Abstract
We have developed a novel continuous flow-through cell separation method using a Percoll density gradient. This method can continuously separate a large number of cells into five fractions according to their densities. In order to apply this method to the separation of basophils, Percoll density gradients were modified to improve basophil enrichment. When a set of Percoll density gradients was prepared (1.071, 1.075, 1.080, 1.084, and 1.090 g Percoll/ml) the basophils in a healthy volunteer were enriched by an average of 23.1% and 63.5%, at Percoll densities of 1.075 g/ml (fraction 3) and 1.080 g/ml (fraction 4), respectively. On average, the yield of basophils was 1.66×105 cells in fraction 3 and 1.61×105 cells in fraction 4 from 9 ml of peripheral blood. The expression of CD203c (cluster of differentiation 203c) on separated basophils was up-regulated by anti-IgE stimulation similar to basophils in whole blood. Histamine release induced by calcium ionophore was also observed in the separated basophils. The present method will be useful for basophil enrichment since it preserves their function without using counterflow elutriation and immunological reagents, and this method will be effective as a preparative separation for cell purification by flow cytometry.
Keywords: Basophil, Cell separation, Centrifugation, Density gradient, Human peripheral blood
1 Introduction
Cell separation and purification are the most important steps in cell research. The cell separation by the density gradient centrifugation with Percoll or Ficoll has been performed for a long time, and is applied for hematopoietic stem cell preparation in these days [1-10]. However, there are some reports that Ficoll based density gradient centrifugation shows a low recovery of cells and gives damages to cells [11-14]. The present continuous flow-through cell separation method using a Percoll density gradient, we developed [15-18], harvested hematopoietic progenitor cells from peripheral blood without a loss of viability.
Recently, allergic disorders such as hay fever, atopic dermatitis, anaphylaxis and asthma are steadily increasing in the world. Many researchers on allergy analyze the functions of mast cells derived from cord blood, because it is hard to harvest matured mast cells present in human tissues [19, 20]. Since the morphology and the function of basophils, present in the peripheral blood, are similar to that of mast cells, basophils have been considered as one of the effector cells or surrogate mast cells playing a major role in allergic inflammatory reactions [21, 22]. However, basophils comprise less than 1% of the leukocyte population in human peripheral blood, which has obviously hampered research into their role in health and diseases. Then, the development of basophil-depleting antibodies and the creation of genetically modified mice that depleted basophils, have accumulated some evidence which suggest that basophils are major regulator cells or initiator cells of immune responses [23-27]. Therefore, recently the study of basophils has developed rapidly and its achievements have become a center of attraction.
Although methods for the purification of basophils with the specific antibody have been developed, the negatively selecting magnetic cell separation method for obtaining pure basohil preparations requires prior enrichment steps. These include erythrocyte sedimentation in hydroxyethyl starch, Ficoll or Percoll density gradient centrifugation and counter-current centrifugal elutriation that require skilled techniques, considerable patience and time. In addition the method suffers from the loss of basophils in the procedure of depleting erythrocytes and harvesting leukocytes. The batch density gradient method using Percoll can enrich basophils, which are viable, appear morphologically normal, and release histamine. However, the procedure of laying discontinuous gradients of Percoll is complicated and the recovery and purity of basophils is relatively low [28-32].
Therefore, we developed a novel continuous flow-through cell separation method using a Percoll density gradient that can continuously separate a large number of cells into five fractions according to their densities and automatically harvest separated cell populations from each gradient layer. This method facilitated the separation of hematopoietic progenitor cells from human buffy coat, cord blood and peripheral blood [16-18].
In the present study, we applied this method to basophil separation from peripheral blood in a single step without using counter-flow elutriation and immunological reagents.
2 Materials and methods
2.1 Materials
The following reagents and materials were obtained from the indicated suppliers: Percoll (GE Healthcare Bio-Science AB, Uppsala, Sweden); 10 × concentrated phosphate-buffered saline without Ca2+ (10 × PBS (−)), 0.5 M ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (BSA), May-Grunwald’s stain solution, Giemsa’s stain solution, and Türk’s reagent (Sigma-Aldrich, St. Louis, MO, USA); fetal bovine serum (FBS) (Nichirei Bioscience, Tokyo, Japan); penicillin (PC) and streptomycin (SM) (Meiji Seika, Tokyo, Japan); trypan blue and trisodium citrate dehydrate (Katayama Chemical, Osaka, Japan); RPMI-1640 (Nissui, Tokyo, Japan); D-glucose (Wako, Osaka, Japan); 3.8% sodium citrate (Fuso, Osaka, Japan); an allergenicity kit (Beckman Coulter, Marseille, France); and a histamine enzyme immunoassay kit (SPI-Bio, Montigny le Bretonneux, France). Trypan blue was prepared as a 0.3% trypan blue solution in PBS (−). Acid citrate dextrose (ACD) was prepared as 3.8% trisodium citrate in a 4.5% D-glucose solution.
