Abstract
PURPOSE
The aim of the study was to determine if increasing post-therapy calcification in peritoneal metastases in recurrent low-grade serous ovarian carcinomas indicated response to therapy.
MATERIALS AND METHODS
Retrospective analysis of patients with histologically-confirmed, recurrent low-grade serous ovarian carcinoma who received treatment at our institution between 2000 and 2014 was performed. Only patients who had calcified tumor implants and showed either interval increase or decrease in tumor calcification following therapy were included in the study. Pre- and post-therapy CT scans of these patients were reviewed by 2 radiologists independently. Changes in the tumor calcification status and tumor deposits size were correlated with serum CA-125 levels. Fisher's exact test was used to assess the association between peritoneal deposit and calcification status with serum CA-125 status.
RESULTS
35 patients were included in the study. Based on serial serum CA 125 levels, 22 patients (63%) had progressive disease, 12 (34%) had partial response and 1 (3%) had stable disease. Using RECIST 1.1, 16 had progressive disease, 3 had partial response and 16 had stable disease. In the patients with progressive disease, post therapy tumor calcification increased in 77% and decreased in 23%. Fischer's exact test showed that serum CA 125 change was significantly associated with change in size of peritoneal deposits and calcification change.
CONCLUSIONS
This preliminary study shows that post-therapy increase in peritoneal implant calcification in low-grade serous ovarian carcinomas is not an indicator of response to therapy.
Introduction
Post therapy tumor calcification may be seen in a wide variety of malignancies [1-7]. In certain malignancies such as lymphoma, colorectal cancers and glioblastoma, tumoral calcification following treatment is considered to represent a sign of response to therapy and is associated with a better prognosis [8-12]. However, in several other tumors, including malignant epithelial ovarian tumors, the significance of post-therapy calcification is unclear.
Among the malignant epithelial ovarian neoplasms, post-treatment tumor calcification is often seen in low-grade serous papillary tumors [13-18]. The histopathological basis of such post-therapy calcification in ovarian malignancies is poorly understood. In particular, it is unclear if calcification following therapy in low-grade serous ovarian cancers is synonymous with response to therapy or progression. However, this is an extremely important question to answer as this directly impacts management.
The aim of the study was to determine if increasing post-therapy calcification in peritoneal metastases in recurrent low-grade serous ovarian carcinomas indicated response to therapy.
Materials and Methods
Patients
This retrospective study was approved by our hospital Institutional Review Board. We retrospectively reviewed the clinical, radiological and pathological records of patients with recurrent low-grade serous ovarian carcinomas treated in our institution from 2000 to 2014. As a tertiary referral cancer center, the majority of the low grade serous ovarian carcinoma patients who present to our institution have recurrent tumor following primary surgery in outside institutions.
Since the aim of the study was to assess the significance of increasing post-therapy calcification as a predictor of tumor response, only those patients who had calcified tumor implants on the CT scan and showed interval increase or decrease in tumor calcification in the CT scan 3 months following therapy were included in the study. 35 patients who satisfied the inclusion criteria were identified and included in the study.
CT Protocol
All the patients underwent oral and intravenous contrast enhanced CT abdomen and pelvis before receiving treatment (chemotherapy or hormonal therapy) and follow-up CT was performed after 3 months of therapy. All patients were instructed to consume 840 ml of oral contrast, which was made up of 40 ml of iohexol 350 (Omnipaque 350; GE healthcare) diluted in 800 ml of water. Patients were encouraged to drink it over 90 minutes. A volume of 100 mL of intravenous contrast agent ioversol (Optiray 320, Maliinckrodt Inc.) was injected manually at a rate of 3 mL/s. After a 30 second scan delay, Lightspeed 64-slice or 16-slice CT scanners (GE Medical Systems, Milwaukee, WI) were used to acquire the contrast-enhanced images craniocaudally with thin collimation (0.625 mm), 120 kVp, and automatic modulation of the milliamperes. Both 5 and 2.5 mm axial images, as well as 2.5×2.5 mm coronal and sagittal reformatted images, were reviewed using our Picture Archiving Communications system (iSite: Philips Medical Systems).
