Abstract
Neuroblastoma (NB) is the most common extra-cranial solid tumor of childhood and is characterized by a wide range of clinical behaviors. Amplification of MYCN is a well-known poor prognostic factor in NB patients. As the MYCN amplification status is usually tested using tumor specimens, lengthy and invasive procedures are unavoidable. To evaluate the possibility of detecting MYCN amplification without invasive procedure, we performed conventional polymerase chain reaction (PCR) analysis to identify MYCN amplification using the preserved serum DNA. PCR of serum DNA was done in 105 NB patients whose MYCN status had been confirmed by fluorescence in situ hybridization. MYCN amplification was evaluated as the ratio of signal intensities between MYCN and NAGK (M/N ratio). When regarding the tissue FISH results as a reference, 10 patients had MYCN-amplified (MNA) NB, and 95 had non-MNA NB. The M/N ratio of the MNA group (median 2.56, range 1.01-3.58) was significantly higher than that of the non-MNA group (median 0.97, range 0.67-5.18) (P < 0.001). In the receiver operating characteristic curve analysis, the area under the curve was 0.957 (95% confidence interval 0.898–1.000; P < 0.001), and it showed 90.9% sensitivity and 97.9% specificity with the selected cut-off value set as 1.6. The detection of MYCN amplification using conventional PCR analysis of serum samples seems to be a simple and promising method to evaluate the MYCN status of NB patients. Further study with a larger set of patients is needed to confirm the accuracy of this result.
Keywords: Neuroblastoma, MYCN Amplification, Polymerase Chain Reaction, Serum DNA
Graphical Abstract
INTRODUCTION
Neuroblastoma (NB) is the most common extra-cranial solid tumor of childhood, and it is characterized by highly heterogeneous clinical courses, ranging from spontaneous regression without any therapeutic intervention to very fatal cases showing rapid progression and death despite modern, intensive and multimodal treatment (1,2,3). The treatment of NB is determined based on risk groups, which take into account factors such as age, stage, histologic category, grade of tumor differentiation, the status of the v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN) oncogene, chromosome 11q status, and DNA ploidy (1). Although low- and intermediate-risk patients generally have a favorable outcome, high-risk patients still show frequent relapses and a poor prognosis.
Amplification of the MYCN oncogene was one of the first reported genetic markers for highly aggressive and advanced stage NB (4). MYCN amplification is observed in approximately 20% of cases and remains a powerful prognostic factor indicating poor outcome (5). The status of MYCN has been determined using Southern blotting or fluorescence in situ hybridization (FISH) of tumor tissues obtained invasively. Recently, several studies detecting MYCN amplification with real-time polymerase chain reaction (PCR) using peripheral blood were reported (6,7,8,9). These studies showed correlation of serum MYCN status with tissue MYCN results, suggesting the usefulness of detecting MYCN amplification with circulating DNA. In this study, we performed conventional PCR analysis using serum specimens from NB patients and compared the results with the MYCN status tested with FISH on tumor tissues.
MATERIALS AND METHODS
Study subjects
Among the patients diagnosed with NB between June 2011 and December 2015, patients who had FISH results for MYCN amplification in their tumor tissues were eligible for this study. Patients without stored serum samples at the time of diagnosis were excluded. A total of 105 patients were enrolled in this study with informed consent from their parents.
Serum preparation, DNA isolation and qualitative PCR
At diagnosis, 4 mL of whole blood was collected using a serum separator tube. After clotting time, blood was centrifuged (2,400 g) at 4 degrees for 5 minutes. Serum was transferred into a 1.5mL Eppendorf tube and stored at -80 degrees freezer. Circulating DNA was extracted from 200 μL of a stored serum sample by using the QIAamp DNA blood mini kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s protocols. Thirty PCR cycles were performed; each cycle consisted of 30 seconds at 94°C, 60 seconds at 60°C, and 30 seconds at 72°C. In addition to MYCN (GenBank accession No. NG 007457), PCR amplification of N-acetylglucosamine kinase (NAGK, GenBank accession No. NM 017567) gene located at 2p12 was performed as a single-copy reference gene. The sequence of primers used for MYCN and NAGK are as follows: MYCN forward, 5'-GTGCTCTCCAATTCTCGCCT-3'; MYCN reverse, 5'-GATGGCCTAGAGGAGGGCT-3'; NAGK forward, 5'-TGGGCAGACACATCGTAGCA-3'; and NAGK reverse, 5'-CACCTTCACTCCCACCTCAAC-3'. PCR products were separated on a 1.5% agarose gel, and densitometric results were analyzed with NIH Image J software (http://rsbweb.nih.gov/ij/) as a ratio of signal intensity. MYCN amplification was evaluated as the MYCN/NAGK (M/N) ratio.
