Abstract
The purpose of the present study was to determine the potential relationships of glycemic control and use of metformin with non-muscle invasive bladder cancer characteristics. We reviewed data from 645 patients with non-muscle invasive bladder cancer between January 2004 and May 2015. We analyzed the association of pre and post-operative glycemic control and use of metformin with clinical characteristics of bladder tumors. We also analyzed the association of glycemic control and use of metformin with recurrence-free and progression-free survivals. Diabetes mellitus patients showed decreased recurrence-free survival (hazard ratio 1.42; 95% confidence interval 1.1–1.9; P = 0.021) and progression-free survival (hazard ratio 1.79; 95% confidence interval 1.1–2.8; P = 0.013). Diabetes mellitus patients with a HbA1c ≥ 7.0% demonstrated a higher rate of progression (P = 0.026). Kaplan-Meier analysis showed that progression-free survival rate was associated with poor baseline glycemic control (P = 0.026) and post-operative glycemic control (P = 0.025). However, use of metformin had no impact on the recurrence (P = 1.00) and progression (P = 0.282). In conclusion, poor baseline and post-operative glycemic control was related with shorter progression-free survival of patients with non-muscle invasive bladder cancer. Use of metformin had no impact on the recurrence and progression. Therefore, tight glycemic control and close follow-up for bladder tumor may be beneficial in patients with poor glycemic control.
Keywords: Diabetes Mellitus, Progression, Recurrence, Bladder Cancer, Metformin
Graphical Abstract
INTRODUCTION
Bladder cancer is the 7th most common cancer in men and the 17th most common in women in the world (1). Up to 85% of patients with bladder cancer present with disease confined to the mucosa (stage Ta and Tis) or submucosa (stage T1) at the time of presentation. However, non-muscle invasive bladder cancer (NMIBC) is characterized by a high risk of recurrence after transurethral resection of an initial tumor. Of these, 15%–61% recur within 1 year, 31%–78% recur within 5 years and 5%–20% progress to muscle invasive disease (2). Therefore, for oncologists who manage NMIBC, individual patient risk assessment of tumor recurrence and progression may guide treatment decisions and surveillance monitoring.
Diabetes mellitus (DM) is thought to be a factor which affects the treatment outcome of malignancies. Epidemiologic evidence indicates that type 2 DM is a risk factor for several cancers like colorectal cancer, breast cancer, endometrial cancer, hepatocellular cancer, but results for bladder cancer have been difficult to interpret and have not been established (3). Several studies have proposed an epidemiologic association between DM and bladder cancer (4,5,6). Indeed, if DM and bladder cancer risk have an association, it can be hypothesized that DM may have an influence on the recurrence or progression of bladder cancer. Also, DM-related status, such as glycemic control, possibly has an important clinical significance with respect to bladder cancer characteristics.
There has been considerable interest in the antitumor properties of metformin. Lin et al. (7) reported that the magnitude of cancer risk reduction and prolonged cancer onset times produced by metformin in patients with type 2 diabetes depended on the dose of metformin. Therefore, we determined the potential association of a history of DM and glycemic control with tumor characteristics and recurrence-free survival (RFS) and progression-free survival (PFS) among patients who underwent transurethral resection (TUR) for NMIBC. Also, we determined whether metformin use had an impact on the characteristics of bladder cancer.
MATERIALS AND METHODS
Study population
We retrospectively reviewed medical records of patients who underwent TUR for bladder tumor between January 2004 and May 2015 at Chonnam National University Hospital and Chonnam National University Hwasun Hospital. In this study, patients meeting the following criteria were selected: 1) histologically diagnosed as having urothelial cancer; 2) the first TUR for bladder tumor should have been performed at our institution and the tumor must be resected completely; 3) from the time of diagnosis of bladder tumor, HbA1c and metformin use should be followed up. Patients who did not have urothelial cancer, did not have HbA1c follow-up, or had carcinoma in situ (CIS) only and muscle invasive disease were excluded from the study. Based on these criteria, 645 patients were enrolled in the present study.