Image analysis
The baseline CT and post-therapy follow-up CT were retrospectively reviewed by two radiologists (RI and PB, who were blinded to the results of CA-125 levels and other clinical details) independently; Any discordance or discrepancy in radiologic findings was mediated by a third radiologist. Tumor response in the non-calcified peritoneal deposits was independently evaluated using response evaluation criteria in solid tumors (RECIST) 1.1 criteria. Tumoral calcification was classified as punctate, amorphous or linear (when more than one type of calcification was present, the dominant pattern of calcification was recorded) (Fig. 1A, 1B, 1C). The radiologists also documented if there had been increase or decrease in the size of calcification in the calcified peritoneal deposits on the post-therapy scan (increase of 5 mm or more was defined as increasing calcification, and a decrease of 5 mm or more was defined as decreasing calcification) (Fig 2). We used a 5 mm cut off for change in tumor calcification status as there are no current standards for evaluating this parameter and using a 5 mm change in size was felt to remove any ambiguity in deciding if there was truly an increase or decrease in post treatment tumoral calcification. Development of any new areas of calcification in a previously non-calcified deposit was also categorized as “increasing calcification” (Fig. 3).
FIG 1A.
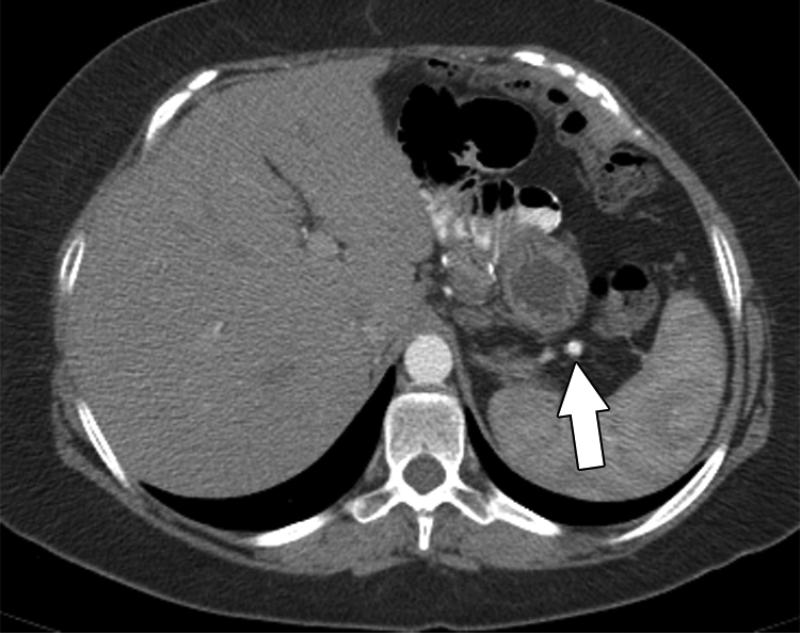
63-year-old female with low-grade serous ovarian cancer. Non-contrast axial CT scan of the abdomen shows peritoneal implants with amorphous type calcification (arrow) within the fissure for the ligament teres.
FIG 1B.
54-year-old female with low-grade serous ovarian cancer. Axial intravenous contrast enhanced CT scan of the abdomen shows peritoneal implants with linear type calcification (arrow).
FIG 1C.
49-year-old female with low-grade serous ovarian cancer. Non-contrast axial CT scan of the abdomen shows peritoneal implants with punctate type calcification (arrow).
Serum CA-125 levels (obtained within 2 weeks of CT scan) were recorded both before and after 3 months of therapy in all patients. The CT findings were compared with serial changes in serum CA-125 levels. Changes in the serum CA-125 levels were categorized as a response according to the Gynecologic Cancer Intergroup serum CA 125 criteria [19,20].
The change in status of serum CA-125, peritoneal deposit, and calcification of patients were summarized using frequencies and percentages. Cochran-Mantel-Haenszel test was used to assess the association between calcification and serum CA-125 status stratified by peritoneal deposit status. Fisher's exact test was used to assess the association between peritoneal deposit and calcification status with serum CA-125 status. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9 (SAS Institute, Cary, NC).