Statistical analyses
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA). Differences between categorical variables were measured using χ2 or Fisher’s exact tests, and differences between continuous variables were calculated with the Mann-Whitney test. Diagnostic performance was assessed by receiver operating characteristic (ROC) curve analysis. The Youden index was used to identify the optimal cut-off points.
Ethics statement
This study was approved by the institutional review board at Samsung Medical Center (IRB No. 2013-07-139), and informed consents were obtained from the parents.
RESULTS
Characteristics of patients according to tissue MYCN status
Of the 105 patients enrolled in this study, 10 had MYCN-amplified (MNA) tumors and 95 had non-MNA tumors, as determined using FISH (Table 1). Among the 10 patients with MNA tumors, 9 patients had stage 4 disease. There were no statistically significant differences in sex and age at diagnosis between the MNA tumor and non-MNA tumor groups. Most MNA tumors showed unfavorable histology, and the grade of tumor differentiation of all of the MNA tumors was undifferentiated or poorly differentiated.
Table 1. Characteristics of patients according to their tissue MYCN result.
| Parameters | FISH results | ||
|---|---|---|---|
|
MYCN amplified n = 10 (9.5 %) |
MYCN non-amplified n = 95 (90.5%) |
P | |
| Sex, No. (%) | 0.321 | ||
| Male | 3 (30.0) | 48 (50.5) | |
| Female | 7 (70.0) | 47 (49.5) | |
| Median age at diagnosis, mon (range) | 22.5 (1–55) | 25.0 (1–231) | 0.760 |
| Stage, No. (%) | 0.238 | ||
| 1 | 0 (0) | 10 (10.5) | |
| 2 | 0 (0) | 21 (22.1) | |
| 3 | 1 (10.0) | 13 (13.7) | |
| 4 | 9 (90.0) | 49 (51.6) | |
| 4S | 0 (0) | 2 (2.1) | |
| INPC classification, No. (%) | 0.038 | ||
| Favorable | 1 (10.0) | 46 (48.4) | |
| Unfavorable | 9 (90.0) | 47 (49.5) | |
| Unknown | 0 (0) | 2 (2.1) | |
| Grade of differentiation, No. (%) | 0.001 | ||
| Undifferentiated | 3 (30.0) | 1 (1.1) | |
| Poorly differentiated | 7 (70.0) | 47 (49.5) | |
| Differentiating | 0 (0) | 17 (17.9) | |
| Ganglioneuroblastoma | 0 (0) | 29 (30.5) | |
| Unknown | 0 (0) | 1 (1.1) | |
| Serum MYCN PCR results, median (range) | 2.56 (1.01–3.58) | 0.97 (0.67–5.18) | < 0.001 |
INPC, International Neuroblastoma Pathology Classification; M/N ratio, MYCN/NAGK ratio.