Data collection
Patients were divided into two groups based on diabetic status: 518 patients (80.3%; group I) did not have DM and 127 patients (19.7%; group II) had DM at the time of surgery. In group I, in order to confirm that they did not have DM at the time of surgery, medication history and preoperative fasting glucose levels were reviewed; Group II patients were identified as having DM at the time of surgery (all type2 DM) on the basis of a history of DM or medical therapy, and preoperatively elevated fasting glucose levels (> 126 mg/dL). We also divided group II into two groups according to the preoperative glycemic control status: 61 patients (48%; group IIA; good glycemic control group) did not have poor glycemic control and 66 patients (52%; group IIB; poor glycemic control group) had poor glycemic control. HbA1c levels were determined at the time of admission for initial TUR for NMIBC. In addition, DM patients were analyzed according to the postoperative glycemic control status. HbA1c levels were checked every three months until the first recurrence or progression and the reference point of good post-operative glycemic control was less than 7.0%. The reference point of good glycemic control after operation was HbA1c levels less than 7.0% at follow-up. Also, we divided the group II into two groups based on the metformin use: 66 patients (52%; group IIC; Non-metformin group) did not take metformin and 61 patients (48%, group IID; metformin group) took metformin.
The clinicopathological factors assessed were as follows: 1) age at the time of surgery, gender, history of smoking and comorbidities (DM, hypertension, serum creatinine); 2) tumor-related factors, including multiplicity, size, pathologic stage (based on the 2009 TNM classification) (8), grade (based on the 2004 World Health Organization (WHO) / International Society of Urological Pathology Consensus (ISUP) classification) (9), with or without intravesical therapy after TUR; and 3) disease status factors, such as recurrence, early recurrence and progression. Early recurrence was defined as recurrence within the first year. The correlation of these various clinicopathological features with preoperative HbA1c levels, postoperative HbA1c levels and history of metformin use was also investigated.
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The distribution of clinicopathological covariates between the two groups was evaluated by the Chi-squared test for categorical variables. Kaplan–Meier analysis was used to assess factors affecting recurrence-free and progression-free survival. RFS was defined as the time from initial TUR to first tumor recurrence (regardless of grade or stage), PFS was defined as the time from initial TUR to tumor progression, any increase in grade (G1/2 to G3) or stage (Ta to T1 or T2, T1 to T2) after repeat TUR for recurrence. Factors included in the univariate survival analysis were age, gender, smoking, DM, hypertension, intravesical treatment, stage, grade, size, multiplicity, and serum creatinine. Multivariate regression analysis using Cox proportional hazards regression model (the stepwise forward procedure) was performed to determine the adjusted hazard ratio (HR) to identify prognostic factors for RFS and PFS. The Mann-Whitney and Chi-squared tests were used for subgroup analysis of patients in Group II (stratified according to HbA1c levels). In all cases, two-tailed P < 0.05 was considered statistically significant.
Ethics statement
The study protocol was approved by the institutional review board of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2015-096). Informed consent was waived by the board.
RESULTS
Baseline characteristics in Groups I and II
The median age of all 645 patients enrolled in the study was 64.6 years, with a median follow-up time of 46 months (range, 3–172 months). Of the 645 patients, 127 patients (19.7%) were determined to have DM at the time of surgery (Group II) and 518 patients (80.3%) did not have DM (Group I). The baseline characteristics were similar in both groups in terms of gender, median follow-up time, smoking status, size, stage, and grade. However, compared with Group I patients, Group II patients were older, had a higher serum creatinine and body mass index (BMI) (P = 0.004) and higher rates of hypertension (P = 0.001), tumor multiplicity (P = 0.036), intravesical treatment (P = 0.002), recurrence (P = 0.018), early recurrence (P = 0.002) and progression (P = 0.010) (Table 1).