Results
The mean age of the patients in the study was 48 (range 17-80 years; median age 59 years). None of the patients had hypercalcemia (mean 9.2 mg/ dl; range 8-10.1) at baseline or during follow-up. Calcified peritoneal implants were seen in all patients. Amorphous pattern calcification was the predominant type of calcification and was seen in 27 patients; 4 patients had punctate calcification, and 3 patients had linear calcification.
Of the 35 patients in the study group, 22 (63%) had progressive disease based on serum CA 125 levels. Out of these 22 patients, 16 showed progressive disease and 6 had stable disease based on RECIST 1.1; Among the 22 patients with progressive disease, post therapy tumor calcification increased in 17 patients (77%) and decreased in 5 patients (23%).
12 (34%) out of the 35 patients were deemed to have response based on > 50% decrease in serum CA-125 levels; of these 12 patients, only 2 demonstrated partial response based on RECIST 1.1 criteria, whilst the other 10 demonstrated stable disease. Among the 12 patients with serum CA 125 response, post-therapy calcification increased in 5 patients (42%) and decreased in 7 patients (58%).
Statistical Analysis
Serum CA125 change was significantly associated with change in size of peritoneal deposits and calcification change (defined as change in size of calcified deposit by 5 mm or more). When serum CA125 increased, the peritoneal deposits and the tumor calcifications increased (Table 1). Association between tumor calcification and serum CA125 stratified by peritoneal status was performed using Cochran-Mantel-Haenszel test. This test did not show any significant overall association between tumor calcification and serum CA125, when stratified by peritoneal status (Table 2).
Table 1.
Summary of peritoneal deposit and calcification status by serum CA125 status. P-values by Fisher's exact test.
| Serum CA125 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| decreased | increased | stable | |||||
| N | % | N | % | N | % | ||
| Peritoneal deposits | |||||||
| decreased | 2 | 16.67 | 0 | 0 | 1 | 100.00 | <0.0001 |
| increased | 0 | 0 | 16 | 72.73 | 0 | 0 | |
| stable | 10 | 83.33 | 6 | 27.27 | 0 | 0 | |
| Calcifications | |||||||
| decreased | 7 | 58.33 | 5 | 22.73 | 1 | 100.00 | 0.04 |
| increased | 5 | 41.67 | 17 | 77.27 | 0 | 0 | |
Table 2.
Summary of calcification and CA125 stratified by peritoneal status. The overall association between calcification and CA125 adjusted for peritoneal status was not significant (p-value = 0.19 by Cochran-Mantel-Haenszel test).
| Peritoneal Deposits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| decreased | increased | stable | ||||||||
| CA125 | CA125 | CA125 | ||||||||
| decreased | stable | increased | decreased | increased | ||||||
| N | % | N | % | N | % | N | % | N | % | |
| Calcifications | ||||||||||
| decreased | 2 | 100.00 | 1 | 100.00 | 0 | 0 | 5 | 50.00 | 5 | 83.33 |
| increased | 0 | 0 | 0 | 0 | 16 | 100.00 | 5 | 50.00 | 1 | 16.67 |
Discussion
Tumor calcification in metastatic ovarian implants is not uncommon, with reported incidence on CT ranging from 8 to 16% [21,22,15]. Among different subtypes of ovarian cancer, calcified metastasis tends to occur more often in low-grade serous ovarian cancers [21,13,14,22,16,17,23]. The exact etiology of tumor calcification in ovarian cancer is still unclear. It is hypothesized that multiple mechanisms may be involved in the pathogenesis of ovarian tumor calcifications, with dystrophic calcification developing secondary to degeneration of the epithelium or in areas of tumor necrosis or may be related to hormonal influence [23].
From a clinical point of view, it is important to know the significance of temporal changes in tumor calcification following therapy and to evaluate if increasing tumoral calcification following therapy correlates with response to therapy or progression.