2.2 Principle and procedure of the flow-through density gradient cell separation method
The apparatus for flow-through density gradient cell separation was designed in our laboratory according to the description in U.S. patent #4,425,112 [33] and was custom made by Pharma-Tech Research Corporation (Baltimore, MD, USA) and Kutsuwa Sangyo Corporation (Hiroshima, Japan).
The separation column (140-mm diameter) consists of a pair of plastic disks that are tightly sealed together. The lower disk has a circular groove 15 mm wide, 2 mm deep and of 8 ml capacity that forms the separation channel, and the upper disk has six inlet and six outlet tubes. The separation column was rotated at 1500 rpm (140 × g) and preservation of the density gradient was confirmed by stroboscopic observation of a set of density media that were each colored with a different dye (Fig. 1). The separation column is mounted on a seal-less continuous flow centrifuge [33-35] and 12 flow tubes exit the centrifuge with a non-twisting mechanism (Fig. 2).
Figure 1.
Stroboscopic photograph of the separation disk.
Percoll density media were differentially colored with various dyes and were pumped into the column.
Figure 2.
Cross-sectional view of the seal-less continuous flow centrifuge.
The motor rotates the gear box with the tube support through a toothed belt. The separation disk rotates at a speed (2ω) double that of the tube support, which allows the flow tubes to rotate without twisting.
A set of Percoll density media was pumped into inlets 2 through 6 and pumped out through the respective outlets at the same flow rates using a multichannel peristaltic pump (Watson-Marlow, Cornwall, UK) under a centrifugal force field, resulting in the natural formation of five circular layers. The layers were preserved throughout the separation channel according to their densities. It took approximately 30 min to form five gradient layers at a flow rate of 0.2 ml/min. The flow rate was then decreased to 0.1 ml/min and PBS (−) stock solution was pumped into the proximal inlet 1 at a flow rate of 0.3 ml/min using another pump. The six density gradient layers were maintained at a steady state for 15 min before cell separation (Fig. 3).
Figure 3.
Principle of the novel continuous flow-through cell separation method.
Sample cell suspension was continuously pumped into inlet 1. A set of Percoll density media was continuously pumped into inlets 2 through 6.
When diluted blood sample was continuously pumped into inlet 1, the cells present in the sample gradually migrated into the five gradient layers until they were suspended in media with the same density and were accumulated on a heavier layer before reaching the outlet of the channel. Consequently, the cells were continuously separated and harvested according to their densities. This separation system is schematically shown in Fig. 4.
Figure 4.
Overview of the novel continuous flow-through cell separation system during cell separation.
2.3 Preparation of a set of isotonic density media
Isotonic Percoll stock medium (1.1263 g/ml, 290 mOsm/l) was prepared under aseptic conditions by adding a 10 × concentrated phosphate-buffered saline solution (PBS (−)) (3014 mOsm/l, pH 7.4) to Percoll (1.132 g/ml) containing BSA, EDTA, PC and SM. The isotonic Percoll stock medium was diluted under aseptic conditions with a PBS (−) stock solution (1.0075 g/ml, 290 mOsm/l) containing BSA, EDTA, PC and SM in order to prepare medium at each density (Table 1). An isotonic arbitrary density medium of Percoll was prepared by mixing two of the above stock solutions. Two sets of isotonic density media for basophil separation were prepared by mixing the appropriate volume of the Percoll stock solution with PBS (−) stock solution to give a final density of 1.070, 1.075, 1.080, 1.085 and 1.090 g/ml and 1.071, 1.075, 1.080, 1.084 and 1.090 g/ml (Table 2). The Percoll density media were kept at 4 °C to prevent the propagation of bacteria and were brought back to room temperature 1 h before the separation and mixed well.
Table 1.