Table 1. Characteristics of patients with bladder tumors.
| Parameters | Total (n = 645) | Group I (n = 518) | Group II (n = 127) | P value |
|---|---|---|---|---|
| Median age, yr | 64.6 | 64.1 | 66.5 | |
| Median follow-up, mon | 46 | 44 | 50 | |
| Male:female ratio | 548:97 | 437:81 | 111:16 | 0.410* |
| Smoking | 314 (48.7) | 253 (48.8) | 61 (48.0) | 0.921* |
| BMI | 23.5 ± 0.12 | 23.3 ± 3.0 | 24.4 ± 3.0 | |
| < 23 | 248 (47.9) | 40 (31.5) | 0.004* | |
| 23-24.9 | 105 (20.3) | 33 (26.0) | ||
| ≥ 25 | 165 (31.9) | 54 (42.5) | ||
| Hypertension | 239 (37.0) | 161 (31.1) | 78 (61.4) | 0.001* |
| Mean (± SD) serum creatinine, mg/dL | 1.07 ± 0.02 | 1.03 ± 0.42 | 1.22 ± 0.83 | 0.001* |
| Tumor multiplicity | ||||
| 1-2 | 355 (55.0) | 296 (57.1) | 59 (46.5) | 0.036* |
| ≥ 3 | 290 (45.0) | 222 (42.9) | 68 (53.5) | |
| Tumor size | ||||
| < 3 cm | 451 (69.9) | 356 (69.7) | 95 (74.8) | 0.277* |
| ≥ 3 cm | 187 (28.9) | 155 (30.3) | 32 (25.2) | |
| Tumor stage | ||||
| Ta | 440 (68.2) | 357 (68.9) | 83 (65.4) | 0.457* |
| T1 | 205 (31.8) | 161 (31.1) | 44 (34.6) | |
| Grade | ||||
| PUNLMP | 22 (3.4) | 15 (2.9) | 7 (5.5) | 0.215* |
| Low | 399 (61.9) | 327 (63.1) | 72 (56.7) | |
| High | 224 (34.7) | 176 (34.0) | 48 (37.8) | |
| Intravesical treatment | ||||
| No | 198 (30.7) | 174 (33.6) | 24 (18.9) | 0.002* |
| Yes | 447 (69.3) | 344 (66.4) | 103 (81.1) | |
| Recurrence | 234 (36.3) | 176 (34.0) | 58 (45.7) | 0.018* |
| Early Recurrence | 145 (22.5) | 103 (19.9) | 42 (33.1) | 0.002* |
| Progression | 86 (13.3) | 60 (11.6) | 26 (20.5) | 0.010* |
Unless indicated otherwise, data are given as the number of patients in each group, with the percentage given in parentheses.
Group I, Patients without diabetes mellitus; Group II, Patients with diabetes mellitus; PUNLMP, Papillary urothelial neoplasm of low malignant potential; BCG, Bacillus Calmette-Guerin; BMI, Body mass index.
*χ2 test.
Association between clinical parameters and survival in patients
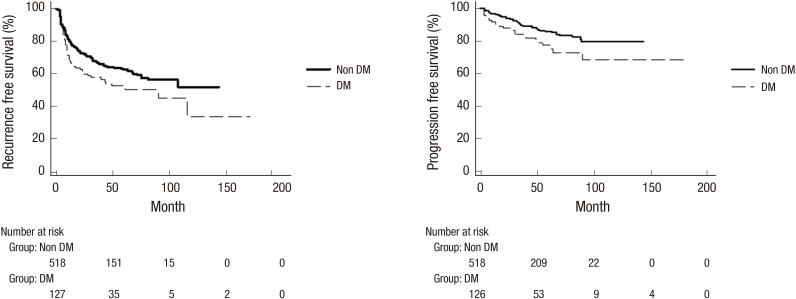
Univariate Cox proportional hazards regression model analysis and Kaplan-Meier analysis showed that DM was associated with decreased RFS (HR, 1.42; 95% confidence interval (CI) 1.06–1.91; P = 0.021) and PFS (HR, 1.79; 95% CI, 1.13–2.84; P = 0.013) (Table 2, Fig. 1). Besides age (≥ median age), multiplicity (≥ 3), high grade (P = 0.001), T1 stage (P = 0.033) and serum creatinine (> 1.5 mg/dL) were related to shorter RFS (P = 0.014), and age (≥ median age), high grade, T1 stage and multiplicity (≥ 3) were related to shorter PFS (P = 0.001) (Table 2). Multivariate regression analysis (Table 3) revealed that old age (P = 0.001), multiplicity (≥ 3) (P = 0.001) and serum creatinine (> 1.5 mg/dL) (P = 0.036) were related to an increased risk of recurrence, whereas old age (P = 0.022), high grade (P = 0.001) and multiplicity (≥ 3) (P = 0.015) were related to an increased risk of progression.