To the best of authors’ knowledge, there has been only a single study that has evaluated the significance of tumor calcification in ovarian carcinoma [21]. Burkill et al. reported that there was no correlation between changes in tumoral calcification on serial CT scans and corresponding serum CA125 levels [21]. However, that study had a heterogeneous group involving several subtypes of ovarian cancers, the radiological response was measured by the older RECIST 1.0 criteria, and there was no clear break down of how many patients showed increasing or decreasing tumor calcification following therapy. We believe our study is the first of its kind which has attempted to evaluate the significance of temporal changes in tumor calcification in the select group of low-grade serous ovarian carcinomas following therapy, with correlation made to serum CA 125 and newly revised RECIST 1.1.
This preliminary study shows that post-therapy increase in peritoneal implant calcification in low-grade serous ovarian carcinomas is not an indicator of response to therapy but rather this may imply progression. In our study, out of the 22 patients who showed progressive disease, 17 had increase in tumor calcification in the follow-up scan. Serum CA125 change was significantly associated with change in size of peritoneal deposits and tumor calcification. When serum CA125 increased, the peritoneal deposits and the tumor calcifications increased. Likewise, among the 12 patients with serum CA 125 response, post-therapy calcification decreased in the in 7 patients.
We believe this is an important addition to the existing fund of knowledge in evaluation of response to therapy in low-grade serous ovarian carcinomas. It is well-known that these tumors are relatively chemoresistant and may potentially benefit from hormonal therapy [24-26]. Objective response by RECIST may be difficult to appreciate in the majority of these patients [24]. In the absence of any significant change in tumor size and serum CA 125 levels, clinicians and radiologists may possibly misinterpret increasing calcification following therapy as tumor response; however, this determination may be erroneous (Fig. 4). Although our study did not show any significant overall association between tumor calcification and serum CA125 when stratified by peritoneal status, there was a trend of increasing calcification being associated with tumor progression, and hence, this finding should be treated with caution. A larger, prospective study would be useful to confirm our preliminary observation. It is possible that functional imaging such as FDG PET CT may have a role in such cases and should be further explored [27-30].
There are several limitations to our study. This is a retrospective study, with its associated inherent bias. This is a preliminary study with a small sample size, and the results should therefore be interpreted with caution. Majority of the patients had primary surgery elsewhere and hence, it was not possible to perform a radiologic- pathologic correlation of the tumoral calcification. We used a 5 mm cut off for change in tumor calcification status as there are no current standards for evaluating this parameter but the authors felt that a 5 mm change was felt to remove any ambiguity in deciding if there was truly an increase or decrease in post treatment tumoral calcification. It is important to note that this study does not evaluate response to treatment regimens (chemotherapy or hormonal therapy) in low-grade serous ovarian cancer as the study had only included patients with calcified tumor implants, who showed either increase or decrease in calcification following therapy. Therefore, the treatment response status of those patients who did not have calcified implants or did not show interval changes in calcification was not evaluated. However, this was not the purpose of our study as we specifically sought to evaluate the significance of increasing post-therapy calcification in tumor implants in low-grade serous ovarian cancer.
Nonetheless, our study clearly demonstrates that increasing post-therapy calcification as seen on CT in peritoneal metastases following therapy for low-grade ovarian serous carcinoma should not be used as an indicator of response to therapy. Large prospective studies using anatomic and functional imaging would be useful for further assessment of tumoral calcification as a potential biomarker for treatment response in low grade serous ovarian carcinomas.
FIG 2A.
50-year-old female with low-grade serous ovarian cancer. A) Baseline intravenous contrast enhanced CT scan of the pelvis shows calcified peritoneal implant (arrow) in the pelvis. B) 3 month follow-up CT scan shows increase in size of the pelvic implant (white arrow). The calcification within the implant has also increased (curved black arrow). This correlated with interval increase in CA-125 levels, consistent with progression.
FIG 2B.
50-year-old female with low-grade serous ovarian cancer. A) Baseline intravenous contrast enhanced CT scan of the pelvis shows calcified peritoneal implant (arrow) in the pelvis. B) 3 month follow-up CT scan shows increase in size of the pelvic implant (white arrow). The calcification within the implant has also increased (curved black arrow). This correlated with interval increase in CA-125 levels, consistent with progression.
FIG 3A.
