depth perception from motion parallax. Vision Research. 2009;49:1969–1978. doi: 10.1016/j.visres.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JF, Bartholomew AN, Burton CL. Aging preserves the ability to perceive 3-D object shape from static but not deforming boundary contours. Acta Psychologica. 2008;129:198–207. doi: 10.1016/j.actpsy.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Norman JF, Cheeseman JR, Pyles J, Baxter MW, Thomason KE, Calloway AB. The effect of age upon the perception of 3-D shape from motion. Vision Research. 2013;93:54–61. doi: 10.1016/j.visres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Norman JF, Clayton AM, Shular CF, Thompson SR. Aging and the perception of depth and 3-D shape from motion parallax. Psychology and Aging. 2004;19:506–514. doi: 10.1037/0882-7974.19.3.506. [DOI] [PubMed] [Google Scholar]
- Norman JF, Crabtree CE, Bartholomew AN, Ferrell EL. Aging and the perception of slant from optical texture, motion parallax, and binocular disparity. Attention, Perception, & Psychophysics. 2009;71:116–130. doi: 10.3758/APP.71.1.116. [DOI] [PubMed] [Google Scholar]
- Norman JF, Dawson TE, Butler AK. The effects of age upon the perception of depth and 3-D shape from differentialmotion and binocular disparity. Perception. 2000;29:1335–1359. doi: 10.1068/p3111. [DOI] [PubMed] [Google Scholar]
- Norman JF, Holmin JS, Beers AM, Cheeseman JR, Ronning C, Stethan AG, Frost AL. Aging and the discrimination of 3-D shape from motion and binocular disparity. Attention, Perception, & Psychophysics. 2012;74:1512–1521. doi: 10.3758/s13414-012-0340-x. [DOI] [PubMed] [Google Scholar]
- Norman JF, Ross HE, Hawkes LM, Long JR. Aging and the perception of speed. Perception. 2003;32:85–96. doi: 10.1068/p3478. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H. An aging nation: The older population in the United States. 2014 Retrieved from http://www.census.gov/prod/2014pubs/p25-1140.pdf. [Google Scholar]
- Owsley C. Aging and vision. Vision Research. 2011;51:1610–1622. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Jr, Sloane ME, Roenker DL, White MF, Overlay ET. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;270:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Ramat S, Zee DS. Ocular motor responses to abrupt interaural head translations in normal humans. Journal of Neurophysiology. 2003;90:887–902. doi: 10.1152/jn.01121.2002. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. Journal of Physiology. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers B, Graham M. Motion parallax as an independent cue for depth perception. Perception. 1979;8:125–134. doi: 10.1068/p080125. [DOI] [PubMed] [Google Scholar]
- Sharpe JA, Sylvester TO. Effects of aging on horizontal smooth pursuit. Investigative Ophthalmology & Visual Science. 1978;17:465–468. [PubMed] [Google Scholar]
- Snowden RJ. Sensitivity to relative and absolute motion. Perception. 1992;21:563–568. doi: 10.1068/p210563. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Braddick OJ. The combination of motion signals over time. Vision Research. 1989;29:1621–1630. doi: 10.1016/0042-6989(89)90143-0. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Braddick OJ. Differences in the processing of short-range apparent motion at small and large displacements. Vision Research. 1990;30:1211–1222. doi: 10.1016/0042-6989(90)90176-l. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Sprenger A, Trillenberg P, Pohlmann J, Herold K, Lencer R, Helmchen C. The role of prediction and anticipation on age-related effects on smooth pursuit eye movements. Annals of the New York Academy of Sciences. 2011;1233:168–176. doi: 10.1111/j.1749-6632.2011.06114.x. [DOI] [PubMed] [Google Scholar]
- Stone LS, Krauzlis RJ. Shared motion signals for human perceptual decisions and oculomotor actions. Journal of Vision. 2003;3:725–736. doi: 10.1167/3.11.7. [DOI] [PubMed] [Google Scholar]
- Stroyan K. Motion parallax is asymptotic to binocular disparity. 2010 http://arxiv.org/abs/1010.0575. [Google Scholar]
- Stroyan K, Nawrot M. Visual depth from motion parallax and eye pursuit. Journal of Mathematical Biology. 2012;64:1157–1188. doi: 10.1007/s00285-011-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill GB, Levitt H. Sequential estimation of points on a psychometric function. The British Journal of Mathematical and Statistical Psychology. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Yu D, Cheung S, Legge GE, Chung STL. Reading speed in the peripheral visual field of older adults: Does it benefit from perceptual learning? Vision Research. 2010;50:860–869. doi: 10.1016/j.visres.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]