Composition of the isotonic Percoll stock medium and the PBS (−) stock solution
| Isotonic Percoll stock medium (1.1263 g/mL, 290 mOsm/L) |
Volume (mL) |
|---|---|
| Percoll (1.132 g/mL, max 25 mOsm/L) | 135.47 |
| PBS (×10) (1.074 g/mL, 3014 mOsm/L) | 13.3 |
| 20% BSA/PBS(−) (1.060 g/mL, 259 mOsm/L) | 0.75 |
| 0.5 M EDTA (1.105 g/mL, 1500 mOsm/L) | 0.3 |
| SM (0.5 g/mL, 870 mOsm/L) | 0.03 |
| PC (100,000 units/mL) | 0.15 |
|
| |
| Total | 150 |
| PBS(−) stock solution (1.0075 g/mL, 290 mOsmL) |
Volume (mL) |
|---|---|
| Distilled water (1.00 g/mL, 0 mOsm/L) | 134.57 |
| PBS (×10) (1.074 g/mL, 3014 mOsm/L) | 14.2 |
| 20% BSA/PBS(−) (1.060 g/mL, 259 mOsm/L) | 0.75 |
| 0.5 M EDTA (1.105 g/mL, 1500 mOsm/L) | 0.3 |
| SM (0.5 g/mL, 870 mOsm/L) | 0.03 |
| PC (100,000 units/mL) | 0.15 |
|
| |
| Total | 150 |
Table 2.
Example of the preparation of each density gradient medium
| Density (D g/mL)a) | 1.070 | 1.075 | 1.080 | 1.085 | 1.090 |
|---|---|---|---|---|---|
| Isotonic Percoll stock medium | |||||
| 1.126 g/mL, 290 mOsm/mL (A mL)a) |
24.0 | 26.0 | 30.0 | 32.0 | 34.0 |
| PBS(−) stock solution | |||||
| 1.007 g/mL, 290 mOsm/L (B mL)a) |
21.48 | 19.76 | 19.16 | 16.94 | 14.96 |
| Density (D g/mL)a) | 1.071 | 1.075 | 1.080 | 1.084 | 1.090 |
|---|---|---|---|---|---|
| Isotonic Percoll stock medium | |||||
| 1.126 g/mL, 290 mOsm/mL (A mL)a) |
24.0 | 26.0 | 30.0 | 32.0 | 34.0 |
| PBS(−) stock solution | |||||
| 1.007 g/mL, 290 mOsm/L (B mL)a) |
20.90 | 19.76 | 19.16 | 17.69 | 14.96 |
Each density medium was prepared in a 50-mL conical tube. The volume of PBS (−) stock solution (B mL) was added to the isotonic Percoll stock medium (A mL) to prepare a desired density solution (D g/mL). That was calculated according to the following formula:
The osmolarity of the set of density media was 288 ± 5 mOsm/l measured using Osmostat OM-6040 (Arkray, Kyoto, Japan).
2.4 Separation of basophils from peripheral blood
Informed consent was obtained from the volunteers after the nature and possible consequences of the present study had been fully explained.
Peripheral blood (11 ml in total) was collected several times each day from a healthy volunteer and from a volunteer with atopic dermatitis. Prior to separation, the anti-coagulant EDTA was added to 2 ml of this sample for leukocyte counting and for measurement of the hematocrit value. The anti-coagulant 3.8% sodium citrate (1 ml) was added to the remaining 9 ml of blood, which was then used for cell separation.
After the steady state of the six density gradient layers was maintained for 15 min, the blood sample was diluted to 20% of the hematocrit value using approximately 10 ml of ACD solution, and was pumped into the proximal inlet 1 at 0.3 ml/min for about 70 min. The supernatant of the sample was pumped out from outlet 1 at a reduced flow rate of 0.24 ml/min due to the change in volume as a result of cell removal.
Outlet 6 was opened to the air to collect excess erythrocytes and to regulate the pressure in the channel (Fig. 4).
Each fraction was collected in 4 ml of ACD solution and was washed by centrifugation at 360 × g for 20 min at room temperature to remove Percoll and then at 120 × g for 10 min with 10% FBS·RPMI-1640 at 4°C.
2.5 Cell counts
Total leukocytes counts were assessed using Türk’s staining and cell viability was assessed by trypan blue dye exclusion. Differential leukocyte counts and basophil purity were determined by microscopic observation of May-Giemsa double-stained cytospin smears. Six hundred leukocytes were counted on the smear before separation, and two hundred leukocytes were counted on each fractionated smear.
2.6 Measurement of basophil activation following anti-IgE stimulation
An allergenicity kit, based on a triple staining protocol, was used for detection of the surface expression of an activation marker (CD203c) on basophils [36].
The concentration of cells in each fraction was adjusted to between 4 × 105 cells/ml to 1 × 107 cells/ml. Each fraction (0.1 ml) was mixed with PBS (−) as a negative control and anti-IgE as a positive control. An antibody mixture, CRTH2-FITC/CD203c-PE/CD3-PC7, was added to each specimen and incubated for 15 min at 37°C in a water bath, protected from light. Each specimen was then fixed during erythrolysis for flow cytometric analysis using FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA).