66-year-old female with low-grade serous ovarian cancer. A) Baseline intravenous contrast enhanced CT scan of the pelvis scan shows non-calcified peritoneal implant (arrow) in the pelvis. B) 3 month follow-up CT scan of the pelvis shows interval development of calcification within the pelvic implant (arrow). This correlated with interval increase in CA-125 levels, consistent with progression.
FIG 3B.
66-year-old female with low-grade serous ovarian cancer. A) Baseline intravenous contrast enhanced CT scan of the pelvis scan shows non-calcified peritoneal implant (arrow) in the pelvis. B) 3 month follow-up CT scan of the pelvis shows interval development of calcification within the pelvic implant (arrow). This correlated with interval increase in CA-125 levels, consistent with progression.
FIG 4A.
29-year-old female with recurrent low-grade serous ovarian cancer. Patient had stable small calcified implants and had been on letrozole A) Axial CT abdomen shows a small 6 mm completely calcified peritoneal implant (arrow) in the left upper quadrant, which had remained stable for more than 12 months. B) Follow up scan performed 6 months later interval increase in size of the calcification (arrow), which now measures 1.5 cm. However, there were no changes in any of the non-calcified implants and the CA 125 levels also did not show any significant interval changes. C) Further follow up CT performed 3 months later shows the implant (arrow) has significantly increased in size to 2.5 cm. There is also interval development of non-calcified soft tissue component (arrowhead) within the lesion, consistent with progression. D) Axial CT also demonstrates new site of metastasis in the gastric wall (arrowhead), consistent with progression. The CA-125 levels had now almost doubled since the first CT scan (16.3 u/ml compared to 9.4 u/ml). Patient was enrolled in a clinical trial and was randomized to receive Trametinib. This case demonstrates that increasing calcification in patients with low grade serous ovarian carcinoma should be viewed with suspicion and closely monitored.
FIG 4B.
29-year-old female with recurrent low-grade serous ovarian cancer. Patient had stable small calcified implants and had been on letrozole A) Axial CT shows a small 6 mm completely calcified peritoneal implant (arrow) in the left upper quadrant, which had remained stable for more than 12 months. B) Follow up scan performed 6 months later interval increase in size of the calcification (arrow), which now measures 1.5 cm. However, there were no changes in any of the non-calcified implants and the CA 125 levels also did not show any significant interval changes. C) Further follow up CT performed 3 months later shows the implant (arrow) has significantly increased in size to 2.5 cm. There is also interval development of non-calcified soft tissue component (arrowhead) within the lesion, consistent with progression. D) Axial CT also demonstrates new site of metastasis in the gastric wall (arrowhead), consistent with progression. The CA-125 levels had now almost doubled since the first CT scan (16.3 u/ml compared to 9.4 u/ml). Patient was enrolled in a clinical trial and was randomized to receive Trametinib. This case demonstrates that increasing calcification in patients with low grade serous ovarian carcinoma should be viewed with suspicion and closely monitored.
FIG 4C.
29-year-old female with recurrent low-grade serous ovarian cancer. Patient had stable small calcified implants and had been on letrozole A) Axial CT shows a small 6 mm completely calcified peritoneal implant (arrow) in the left upper quadrant, which had remained stable for more than 12 months. B) Follow up scan performed 6 months later interval increase in size of the calcification (arrow), which now measures 1.5 cm. However, there were no changes in any of the non-calcified implants and the CA 125 levels also did not show any significant interval changes. C) Further follow up CT performed 3 months later shows the implant (arrow) has significantly increased in size to 2.5 cm. There is also interval development of non-calcified soft tissue component (arrowhead) within the lesion, consistent with progression. D) Axial CT also demonstrates new site of metastasis in the gastric wall (arrowhead), consistent with progression. The CA-125 levels had now almost doubled since the first CT scan (16.3 u/ml compared to 9.4 u/ml). Patient was enrolled in a clinical trial and was randomized to receive Trametinib. This case demonstrates that increasing calcification in patients with low grade serous ovarian carcinoma should be viewed with suspicion and closely monitored.
FIG 4D.