Table 2. Factors affecting recurrence and progression-free survival in total patients using univariate survival analysis.
| Factors | Recurrence-free survival | Progression-free survival | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (≥ median age) | 1.02 (1.01-1.04) | 0.001 | 1.04 (1.02-1.06) | 0.001 |
| Gender (female) | 0.88 (0.60-1.27) | 0.485 | 1.05 (0.58-1.89) | 0.880 |
| BMI (≥ 25) | 0.99 (0.95-1.03) | 0.597 | 0.96 (0.90-1.03) | 0.288 |
| DM | 1.42 (1.06-1.91) | 0.021 | 1.79 (1.13-2.84) | 0.013 |
| Hypertension | 1.95 (0.92-1.55) | 0.183 | 1.33 (0.87-2.04) | 0.193 |
| Intravesical treatment | 1.22 (0.92-1.63) | 0.172 | 0.99 (0.63-1.56) | 0.975 |
| Grade (high grade) | 1.55 (1.19-2.01) | 0.001 | 4.57 (2.92-7.15) | 0.001 |
| Stage (T1) | 1.34 (1.02-1.74) | 0.033 | 3.67 (2.38-5.65) | 0.001 |
| Multiplicity (≥ 3) | 1.80 (1.39-2.33) | 0.001 | 2.55 (1.63-3.98) | 0.001 |
| Size (≥ 3 cm) | 0.97 (0.73-1.29) | 0.853 | 1.04 (0.65-1.65) | 0.879 |
| Creatinine (> 1.5 mg/dL) | 1.24 (1.04-1.47) | 0.014 | 1.23 (0.98-1.56) | 0.078 |
| Smoking | 0.90 (0.70-1.17) | 0.471 | 0.79 (0.51-1.21) | 0.282 |
Fig. 1.
Recurrence and progression-free survival according to diabetes mellitus status.
Table 3. Recurrence and progression-free survival in total patients using multivariate survival analysis.
| Factors | Recurrence-free survival | Progression-free survival | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age (≥ median age) | 1.02 (1.09-1.30) | 0.001 | 1.02 (1.04-1.08) | 0.022 |
| DM | 1.22 (0.89-1.67) | 0.211 | 1.54 (0.95-2.50) | 0.080 |
| Grade (high grade) | 1.36 (0.93-1.97) | 0.105 | 2.67 (1.47-4.85) | 0.001 |
| Multiplicity (≥ 3) | 1.61 (1.22-2.10) | 0.001 | 1.76 (1.11-2.79) | 0.015 |
| Creatinine (>1.5 mg/dL) | 1.22 (1.01-1.48) | 0.036 | 1.12 (0.87-1.45) | 0.352 |
Subgroup analysis of DM patients according to pre-operative HbA1c levels
We divided DM patients into subgroups according to pre-operative HbA1c levels (≥ 7.0% vs. < 7.0%); 61 patients had good glycemic control (HbA1c < 7.0%) and 66 patients had poor glycemic control (HbA1c ≥ 7.0%). Compared with patients who had good glycemic control, the poor glycemic control group demonstrated a significantly higher rate of intravesical treatment (P = 0.006) and progression rate (P = 0.026). In addition, patients with good pre-operative HbA1c levels were found to have good post-operative HbA1c levels (Table 4).
Table 4. Characteristics of subjects with diabetes mellitus and bladder cancer according to the pre-operative HbA1c levels.