2.7 Histamine release induced by calcium ionophore
A histamine enzyme immunoassay kit was used for the quantification of histamine. After cell separation, the concentration of leukocytes in fractions 3 (density, 1.075 g/ml) and 4 (density, 1.080 g/ml) was adjusted to 5 × 105 cells/ml. An aliquot (0.1 ml) of the cells was then suspended in 10% FBS·RPMI-1640 containing calcium (400 μl), and was pre-incubated for 20 min at 37°C. Next, 10% FBS·RPMI-1640 (100 μl) or 25 μM calcium ionophore A23187 (100 μl) was added and the cells were incubated for a further 20 min at 37°C. All tubes were allowed to stand on ice for 5 min and were then centrifuged (1000 × g for 5 min at 4°C). Each supernatant was collected and frozen at −30°C until assay. The cell pellets were resuspended in 10% FBS·RPMI-1640 (500 μl), and were lysed by sonication and freeze-thawing. After centrifugation (1000 × g for 5 min at 4°C), each cell lysate was collected and frozen at −30°C until assay.
The cell supernatants and cell lysates were diluted based on the basophil count of the cell fraction to a dilution that corresponded to 1 × 103 basophils, and were then assayed using the histamine enzyme immunoassay kit.
The percentage of histamine release was determined from the total histamine content in the supernatant and the cell lysate. The net histamine release was calculated by subtracting the histamine remaining in the cell lysate.
3 Results
3.1 Separation of basophils from peripheral blood
Peripheral blood from a healthy volunteer and a volunteer with atopic dermatitis were separated using a set of Percoll isotonic density gradient media (1.070, 1.075, 1.080, 1.085 and 1.090 g /ml). Lymphocytes were concentrated in fraction 2 (density, 1.070 g/ml), which comprised 96.4% and 97.8%, respectively, of the cells in fraction 2 together with some monocytes and platelets. Neutrophils were in fraction 6 (density, 1.090 g/ml), which comprised 92.0% and 82.4%, respectively, of the cells in fraction 6 together with some eosinophils and a large number of erythrocytes. A higher number of neutrophils and eosinophils were present both in the peripheral blood and in fraction 4 of the volunteer with atopic dermatitis than in that of the healthy volunteer. Basophils were enriched in fractions 3 (density, 1.075 g/ml) and 4 (density, 1.080 g/ml) and were mainly mixed with lymphocytes (Figs. 5, 6 and Table 3).
Figure 5.
Differential leukocyte counts of cells separated from a healthy volunteer.
Basophils were concentrated in fractions 3 and 4, which included fewer cells than fractions 2 and 6.
Figure 6.
Differential leukocyte counts of cells separated from a patient with atopic dermatitis.
Some neutrophils and eosinophils were included in fraction 4.
Table 3.
Comparison of basophils separated using two sets of density media from a healthy volunteer and from a patient with atopic dermatitis
| Before separation Peripheral blood (9 mL) |
Enriched basophils (×105 cells) |
Purity of basophils (%) |
Total cell recovery (%) |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Healthy volunteer |
Leukocytes (×106 cells) |
Basophils (×105 cells) |
Fraction 3 1.075 g/mL |
Fraction 4 1.080 g/mL |
Fraction 3 1.075 g/mL |
Fraction 4 1.080 g/mL |
|
| 1sta) | 48.8 | 5.9 | 0.43 | 0.66 | 16.5 | 62.8 | 70.4 |
|
| |||||||
| 2ndb) | 61.5 | 4.3 | 3.20 | 1.32 | 51.0 | 65.0 | 66.0 |
| 3rdb) | 42.6 | 3.4 | 0.89 | 2.00 | 12.0 | 71.5 | 55.9 |
| 4thb) | 59.7 | 6.0 | 0.90 | 1.52 | 6.3 | 53.9 | 60.0 |
| Before separation Peripheral blood (9 mL) |
Enriched basophils (×105 cells) |
Purity of basophils (%) |
Total cell recovery (%) |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Atopic dermatitis patient |
Leukocytes (×106 cells) |
Basophils (×105 cells) |
Fraction 3 1.075 g/mL |
Fraction 4 1.080 g/mL |
Fraction 3 1.075 g/mL |
Fraction 4 1.080 g/mL |
|
| 1sta) | 70.3 | 12.0 | 0.97 | 1.74 | 23.8 | 50.8 | 75.6 |
|
| |||||||
| 2ndb) | 74.7 | 5.2 | 3.06 | 2.62 | 22.0 | 57.5 | 76.8 |
A set of density gradient media of densities of 1.070, 1.075, 1.080, 1.085 and 1.090 g/mL was used.
A set of density gradient media of densities of 1.071, 1.075, 1.080, 1.084 and 1.090 g/mL was used.