29-year-old female with recurrent low-grade serous ovarian cancer. Patient had stable small calcified implants and had been on letrozole A) Axial CT shows a small 6 mm completely calcified peritoneal implant (arrow) in the left upper quadrant, which had remained stable for more than 12 months. B) Follow up scan performed 6 months later interval increase in size of the calcification (arrow), which now measures 1.5 cm. However, there were no changes in any of the non-calcified implants and the CA 125 levels also did not show any significant interval changes. C) Further follow up CT performed 3 months later shows the implant (arrow) has significantly increased in size to 2.5 cm. There is also interval development of non-calcified soft tissue component (arrowhead) within the lesion, consistent with progression. D) Axial CT also demonstrates new site of metastasis in the gastric wall (arrowhead), consistent with progression. The CA-125 levels had now almost doubled since the first CT scan (16.3 u/ml compared to 9.4 u/ml). Patient was enrolled in a clinical trial and was randomized to receive Trametinib. This case demonstrates that increasing calcification in patients with low grade serous ovarian carcinoma should be viewed with suspicion and closely monitored.
Acknowledgments
Funding: This study was supported by MD Anderson Cancer Center Support Grant No. NIH/NCI P30 CA016672 from the National Cancer Institute, National Institutes of Health
Footnotes
Disclosures: None (All the authors confirm that there are no relevant disclosures)
Submission Declaration: All the authors confirm that this manuscript has not been published previously, and that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Compliance with ethical standards
Conflict of interest: All authors confirm that there are no relevant conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.” Being a retrospective study, formal consent was not required.
This article does not contain any studies with animals performed by any of the authors.
Informed consent: IRB approval was obtained for a waiver of Informed Consent/Authorization because this is a retrospective chart review that involves no diagnostic or therapeutic intervention, as well as no direct patient contact and no patient identifiers. A consent waiver was granted by the institutional review board.
Contributor Information
Dhakshinamoorthy Ganeshan, University of Texas MD Anderson Cancer Center, Department of Diagnostic Radiology, Pickens Academic Tower, 1400 Pressler Street, Unit 1473, Houston, TX 77030-4009, Telephone: 404-426-0278, Fax: 713-745-1151.
Priya Bhosale, University of Texas MD Anderson Cancer Center, Department of Diagnostic Radiology, Pickens Academic Tower, 1400 Pressler Street, Unit 1473, Houston, TX 77030-4009.
Wei Wei, University of Texas MD Anderson Cancer Center, Department of Biostastics, 1400 Pressler Street, Houston, TX 77030-4009.
Preetha Ramalingam, University of Texas MD Anderson Cancer Center, Department of Pathology, Pickens Academic Tower, 1400 Pressler Street, Unit 1473, Houston, TX 77030-4009.
Eniola Mudasiru-Dawodu, University of Texas MD Anderson Cancer Center, Department of Diagnostic Radiology, Pickens Academic Tower, 1400 Pressler Street, Unit 1473, Houston, TX 77030-4009.
David Gershenson, University of Texas MD Anderson Cancer Center, Department of Gynecologic Oncology &Reproductive Medicine, 1400 Pressler Street, Houston, TX 77030-4009.
Charlotte Sun, University of Texas MD Anderson Cancer Center, Department of Gynecologic Oncology &Reproductive Medicine, 1400 Pressler Street, Houston, TX 77030-4009.
Revathy Iyer, University of Texas MD Anderson Cancer Center, Department of Diagnostic Radiology, Pickens Academic Tower, 1400 Pressler Street, Unit 1473, Houston, TX 77030-4009.