| Parameters | HbA1c < 7.0 (n = 61) | HbA1c ≥ 7 (n = 66) | P value |
|---|---|---|---|
| BMI (≥ 25) | 24.6 ± 2.7 | 24.3 ± 3.3 | 0.591* |
| Creatinine (> 1.5 mg/dL) | 1.092 ± 0.42 | 1.347 ± 1.07 | 0.085* |
| Hypertension | 38 (62.3) | 40 (60.6) | 0.857* |
| Tumor stage | |||
| Tis | 0 (0) | 0 (0) | 0.064* |
| Ta | 45 (73.8) | 38 (57.6) | |
| T1 | 16 (26.2) | 28 (42.4) | |
| Grade | |||
| PUNLMP | 4 (6.6) | 3 (4.5) | 0.730* |
| Low | 36 (59.0) | 36 (54.2) | |
| High | 21 (34.4) | 28 (42.4) | |
| Tumor size | |||
| < 3 cm | 45 (73.8) | 50 (75.8) | 0.840* |
| ≥ 3 cm | 16 (26.2) | 16 (24.2) | |
| Tumor multiplicity | |||
| < 3 | 30 (49.2) | 29 (43.9) | 0.596* |
| ≥ 3 | 31 (50.8) | 37 (56.1) | |
| Intravesical treatment | 43 (70.5) | 60 (90.9) | 0.006* |
| Recurrence | 28 (45.9) | 30 (45.5) | 1.000* |
| Early recurrence | 20 (32.8) | 22 (33.3) | 1.000* |
| Progression | 7 (11.5) | 19 (28.8) | 0.026* |
| Metformin use | 30 (49.2) | 31 (47.0) | 0.860* |
| Post-operative HbA1c (< 7.0%) | 54 (88.5) | 14 (21.2) | 0.001* |
| Smoking | 31 (50.8) | 30 (45.5) | 0.596* |
Unless indicated otherwise, data are given as the number of patients in each group, with the percentage given in parentheses.
PUNLMP, Papillary urothelial neoplasm of low malignant potential; BMI, Body mass index.
*χ2 test.
Association between clinical parameters and survival in DM patients
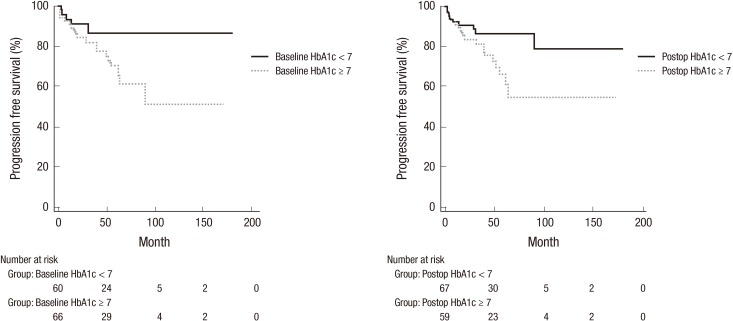
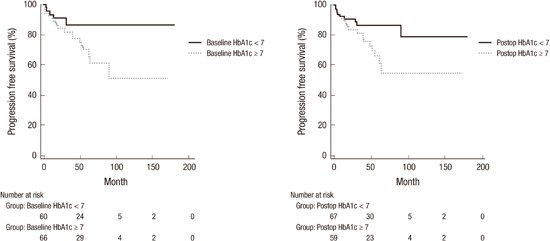
Univariate Cox proportional hazards regression model analysis and Kaplan-Meier analysis according to DM control status showed that any factors were not associated with RFS and that BMI (≥ 25) (P = 0.038), high grade (P = 0.005), T1 stage (P = 0.001), multiplicity (≥ 3) (P = 0.035), baseline HbA1c (≥ 7%) (P = 0.033) and post-operative HbA1c (≥ 7%) (P = 0.032) were related to shorter PFS (Table 5, Fig. 2). Multivariate survival analysis revealed that any factors were not associated with RFS and PFS (P = 0.223).
Table 5. Factors affecting recurrence and progression-free survival in diabetes mellitus patients using univariate survival analysis.