Accuracy of serum MYCN PCR results
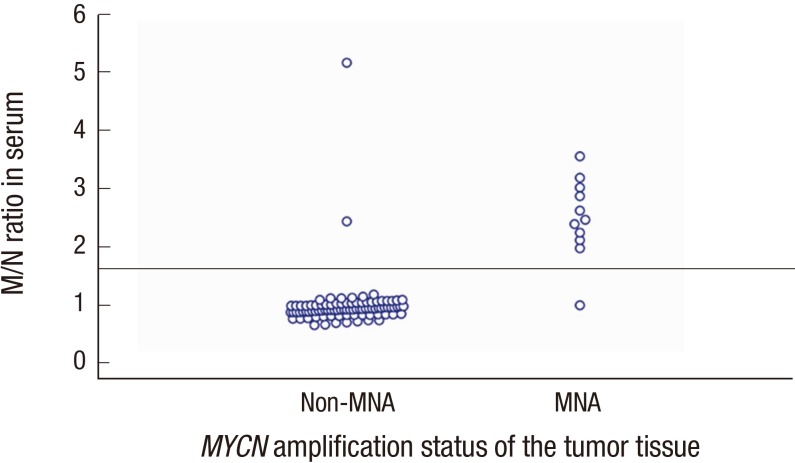
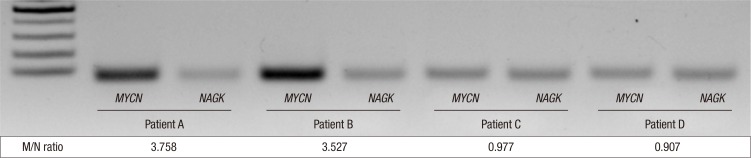
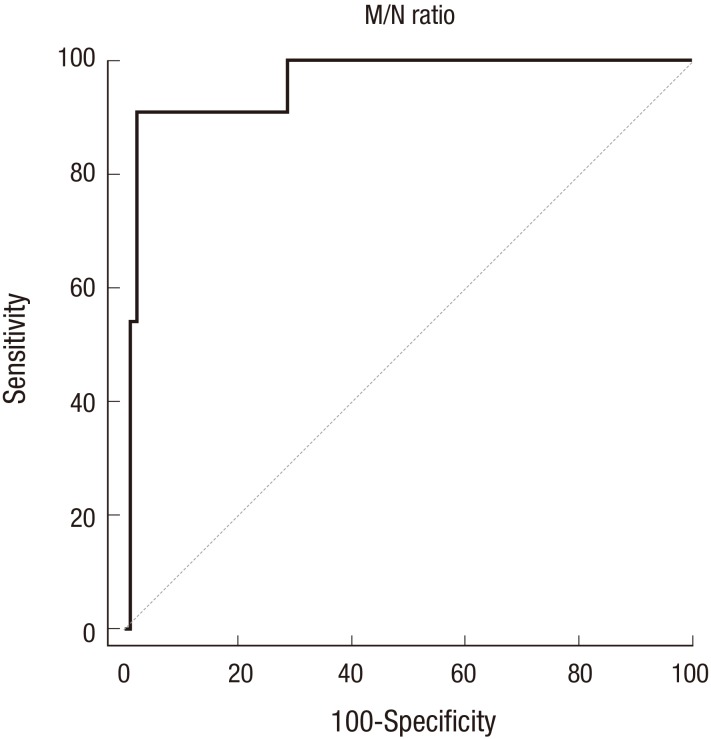
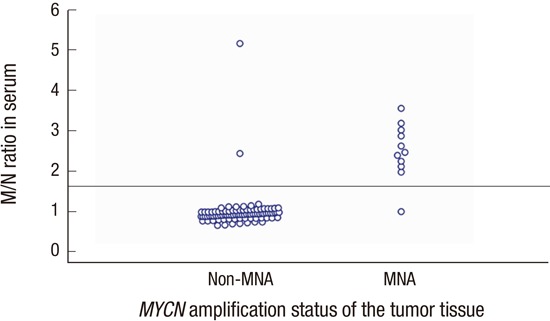
The serum M/N ratio ranged from 0.67 to 5.18, and the M/N ratio of the MNA NB group was significantly higher than that of the non-MNA NB group (P < 0.001). The distributions of M/N ratios in the MNA and non-MNA tumor groups are shown in Fig. 1. PCR results of representative cases of MNA tumors and non-MNA tumors are shown in Fig. 2. In the ROC curve analysis, the area under the curve was 0.957 (95% confidence interval [CI], 0.898–1.000; P < 0.001), and it showed 90.9% sensitivity and 97.9% specificity with the selected cut-off value for M/N ratio set as 1.6 (Fig. 3).
Fig. 1.
Distributions of MYCN/NAGK (M/N) ratios in MYCN-amplified (MNA) and non-MNA tumors. The M/N ratio of serum DNA according to the MYCN tissue status is shown. The line in the middle indicates cut off level (1.6).
Fig. 2.
Detection of MYCN amplification in serum using PCR analysis. Patients A and B had MYCN-amplified (MNA) tumors, while patients C and D had non-MNA tumors. The MYCN/NAGK (M/N) ratios of patient A, B, C and D were 3.758, 3.527, 0.977, and 0.907, respectively. The very left lane is ladder marker DNA.
Fig. 3.