References
- 1.Agarwal A, Yeh BM, Breiman RS, Qayyum A, Coakley FV. Peritoneal calcification: causes and distinguishing features on CT. AJR American journal of roentgenology. 2004;182(2):441–445. doi: 10.2214/ajr.182.2.1820441. doi:10.2214/ajr.182.2.1820441. [DOI] [PubMed] [Google Scholar]
- 2.Hale HL, Husband JE, Gossios K, Norman AR, Cunningham D. CT of calcified liver metastases in colorectal carcinoma. Clinical radiology. 1998;53(10):735–741. doi: 10.1016/s0009-9260(98)80315-2. [DOI] [PubMed] [Google Scholar]
- 3.Lewis RB, Lattin GE, Jr., Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2010;30(6):1445–1464. doi: 10.1148/rg.306105523. doi:10.1148/rg.306105523. [DOI] [PubMed] [Google Scholar]
- 4.Mc Auley G, Jagannathan J, O'Regan K, Krajewski KM, Hornick JL, Butrynski J, Ramaiya N. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR American journal of roentgenology. 2012;198(1):W31–37. doi: 10.2214/AJR.11.6927. doi:10.2214/ajr.11.6927. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Levy AD, Rohrmann CA, Jr., Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003;23(3):645–662. doi: 10.1148/rg.233025134. doi:10.1148/rg.233025134. [DOI] [PubMed] [Google Scholar]
- 6.Scatarige JC, Fishman EK, Saksouk FA, Siegelman SS. Computed tomography of calcified liver masses. Journal of computer assisted tomography. 1983;7(1):83–89. doi: 10.1097/00004728-198302000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N. Primary dedifferentiated liposarcoma of the retroperitoneum. Prognostic significance of computed tomography and magnetic resonance imaging features. Journal of computer assisted tomography. 2003;27(5):799–804. doi: 10.1097/00004728-200309000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Bahr O, Hattingen E, Rieger J, Steinbach JP. Bevacizumab-induced tumor calcifications as a surrogate marker of outcome in patients with glioblastoma. Neuro-oncology. 2011;13(9):1020–1029. doi: 10.1093/neuonc/nor099. doi:10.1093/neuonc/nor099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(33):5344–5351. doi: 10.1200/JCO.2008.17.5299. doi:10.1200/jco.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 10.Bohlman ME, Lichtenfeld JL, Goldman SM. Lymph node calcification secondary to chemotherapy for histiocytic lymphoma. Diagnostic imaging. 1981;50(5):259–262. [PubMed] [Google Scholar]
- 11.Brereton HD, Johnson RE. Calcification in mediastinal lymph nodes after radiation therapy of Hodgkin's disease. Radiology. 1974;112(3):705–707. doi: 10.1148/112.3.705. doi:10.1148/112.3.705. [DOI] [PubMed] [Google Scholar]
- 12.Easson AM, Barron PT, Cripps C, Hill G, Guindi M, Michaud C. Calcification in colorectal hepatic metastases correlates with longer survival. Journal of surgical oncology. 1996;63(4):221–225. doi: 10.1002/(SICI)1096-9098(199612)63:4<221::AID-JSO2>3.0.CO;2-E. doi:10.1002/(sici)1096-9098(199612)63:4<221::aid-jso2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JM, Tirumani SH, Kim KW, Saboo SS, Baez JC, Shinagare AB. Malignant abdominal rocks: where do they come from? Cancer imaging : the official publication of the International Cancer Imaging Society. 2013;13(4):527–539. doi: 10.1102/1470-7330.2013.0048. doi:10.1102/1470-7330.2013.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper RA. Calcified ovarian metastases. AJR American journal of roentgenology. 2002;178(1):243. doi: 10.2214/ajr.178.1.1780243. doi:10.2214/ajr.178.1.1780243b. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DG, Hill MC, Hill S, Zaloudek C. Serous carcinoma of the ovary: CT identification of metastatic calcified implants. Radiology. 1986;158(3):649–652. doi: 10.1148/radiology.158.3.3945732. doi:10.1148/radiology.158.3.3945732. [DOI] [PubMed] [Google Scholar]
- 16.Moncada R, Cooper RA, Garces M, Badrinath K. Calcified metastases from malignant ovarian neoplasm. Review of the literature. Radiology. 1974;113(1):31–35. doi: 10.1148/113.1.31. doi:10.1148/113.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfo I, Blandino A, Gaeta M, Racchiusa S, Freni O. Calcified peritoneal metastases from papillary cystadenocarcinoma of the ovary: CT features. Journal of computer assisted tomography. 1986;10(3):545–546. [PubMed] [Google Scholar]