Using a set of modified density gradient media with densities of 1.071, 1.075, 1.080, 1.084 and 1.090 g/ml, peripheral blood from the same healthy volunteer was separated three more times. Basophils were enriched in fraction 3 (density, 1.075 g/ml) and fraction 4 (density, 1.080 g/ml), which represented, on average, 23.1% and 63.5%, respectively, of the total cells collected in the fraction (Table 3). A differential leukocyte count of the blood of the atopic dermatitis volunteer is shown in Fig. 7. Many eosinophils and neutrophils were excluded from fraction 4. Cell viabilities, measured by trypan blue dye exclusion, were greater than 96% in all fractions.
Figure 7.
Differential leukocyte counts of cells separated from a patient with atopic dermatitis using a modified density gradient.
The number of neutrophils and eosinophils was decreased in fraction 4.
Basophils that were separated into fractions 3 and 4 had characteristic dark granules in the cytoplasm that overlaid the nucleus. A higher number of erythrocytes were found in heavier fractions (Fig. 8).
Figure 8.
Separated basophils stained with May-Giemsa double staining.
(A) fraction 3 (density = 1.075 g/ml): purity of basophils was 12.0%, (B) fraction 4 (density = 1.080 g/ml): purity of basophils was 71.5%.
3.2 Identification of basophils using an allergenicity kit
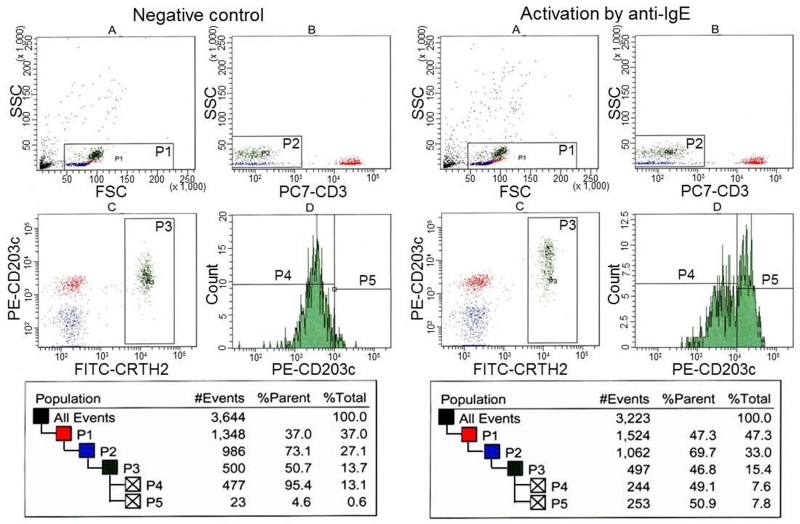
Figure 9 shows the gating method used to identify 500 basophils in the peripheral blood by flow cytometry before cell separation. Histogram A of control and anti-IgE-activated samples displays all events. Region P1 included all leukocytes, while excluding a large amount of debris. Histogram B displays events from region P1. Region P2 was adjusted to include basophils, monocytes and B-lymphocytes, while excluding a large number of granulocytes and CD3-positive T-lymphocytes. Histogram C displays events from region P2. Region P3 was adjusted to include CRTH2-positive, CD203c-positive, and CD3-negative cells (i.e., basophils). Histogram D shows cell counts from region P3. Region P4 included 95% of the basophils and defined the region of non-activated basophils. Basophils accounted for 0.83% of the cells in this sample, which was calculated from cell counts in region P3 and region P1.
Figure 9.
Detection of basophils in whole blood of a healthy volunteer by flow cytometry.
Basophils were included in the region “P3”. Basophils activated by anti-IgE were present in region 5.
The histograms on the right show that the CD203c antigen was up-regulated after activation by anti-IgE. Region P5 defined the activated basophils and indicated that 48.8% of the basophils were activated.
When fraction 3 was analyzed (Fig. 10), erythrocytes and granulocytes were not detected in histograms A and B, since a large majority of these cells were already removed and, in histogram C, the basophils expressed CD203c antigens similar to the basophils in peripheral blood. After activation by anti-IgE, CD203c was up-regulated in 50.9% of the basophils, as shown in region P5.
Figure 10.
Detection of basophils separated in fraction 3 from a healthy volunteer by flow cytometry.
3.3 Histamine release by calcium ionophore stimulation
Histamine release was measured based on 1 × 103 basophils. Histamine was spontaneously released from 14.6% and 12.9% of the basophils in fractions 3 and 4, respectively. After calcium ionophore stimulation, histamine was released from 90.5% and 89.3% of the basophils in fractions 3 and 4, respectively (Fig. 11). The total histamine levels determined using 103 basophils from fractions 3 and 4 were approximately 1.23 ± 0.17 pg/basophil.