| Factors | Recurrence-free survival | Progression-free survival | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (≥ median age) | 0.99 (0.97-1.02) | 0.577 | 1.04 (0.99-1.08) | 0.117 |
| Gender (female) | 1.02 (0.46-2.25) | 0.959 | 1.37 (0.47-3.97) | 0.567 |
| BMI (≥ 25) | 1.02 (0.93-1.11) | 0.701 | 0.87 (0.77-0.99) | 0.038 |
| Hypertension | 1.53 (0.89-2.66) | 0.128 | 1.70 (0.73-3.95) | 0.216 |
| Intravesical treatment | 0.76 (0.40-1.44) | 0.406 | 0.92 (0.35-2.44) | 0.866 |
| Grade (high grade) | 1.04 (0.60-1.77) | 0.898 | 3.09 (1.41-6.76) | 0.005 |
| Stage (T1) | 1.14 (0.66-1.96) | 0.643 | 3.69 (1.68-8.11) | 0.001 |
| Multiplicity (≥ 3) | 1.48 (0.87-2.51) | 0.146 | 2.54 (1.07-6.05) | 0.035 |
| Size (≥ 3 cm) | 0.91 (0.50-1.66) | 0.748 | 0.77 (0.29-2.06) | 0.604 |
| Creatinine (> 1.5 mg/dL) | 1.06 (0.78-1.43) | 0.708 | 1.05 (0.71-1.56) | 0.791 |
| Pre-operative HbA1c (≥ 7%) | 0.94 (0.56-1.58) | 0.826 | 2.57 (1.08-6.13) | 0.033 |
| Post-operative HbA1c (≥ 7%) | 0.87 (0.51-1.46) | 0.605 | 2.43 (1.08-5.48) | 0.032 |
| Metformin use | 1.07 (0.64-1.80) | 0.795 | 1.52 (0.70-3.33) | 0.286 |
| Smoking | 1.06 (0.63-1.79) | 0.816 | 0.89 (0.41-1.95) | 0.777 |
Fig. 2.
Progression-free survival according to pre and post-operative glycemic control.
Subgroup analysis of DM patients according to metformin use
We divided DM patients into subgroups according to the use of metformin; there were 66 never-users of metformin and 61 ever-users of metformin. But, metformin use was not associated with progression (P = 0.282) and recurrence (P = 1.00).
DISCUSSION
Recently, the prevalence of diabetes mellitus and cancer is increasing. It is not surprising that the incidence of concomitant occurrence of two diseases will increase. Diabetes was found to be common in some cancers, including breast, colorectal, endometrial, liver, and pancreatic cancers (3,10,11,12,13). Epidemiologic studies of diabetes and the risk of bladder cancer have reported inconsistent results. Several cohort studies have reported that diabetes is related to a significant 1.3 to 2.5-fold increased risk of bladder cancer (14,15,16). Conversely, others have failed to find any association between the two disease entities (17,18,19). A recent updated meta-analysis of 36 observational studies showed that DM was associated with an increased risk of bladder cancer (the summary RR, 1.35; 95% CI, 1.17-1.56; P < 0.001) (20). However, there are limited data regarding the relationship between glycemic control of DM and recurrence or progression of bladder cancer. Hence, the present study focused on DM and glycemic control and whether it affected the RFS and PFS.
The risks for both recurrence and progression in NMIBC are associated with multiple tumor-related factors, including histologic grade, depth of invasion, multiplicity, tumor size, tumor morphology, the presence or absence of vascular or lymphatic invasion, and the presence or absence of CIS. Although these tumor-related factors provide some prognostic information, they ultimately fail to clearly evaluate the malignant potential of individual tumors. Therefore, there is a need to determine other clinical factors to predict tumor recurrence or progression in patients with NMIBC. In a study of clinical risk factors, Joo et al. (21) reported that patients with NMIBC who had high serum creatinine levels (1.5 mg/dL) had more frequent recurrence, but not progression, than those with low serum creatinine levels. In addition, the authors suggested that DM may affect tumor recurrence (HR, 2.06; 95% CI, 0.89–4.72; P = 0.091). Our results for DM reinforce these findings (for recurrence, HR, 1.42; 95% CI, 1.1–1.9; P = 0.021; for progression, HR, 1.79; 95% CI, 1.1–2.8; P = 0.013); furthermore, results for high serum creatinine level (HR, 1.24; 95% CI, 1.0-1.5; P = 0.014) were consistent with those in the study by Joo et al. (21), with no significant association being found between serum creatinine and progression in the present study.
The possible mechanisms underlying the association of diabetes with bladder cancer risk are uncertain. In type 2 diabetes, insulin resistance leads to a state of hyperinsulinemia (22,23). Insulin has mitogenic properties and could stimulate tumor growth by increasing bioactive IGF-I, which in turn stimulates cell proliferation and inhibits apoptosis (24). In the circulation, IGF-I binds mainly to the main IGF binding protein, IGFBP-3 (25). Several epidemiological studies have implicated IGF-I and IGFBP-3 in the development of prostate, breast and colorectal cancers (26). IGF-I and IGFBP-3 may also play a role in the development of bladder cancer. In a US case–control study, statistically significantly higher plasma IGF-I concentrations and a higher molar ratio of IGF-I to IGFBP-3 were observed in patients with bladder cancer compared with controls (27).