Receiver operating characteristic (ROC) curve constructed using the MYCN/NAGK (M/N) ratio. In the ROC curve analysis, the area under the curve was 0.957 (95% confidence interval, 0.898–1.000; P < 0.001), and it showed 90.9% sensitivity and 97.9% specificity with the selected M/N ratio cut-off value set of 1.6.
When regarding the tissue FISH results as a reference, false-positive results were obtained in the serum PCR analysis for 2 patients, and a false-negative result was obtained in 1 patient. One patient with false-positive results (0.67 of FISH MYCN/CEP2 ratio and 5.18 of serum M/N ratio) had poorly differentiated NB and stage 4 disease with distant lymph node metastasis. Initial lactate dehydrogenase (LDH) and neuron specific enolase (NSE) levels were 4,265 IU/L and 350 ng/mL, respectively. After 3 cycles of chemotherapy, tumor volume reduction was measured as 72.7%. The other patient had poorly differentiated NB with bone marrow and bone metastasis. The FISH MYCN/CEP2 ratio of the tumor tissue was 1.01 and the serum M/N ratio was 2.46. The LDH and NSE levels were 788 IU/L and 58.5 ng/mL at diagnosis, and tumor volume reduction after 3 cycles of chemotherapy was 41.3%. Both patients are alive without disease after treatment according to the high risk NB protocol.
The patient who had a false-negative result was a 21-month-old boy diagnosed with poorly differentiated NB with bone metastasis. The FISH MYCN/CEP2 ratio and serum M/N ratio were > 20 and 1.007, respectively.
Relapsed patient
Among 105 patients, 1 patient had a recurrent MNA tumor. PCR analysis with the serum sample from this patient was performed with both the specimens from the initial diagnosis and the relapse, and high M/N ratios were observed in both samples (2.643 at diagnosis and 2.407 at relapse).
Neonatal NB
Fifteen patients were infants less than 3 months old, and 1 of them had MNA NB. Five patients were diagnosed with stage 1 or 4S disease after surgery, and they were in remission without chemotherapy. In these patients, no discrepancies were observed between the results of serum PCR and FISH with tumor tissue.
DISCUSSION
In this study, we used conventional PCR analysis to evaluate MYCN amplification using serum samples from NB patients. The MNA NB group had significantly higher M/N ratios compared to those of the non-MNA group, and the PCR results correlated well with FISH results with high sensitivity and specificity that were similar to the results reported previously using real-time PCR (6,7,8,9). The advantages of conventional PCR include the ready access to conventional thermocyclers in almost all research facilities and the low cost.
MYCN amplification is a known poor prognostic factor of NB. The International Neuroblastoma Risk Group Biology Committee agreed that MYCN status should be evaluated in every resected neuroblastic tumor, including the Schwann cell stroma-rich categories (10). Recommended and accepted techniques to detect MYCN amplification in tumor tissue include interphase FISH, PCR, array-based comparative genomic hybridization and multiplex ligation-dependent probe amplification, but interphase FISH is usually preferred and used in many institutions (11).
The results of our study along with those of previous studies showed that measuring MYCN amplification with serum samples could be used to predict the MYCN status of tumor tissue noninvasively. This method can be adjusted usefully in several clinical settings. Gotoh et al. (7) demonstrated that the serum M/N ratio decreased in the patients in remission but increased beyond the cut-off value in the patients who relapsed or failed to achieve remission. Therefore, the serum M/N ratio could be used as a marker to monitor treatment response and predict relapse. MYCN PCR could be performed using cerebrospinal fluid (CSF) as well as serum, and the high number of MYCN copies in the CSF might indicate the presence of metastatic NB of the central nervous system (12).
One good candidate for the application of serum MYCN test is in infants with a congenital suprarenal mass. The universal use of ultrasonography in the prenatal period leads to the increased detection of suprarenal masses in neonates. The differential diagnosis for a congenital suprarenal mass includes adrenal hemorrhage, adrenal hyperplasia, renal dysplasia, pulmonary sequestration, NB and so on (13,14). When the suprarenal mass is suspected to be NB, expectant observation can be considered because a high rate of spontaneous regression has been observed in infants with NB (15,16). However, if the tumor tissue has MYCN amplification, the infants have to be classified as a high-risk group requiring intensive, multimodal treatment before progression (17,18,19). PCR can easily be performed by using peripheral blood for this group of patients to detect MYCN amplification and to decide the treatment strategy without obtaining tumor tissue invasively. In this study, 5 infants less than 3 months of age underwent an operation and they were diagnosed with a stage 1 or 4S non-MNA NB, and there was no discrepancy between the serum PCR and FISH results in these patients.