Figure 11.
Histamine release induced by the Calcium ionophore A23187.
Histamine released from 103 basophils in fractions 3 and 4 was measured by enzyme immunoassay.
4 Discussion
The original cell separation apparatus, which had a separation channel with a capacity of 160- ml, was developed for separating a large quantity of cells for transfusion medicine [16]. The present apparatus, which has a miniature separation channel of 8 ml, was newly manufactured for separating various cells at many facilities in the future [15, 17, 18].
Blood was diluted with ACD solution to a hematocrit value of 20% before separation. As a result, the blood volume almost doubled and the density decreased to approximately 1.03 g/ml. It took about 70 min to apply 20 ml of the diluted blood into the separation column using a flow rate of 0.3 ml/min. It then took about 15 min for the cells to flow-through the cell separation channels, and more 20 min to complete the harvesting the eluted cells in each fraction. The volume of the density gradient media indicated in Table 2 is sufficient for two complete separations.
In order to observe small changes in separated cell populations induced by small alterations in the density gradient, peripheral blood derived from the same subject was aliquoted and each aliquot was individually separated in order to cancel out the influence of differences between individuals. To confirm the distribution of the basophils, the density gradient media were first prepared at a density starting from 1.070 g/ml and increasing by 0.005 g/ml increments up to 1.090 g/ml. Blood samples from a healthy volunteer and a volunteer with atopic dermatitis were then separated. The separated cells were washed 3 times, and then cell viability evaluation, cell counting and cytospin preparations were carried out. Washing induced a greater loss of cells in the fractions with fewer cells (Table 3).
The density gradient was then modified to improve enrichment of the basophils in fractions 3 and 4. The density of inlet 2 was increased from 1.070 g/ml to 1.071 g/ml to reduce the number of lymphocytes migrating into fraction 3. The density of inlet 5 was reduced from 1.085 g/ml to 1.084 g/ml to increase the number of neutrophils migrating into fraction 5. As expected, the neutrophils and eosinophils in fraction 4 tended to migrate to the heavier fraction 5 and the purity of the basophils in fraction 4 remained stable at about 60%. However, the purity of the basophils in fraction 3 did not increase, since large amounts of lymphocytes were originally present in the adjacent fraction 2 (Figs. 6, 7, Table 3). By using this modified Percoll gradient, this single step method resulted in a good yield of basophils of, on average, 1.6 × 105 cells in fraction 4 from 9 ml of peripheral blood. This procedure also allowed enrichment of basophils in a single step, which provides an advantage over previous two-step methods [28-32, 37, 38]. The present cell separation apparatus, and the utilization of isotonic Percoll density gradient media, would result in a better yield than that achieved by other techniques by decreasing the loss of cells at harvesting and by causing less damage to the cells. The use of isotonic Percoll density gradient media with antibiotics would make it possible to separate various kinds of cells under aseptic conditions for further research.
Following two washes, the separated cells were immediately applied to tests of function such as CD203c expression and/or histamine release. The analysis of CD203c expression by flow cytometry indicated that IgE receptors (FcεR1) on the enriched basophils were well preserved, since the CD203c antigen was up-regulated after activation by anti-IgE similar to the intact basophils in peripheral blood (Figs. 9, 10).
Since the remaining CD3-positive cells in fractions 3 and 4 were CRTH2-negative (data not shown), CRTH2-positive cell populations in fractions 3 and 4 can be easily defined as basophils without CD3 staining. Furthermore, the enriched basophils populations in fractions 3 and 4 could be gated in the histogram on the basis of side scatter characteristics (SSC) and front scatter characteristics (FSC) without specific antibodies (Fig. 10A), as previously demonstrated [28].
Fractions 3 and 4 contained no eosinophils, which are known to neutralize histamine by releasing histaminase and which, if present, could confuse interpretation of histamine release from basophils. Histamine release was directly analyzed using fractions 3 and 4 without any pretreatment. The levels of histamine released and the total histamine levels determined using 103 basophils from fractions 3 and 4 (approximately 1.23 ± 0.17 pg/basophil) were consistent with previously reported values [29]. These results suggested that the separated basophils preserved their ability to release histamine following calcium influx and that the present method could remove cells that interfere with the histamine release test.
The present method does not require specific antibodies or special skills. Furthermore, in the case of cells isolated from tissues instead of blood cells, the lack of erythrocytes means that the cell preparation would not need to be diluted, the sample application time would be faster due to the low volume and density of the sample, and it would not be necessary to consider the change in volume as a result of cell removal. Outlet 1 should be opened to the air instead of outlet 6 to collect the supernatant of the sample [15, 16].