An additional purpose of the present study was to evaluate tumor characteristics according to HbA1c. Poor glycemic control was associated with progression, even though more patients with poor glycemic control received intravesical treatment than patients with good glycemic control. Also, baseline HbA1c (≥ 7%) (P = 0.033) and post-operative HbA1c (≥ 7%) (P = 0.032) were related to shorter PFS. Notably, poor glycemic control was not associated with tumor characteristics and recurrence. The finding of no significant difference in the recurrence can be explained as that the baseline HbA1c (≥ 7%) group received more intravesical treatment, although there was no difference in tumor characteristics between controlled and uncontrolled diabetic patients. As known, intravesical treatment could decrease the recurrence rate in patients with NMIBC. In spite of intravesical treatment, the HbA1c (≥ 7%) group had a higher progression rate. Therefore, early active treatment and good postoperative glycemic control might be necessary in the HbA1c (≥ 7%) group.
The importance of glycemic control has been highlighted in other types of cancer. Siddiqui et al. (28) reported that poor glycemic control, as judged by the HbA1c level, independently predicted the early onset of colorectal cancer, a more advanced stage at the time of presentation, and poorer 5-year survival. Tai et al. (29) reported that poorly controlled diabetic patients (HbA1c ≥ 7.0%) with upper urinary tract urothelial carcinoma exhibited a shorter duration of RFS in bladder cancer as compared to those with good glycemic controlled DM and without DM (log-rank test, P < 0.001 and < 0.001, respectively).
Metformin has been demonstrated to exert anticancer effects in several types of cancer, including those affecting the liver, colon, breast, pancreas, and prostate (7). However, the utility of metformin in the prevention and treatment of bladder cancer has not been specifically investigated. Rieken et al. (30) reported that patients with DM who did not take metformin had a greater risk of disease recurrence (HR 1.45, 95% CI 1.09–1.94, P = 0.01) and progression (HR 2.38, 95% CI 1.40-4.06, P = 0.001). DM with metformin use was independently associated with a lower risk of disease recurrence (HR: 0.50, 95% CI 0.27–0.94, P = 0.03). However, our results regarding metformin use are inconsistent with those in the study by Rieken et al. (30), with no significant association being found between metformin use and recurrence or progression. This difference might be explained by the previous study not including data about serum creatinine, BMI and DM control (HgbA1c). Metformin is generally recommended as the 1st choice drug in patients with early DM, but is not recommended in patients with renal insufficiency because of fears about lactic acid accumulation (33). In this study, renal insufficiency and poor DM control were related with poor prognosis of patients with NMIBC. So the effect of metformin in the previous study could be influenced by DM control or other parameters including BMI and creatinine clearance. In this study, use of metformin had no impact on the recurrence and progression of bladder cancer when there was no difference about DM control, serum creatinine, and BMI between metformin ever-users and metformin never-users.
In previous studies showing that metformin affected the outcome of cancer, they have provided the following biochemical basis. First, the mechanisms may involve AMP-activated protein kinase (AMPK)-dependent and AMPK-independent pathways (31,32). The activation of AMPK is indirect and appears to act via inhibition of mitochondrial complex I in the respiratory chain resulting in an increase in the AMP:ATP ratio (31). Metformin may inhibit cell cycle progression and cell proliferation through down-regulating cyclin D1 as an AMPK-dependent effect in breast cancer cells, or activating the HIF target gene REDD1 leading to the inhibition of the mammalian target of rapamycin (mTOR) pathway and cell cycle arrest in prostate cancer cells (31). Second, metformin inhibits hepatic glucose output and improves insulin sensitivity with lowering of circulating levels of insulin and glucose, reduces insulin-like growth factors (IGFs), decreases Akt phosphorylation, and inhibits the crosstalk between receptors of insulin/IGF1 and G protein-coupled receptor signaling pathways (31). Although it is reasonable to speculate that metformin may prevent the development of bladder cancer through these molecular mechanisms, clearly, more basic in vitro and in vivo studies are warranted.