In this study, false-positive results were obtained for 2 patients and a false-negative result was obtained for 1 patient when we considered the tissue FISH results as a reference. Conventional PCR is very simple technique and can be applied easily in research facilities, but it provides qualitative results only. Quantitative results were analyzed with as a ratio of signal intensity between MYCN and NAGK in this study, but this method cannot be precise as real-time PCR, although sensitivity and specificity in this study were similar to the results reported previously using real-time PCR.
High LDH and NSE were known to be associated with MYCN positive NB (20), and we previously reported that greater tumor volume reduction was observed in patients with MYCN positive tumors (21). In our study, 1 patient with false positive result showed clinical features similar to the MYCN positive NB in that this patient had high serum LDH and NSE level at diagnosis and showed good early response to chemotherapy. In this patient, the possibility of inaccurate FISH results cannot be excluded because the results of FISH on tumor tissue can be affected by the quality of the tissue specimen.
False-negative results can be partly explained by the possibility of leukocyte contamination during the preparation of the serum sample; in this case, dilution of tumor DNA with white blood cell (WBC) DNA can lower the M/N ratio in MNA tumors (7). Therefore, the importance of removing WBCs from serum should be emphasized during the handling of the serum sample. An additional high-speed centrifugation step (16,000 g for 5 minutes) before freezing or after thawing the serum sample was found to eliminate cellular contamination (22). By using the standardized protocol with appropriate centrifugation methods, WBC contamination could be minimized and WBC-free serum could be reliably achieved.
In summary, the detection of MYCN amplification using conventional PCR analysis of serum samples seems to be a simple and promising method to evaluate the MYCN status of NB. This noninvasive and simple technique can be used in many clinical settings such as monitoring relapse or deciding the treatment strategy of a congenital suprarenal mass. Further study with a larger set of patients is needed to confirm this result.
Footnotes
Funding: This study was supported by a grant of the Korean Health Technology R & D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1521).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Sung KW. Data collection: Ma Y, Lee JW, Park SJ, Sung KW. Data analysis: Ma Y, Lee JW, Yi ES, Choi YB, Yoo KH, Sung KW, Koo HH. Writing: Ma Y, Lee JW. Revision: Yi ES, Choi YB, Yoo KH, Sung KW, Koo HH. Final approval: all authors.
References
- 1.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens C, Irwin M. Neuroblastoma: the impact of biology and cooperation leading to personalized treatments. Crit Rev Clin Lab Sci. 2012;49:85–115. doi: 10.3109/10408363.2012.683483. [DOI] [PubMed] [Google Scholar]
- 3.Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KK, Hogarty M, COG Neuroblastoma Committee Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013;60:985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 5.Janoueix-Lerosey I, Schleiermacher G, Michels E, Mosseri V, Ribeiro A, Lequin D, Vermeulen J, Couturier J, Peuchmaur M, Valent A, et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27:1026–1033. doi: 10.1200/JCO.2008.16.0630. [DOI] [PubMed] [Google Scholar]
- 6.Combaret V, Audoynaud C, Iacono I, Favrot MC, Schell M, Bergeron C, Puisieux A. Circulating MYCN DNA as a tumor-specific marker in neuroblastoma patients. Cancer Res. 2002;62:3646–3648. [PubMed] [Google Scholar]