5 Concluding remarks
Flow cytometry, which employs specific antibodies and a laser beam, is a popular method for purifying target cells, but needs a preparative separation for efficient purification. The present method does not need specific antibodies or any special skill such as that of making Percoll layers. The single step cell separation in the present method enriched basophils to the same level as the two step enrichment obtained by using the batch density gradient method and counterflow elutriation. The present method would be suitable as a preparative cell separation method for various cells by using various combinations of Percoll density gradients.
Acknowledgements
The authors would like to thank Mr. Makoto Naruse in Central Research Laboratories for his contribution towards flow cytometric analysis.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- PBS(−)
phosphate-buffered saline without Ca2+
- PC
penicillin
- SM
streptomycin
- ACD
acid citrate dextrose
- AD
atopic dermatitis
- CD
cluster of differentiation
- CRTH2
chemoattractant receptorhomologous molecule expressed on Th2 cells
- FITC
fluorescein isothiocyanate
- PE
phycoerithrin
- PC7
phycoerithrin-cyanin 7
Footnotes
The authors have declared no conflict of interest.
Other Techniques
References
- [1].Ellis WM, Georgiou GM, Roberton DM, Johnson GR. The use of discontinuous Percoll gradients to separate populations of cells from human bone marrow and peripheral blood. J. Immunol. Methods. 1984;66:9–16. doi: 10.1016/0022-1759(84)90242-4. [DOI] [PubMed] [Google Scholar]
- [2].Vincent R, Nadeau D. Adjustment of the osmolality of Percoll for the isopycnic separation of cells and cell organelles. Anal. Biochem. 1984;141:322–328. doi: 10.1016/0003-2697(84)90049-6. [DOI] [PubMed] [Google Scholar]
- [3].Yurasov SV, Flasshove M, Rafii S, Moore MA. Density enrichment and characterization of hematopoietic progenitors and stem cells from umbilical cord blood. Bone Marrow Transplant. 1996;17:517–525. [PubMed] [Google Scholar]
- [4].Regidor C, Posada M, Monteagudo D, Garaulet C, Somolinos N, Forés R, Briz M, Fernández MN. Umbilical cord blood banking for unrelated transplantation: evaluation of cell separation and storage methods. Exp. Hematol. 1999;27:380–385. doi: 10.1016/s0301-472x(98)00016-2. [DOI] [PubMed] [Google Scholar]
- [5].Pertoft H. Fractionation of cells and subcellular particles with Percoll. J. Biochem. Biophys. Methods. 2000;44:1–30. doi: 10.1016/s0165-022x(00)00066-x. [DOI] [PubMed] [Google Scholar]
- [6].Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol. Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- [7].Florian HS, Torsten T, Nicola K, Andreas MZ, Stefanie D. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur. Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- [8].Birgit A, Torsten T, Florian HS, Chang-Hwan Y, David L, Jens K, Volker S, Erhard S, Andreas MZ, Stefanie D. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J. Am. Coll. Cardiol. 2010;55:1385–1394. doi: 10.1016/j.jacc.2009.10.059. [DOI] [PubMed] [Google Scholar]
- [9].Nikodemova M, Watters JJ. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J. Neuroinflammation. doi: 10.1186/1742-2094-9-147. DOI: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pösel C, Möller K, Fröhlich W, Schulz I, Boltze J, Wagner DC. Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One. 2012;7:e50293. doi: 10.1371/journal.pone.0050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van Beem RT, Hirsch A, Lommerse IM, Zwaginga JJ, Noort WA, Biemond BJ, Piek JJ, van der Schoot CE, Voermans C. Recovery and functional activity of mononuclear bone marrow and peripheral blood cells after different cell isolation protocols used in clinical trials for cell therapy after acute myocardial infarction. EuroIntervention. 2008;4:133–138. doi: 10.4244/eijv4i1a21. [DOI] [PubMed] [Google Scholar]
- [12].Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10:203–211. doi: 10.1080/14653240701851324. [DOI] [PubMed] [Google Scholar]
- [13].Nieto JC, Cantó E, Zamora C, Ortiz MA, Juárez C, Vidal S. Selective loss of chemokine receptor expression on leukocytes after cell isolation. PLoS One. 2012;7:e31297. doi: 10.1371/journal.pone.0031297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Naranbhai V, Bartman P, Ndlovu D, Ramkalawon P, Ndung’u T, Wilson D, Altfeld M, Carr WH. Impact of blood processing variations on natural killer cell frequency, activation, chemokine receptor expression and function. J. Immunol. Methods. 2011;366:28–35. doi: 10.1016/j.jim.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shiono H, Okada T, Ito Y. Application of a novel continuous-flow cell separation method for separation of cultured human mast cells. J. Liq. Chromatogr. Rel. Technol. 2005;28:2071–2083. [Google Scholar]