The present study has several limitations. First, we did not consider the potential impact of biochemical data such as levels of glucose, insulin, C-peptide, and IGFs. Second, we did not assess the duration of DM, which may have been affected by recall bias or a period of undetected DM. Third, our results might have been affected by a selection bias because of the possibility of undiagnosed DM among patients who were classified as not having DM (Group I). Fourth, the baseline HbA1c (≥ 7%) group received more intravesical treatment, because patients with HbA1c ≥ 7.0% exhibited trends of higher rate of multiplicity, tumor grade and T1 stage. This might explain the finding that DM control was not related with shorter RFS of patients with NMIBC.
However, this study presented two significant facts. First, the blood sugar level might be controlled before and after TUR-BT in DM patients with NMIBC to expect a good result for the tumor. Second, the urologic clinician must consider controlling the blood sugar level after TUR-BT in DM patients and more aggressive care and follow-up are needed in DM patients with poor DM control.
DM patients with NMIBC showed decreased RFS and PFS. Poor baseline DM control and post-operative DM control were related with shorter PFS of patients with NMIBC. Use of metformin had no impact on the recurrence and progression. Therefore, tight glycemic control and close follow-up for bladder tumor may be beneficial in patients with poor glycemic control.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception and design: Jung SI, Hwang EC. Performing the experiments: Ahn JH, Jung SI. Data acquisition: Ahn JH, Yim SU, Kim SW. Data analysis and interpretation: Jung SI, Hwang EC. Statistical analysis: Hwang EC. Drafting of the manuscript: Ahn JH. Critical revision of the manuscript: Jung SI, Hwang EC. Approval of final manuscript: all authors.
References
- 1.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijden AG, Witjes JA. Recurrence, progression, and follow-up in non-muscle-invasive bladder cancer. Eur Urol Suppl. 2009;8:556–562. [Google Scholar]
- 3.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie T, Zens MS, Ferrara A, Schned A, Karagas MR. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer. 2011;117:1552–1556. doi: 10.1002/cncr.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang H, Yao B, Yan Y, Xu H, Liu Y, Tang H, Zhou J, Cao L, Wang W, Zhang J, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther. 2013;15:914–922. doi: 10.1089/dia.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49:2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 7.Lin HC, Kachingwe BH, Lin HL, Cheng HW, Uang YS, Wang LH. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: a 6-year follow-up study. Pharmacotherapy. 2014;34:36–45. doi: 10.1002/phar.1334. [DOI] [PubMed] [Google Scholar]
- 8.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. West Sussex: Wiley-Blackwell; 2009. [Google Scholar]
- 9.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: the 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 12.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi A, Folsom AR, Anderson KE, Iowa Women’s Health Study Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women’s Health Study. Cancer. 2002;95:2316–2323. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 16.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Larsson SC, Andersson SO, Johansson JE, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44:2655–2660. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Gale EA, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92:2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zendehdel K, Nyrén O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Wang X, Shen Z, Lu Y, Zhong S, Xu C. Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer. 2013;13:310. doi: 10.1186/1471-2407-13-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joo JS, Kim JS, Jung SI, Kang TW, Kwon DD, Choi C, Park KS, Ryu SB. The prognostic significance of elevated serum creatinine for the recurrence and progression in superficial bladder tumors. Korean J Urol. 2007;48:927–932. [Google Scholar]
- 22.Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach LA, Rechler MM. Insulin-like growth factors and diabetes. Diabetes Metab Rev. 1992;8:229–257. doi: 10.1002/dmr.5610080304. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 25.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 26.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, Wu X. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol. 2003;169:714–717. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci. 2008;53:2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 29.Tai YS, Chen CH, Huang CY, Tai HC, Wang SM, Pu YS. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev. 2015;31:307–314. doi: 10.1002/dmrr.2614. [DOI] [PubMed] [Google Scholar]
- 30.Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, Lotan Y, Karakiewicz PI, Fajkovic H, Babjuk M, et al. Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU Int. 2013;112:1105–1112. doi: 10.1111/bju.12448. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 32.Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]