- 18.Teplick JG, Haskin ME, Alavi A. Calcified intraperitoneal metastases from ovarian carcinoma. AJR American journal of roentgenology. 1976;127(6):1003–1006. doi: 10.2214/ajr.127.6.1003. doi:10.2214/ajr.127.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K, Vergote I, Cervantes A, Vermorken J. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). Journal of the National Cancer Institute. 2004;96(6):487–488. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 20.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, du Bois A, Kristensen G, Jakobsen A, Sagae S, Greven K, Parmar M, Friedlander M, Cervantes A, Vermorken J. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21(2):419–423. doi: 10.1097/IGC.0b013e3182070f17. doi:10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 21.Burkill GJ, Allen SD, A'Hern R P, Gore ME, King DM. Significance of tumour calcification in ovarian carcinoma. The British journal of radiology. 2009;82(980):640–644. doi: 10.1259/bjr/12716831. doi:10.1259/bjr/12716831. [DOI] [PubMed] [Google Scholar]
- 22.Franchi M, La Fianza A, Babilonti L, Bolis PF, Dore R, Legnani L, Di Maggio E. Serous carcinoma of the ovary: value of computed tomography in detection of calcified pleural and pulmonary metastatic implants. Gynecologic oncology. 1990;39(1):85–88. doi: 10.1016/0090-8258(90)90405-a. [DOI] [PubMed] [Google Scholar]
- 23.Silva EG, Deavers MT, Parlow AF, Gershenson DM, Malpica A. Calcifications in ovary and endometrium and their neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology. Inc. 2003;16(3):219–222. doi: 10.1097/01.MP.0000057236.96797.07. doi:10.1097/01.mp.0000057236.96797.07. [DOI] [PubMed] [Google Scholar]
- 24.Gershenson DM, Sun CC, Iyer RB, Malpica AL, Kavanagh JJ, Bodurka DC, Schmeler K, Deavers M. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecologic oncology. 2012;125(3):661–666. doi: 10.1016/j.ygyno.2012.02.037. doi:10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguera IR, Sun CC, Broaddus RR, Branham D, Levenback CF, Ramirez PT, Sood AK, Coleman RL, Gershenson DM. Phase II trial of imatinib mesylate in patients with recurrent platinum- and taxane-resistant low-grade serous carcinoma of the ovary, peritoneum, or fallopian tube. Gynecologic oncology. 2012;125(3):640–645. doi: 10.1016/j.ygyno.2012.02.034. doi:10.1016/j.ygyno.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Romero I, Sun CC, Wong KK, Bast RC, Jr., Gershenson DM. Low-grade serous carcinoma: new concepts and emerging therapies. Gynecologic oncology. 2013;130(3):660–666. doi: 10.1016/j.ygyno.2013.05.021. doi:10.1016/j.ygyno.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal K, Bhattacharya A, Harisankar CN, Abrar ML, Dhaliwal LK, Mittal BR. F-18 fluoride uptake in calcified extraosseous metastases from ovarian papillary serous adenocarcinoma. Clinical nuclear medicine. 2012;37(1):e22–23. doi: 10.1097/RLU.0b013e3182392441. doi:10.1097/RLU.0b013e3182392441. [DOI] [PubMed] [Google Scholar]
- 28.Dong A, Wang Y, Zuo C. FDG PET/CT in serous psammocarcinoma of the ovary. Clinical nuclear medicine. 2014;39(5):453–455. doi: 10.1097/RLU.0b013e318286bdfc. doi:10.1097/RLU.0b013e318286bdfc. [DOI] [PubMed] [Google Scholar]
- 29.Hu SL, Zhou ZR, Zhang YJ. Calcified metastases from ovarian carcinoma highlighted by F-18 FDG PET/CT: report of two cases. Abdominal imaging. 2012;37(4):675–679. doi: 10.1007/s00261-011-9800-3. doi:10.1007/s00261-011-9800-3. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi S, Lucchini M, Schmeler KM, Coleman RL, Gershenson DM, Munsell MF, Macapinlac HA, Ramirez PT. Utility of 18F-FDG PET/CT in follow-up of patients with low-grade serous carcinoma of the ovary. Gynecologic oncology. 2014;133(1):100–104. doi: 10.1016/j.ygyno.2014.02.008. doi:10.1016/j.ygyno.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]