- 7.Gotoh T, Hosoi H, Iehara T, Kuwahara Y, Osone S, Tsuchiya K, Ohira M, Nakagawara A, Kuroda H, Sugimoto T. Prediction of MYCN amplification in neuroblastoma using serum DNA and real-time quantitative polymerase chain reaction. J Clin Oncol. 2005;23:5205–5210. doi: 10.1200/JCO.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Combaret V, Hogarty MD, London WB, McGrady P, Iacono I, Brejon S, Swerts K, Noguera R, Gross N, Rousseau R, et al. Influence of neuroblastoma stage on serum-based detection of MYCN amplification. Pediatr Blood Cancer. 2009;53:329–331. doi: 10.1002/pbc.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima M, Hiyama E, Fukuba I, Yamaoka E, Ueda Y, Onitake Y, Kurihara S, Sueda T. Detection of MYCN amplification using blood plasma: noninvasive therapy evaluation and prediction of prognosis in neuroblastoma. Pediatr Surg Int. 2013;29:1139–1145. doi: 10.1007/s00383-013-3374-9. [DOI] [PubMed] [Google Scholar]
- 10.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 11.Ambros PF, Ambros IM, Brodeur GM, Haber M, Khan J, Nakagawara A, Schleiermacher G, Speleman F, Spitz R, London WB, et al. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimoto T, Inoue M, Tokimasa S, Yagyu S, Iehara T, Hosoi H, Kawa K. Detection of MYCN DNA in the cerebrospinal fluid for diagnosing isolated central nervous system relapse in neuroblastoma. Pediatr Blood Cancer. 2011;56:865–867. doi: 10.1002/pbc.22925. [DOI] [PubMed] [Google Scholar]
- 13.Sauvat F, Sarnacki S, Brisse H, Medioni J, Rubie H, Aigrain Y, Gauthier F, Audry G, Helardot P, Landais P, et al. Outcome of suprarenal localized masses diagnosed during the perinatal period: a retrospective multicenter study. Cancer. 2002;94:2474–2480. doi: 10.1002/cncr.10502. [DOI] [PubMed] [Google Scholar]
- 14.Maki E, Oh K, Rogers S, Sohaey R. Imaging and differential diagnosis of suprarenal masses in the fetus. J Ultrasound Med. 2014;33:895–904. doi: 10.7863/ultra.33.5.895. [DOI] [PubMed] [Google Scholar]
- 15.Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel-Walter HG, Schwabe D, Schilling FH, Benz-Bohm G, Berthold F. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 16.Nuchtern JG, London WB, Barnewolt CE, Naranjo A, McGrady PW, Geiger JD, Diller L, Schmidt ML, Maris JM, Cohn SL, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg. 2012;256:573–580. doi: 10.1097/SLA.0b013e31826cbbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minard V, Hartmann O, Peyroulet MC, Michon J, Coze C, Defachelle AS, Lejars O, Perel Y, Bergeron C, Boutard P, et al. Adverse outcome of infants with metastatic neuroblastoma, MYCN amplification and/or bone lesions: results of the French Society of Pediatric Oncology. Br J Cancer. 2000;83:973–979. doi: 10.1054/bjoc.2000.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canete A, Gerrard M, Rubie H, Castel V, Di Cataldo A, Munzer C, Ladenstein R, Brichard B, Bermúdez JD, Couturier J, et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European Neuroblastoma Experience. J Clin Oncol. 2009;27:1014–1019. doi: 10.1200/JCO.2007.14.5839. [DOI] [PubMed] [Google Scholar]
- 19.Di Cataldo A, Dau D, Conte M, Parodi S, De Bernardi B, Giuliano M, Pession A, Viscardi E, Luksch R, Castellano A, et al. Diagnostic and prognostic markers in infants with disseminated neuroblastoma: a retrospective analysis from the Italian Cooperative Group for Neuroblastoma. Med Sci Monit. 2009;15:MT11–8. [PubMed] [Google Scholar]
- 20.Tonini GP, Boni L, Pession A, Rogers D, Iolascon A, Basso G, Cordero di Montezemolo L, Casale F, Pession A, Perri P, et al. MYCN oncogene amplification in neuroblastoma is associated with worse prognosis, except in stage 4s: the Italian experience with 295 children. J Clin Oncol. 1997;15:85–93. doi: 10.1200/JCO.1997.15.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Yoo SY, Kim JS, Sung KW, Jeon TY, Choi JY, Moon SH, Son MH, Lee SH, Yoo KH, Koo HH. The degree of tumor volume reduction during the early phase of induction chemotherapy is an independent prognostic factor in patients with high-risk neuroblastoma. Cancer. 2013;119:656–664. doi: 10.1002/cncr.27775. [DOI] [PubMed] [Google Scholar]
- 22.Swinkels DW, Wiegerinck E, Steegers EA, de Kok JB. Effects of blood-processing protocols on cell-free DNA quantification in plasma. Clin Chem. 2003;49:525–526. doi: 10.1373/49.3.525. [DOI] [PubMed] [Google Scholar]