- [16].Ito Y, Shinomiya K. A new continuous-flow cell separation method based on cell density: principle, apparatus, and preliminary application to separation of human buffy coat. J. Clin. Apheresis. 2001;16:186–191. doi: 10.1002/jca.1032. [DOI] [PubMed] [Google Scholar]
- [17].Shiono H, Ito Y. Novel method for continuous cell separation by density gradient centrifugation: evaluation of a miniature separation column. Prep. Biochem. Biotechnol. 2003;33:87–100. doi: 10.1081/PB-120021434. [DOI] [PubMed] [Google Scholar]
- [18].Shiono H, Chen HM, Okada T, Ito Y. Colony-forming cell assay for human hematopoietic progenitor cells harvested by a novel continuous-flow cell separation method. J. Chromatogr. A. 2007;1151:153–157. doi: 10.1016/j.chroma.2007.01.021. [DOI] [PubMed] [Google Scholar]
- [19].Amano H, Kurosawa M, Ishikawa O, Chihara J, Miyachi Y. Cultured human mast cells derived from umbilical cord blood cells in the presence of stem cell factor and interleukin-6 cannot be a model of human skin mast cells: fluorescence microscopic analysis of intracellular calcium ion mobilization. J. Dermatol. Sci. 2000;24:146–152. doi: 10.1016/s0923-1811(00)00121-3. [DOI] [PubMed] [Google Scholar]
- [20].Shiohara M, Koike K. Regulation of mast cell development. Chem. Immunol. Allergy. 2005;87:1–21. doi: 10.1159/000087566. [DOI] [PubMed] [Google Scholar]
- [21].Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood. 2000;96:4028–4038. [PubMed] [Google Scholar]
- [22].Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp. Dermatol. 2006;15:855–864. doi: 10.1111/j.1600-0625.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- [23].Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- [24].Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat. Rev. Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- [26].Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kepley C, Craig S, Schwartz L. Purification of human basophils by density and size alone. J. Immunol. Methods. 1994;175:1–9. doi: 10.1016/0022-1759(94)90326-3. [DOI] [PubMed] [Google Scholar]
- [29].Gibbs BF, Noll T, Falcone FH, Hass H, Vollmer E, Vollrath I, Wolff HH, Amon U. A three-step procedure for the purification of human basophils from buffy coat blood. Inflamm. Res. 1997;46:137–142. doi: 10.1007/s000110050537. [DOI] [PubMed] [Google Scholar]
- [30].Haisch K, Gibbs BF, Körber H, Ernst M, Grage-Griebenow E, Schlaak M, Haas H. Purification of morphologically and functionally intact human basophils to near homogeneity. J. Immunol. Methods. 1999;226:129–137. doi: 10.1016/s0022-1759(99)00059-9. [DOI] [PubMed] [Google Scholar]
- [31].Tsang S, Hayashi M, Zheng X, Campbell A, Schellenberg RR. Simplified purification of human basophils. J. Immunol. Methods. 2000;233:13–20. doi: 10.1016/s0022-1759(99)00182-9. [DOI] [PubMed] [Google Scholar]
- [32].Gibbs BF, Papenfuss K, Falcone FH. A rapid two-step procedure for the purification of human peripheral blood basophils to near homogeneity. Clin. Exp. Allergy. 2008;38:480–485. doi: 10.1111/j.1365-2222.2007.02919.x. [DOI] [PubMed] [Google Scholar]
- [33].Ito Y. Flow-through centrifuge free of rotating seals particularly for plasmapheresis. 1984 US patent 4,425,112. publ. date January 10.
- [34].Ito Y, Suaudeau J, Bowman RL. New flow-through centrifuge without rotating seals applied to plasmapheresis. Science. 1975;189:999–1000. doi: 10.1126/science.1220011. [DOI] [PubMed] [Google Scholar]
- [35].Ito Y. In: Apheresis: Principles and Practice. McLeod BC, Price TH, Drew MJ, editors. AABB Press; Bethesda, MD: 1997. pp. 9–13. [Google Scholar]
- [36].Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin. Mol. Allergy. 2005;3:9–16. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Miroli AA, James BM, Spitz M. Single step enrichment of human peripheral blood basophils by Ficoll-Paque centrifugation. J. Immunol. Methods. 1986;88:91–96. doi: 10.1016/0022-1759(86)90055-4. [DOI] [PubMed] [Google Scholar]
- [38].Raghuprasad PK. A rapid simple method of basophil purification by density centrifugation on Percoll. J. Immunol. 1982;129:2128–2133. [PubMed] [Google Scholar]