Abstract
We aimed to compare fentanyl, remifentanil and dexmedetomidine with respect to hemodynamic stability, postoperative pain control and achievement of sedation at the postanesthetic care unit (PACU). In this randomized double-blind study, 90 consecutive total laparoscopic hysterectomy patients scheduled for elective surgery were randomly assigned to receive fentanyl (1.0 µg/kg) over 1 minute followed by a 0.4 µg/kg/hr infusion (FK group, n = 30), or remifentanil (1.0 µg/kg) over 1 minute followed by a 0.08 µg/kg/min infusion (RK group, n = 30), or dexmedetomidine (1 µg/kg) over 10 minutes followed by a 0.5 µg/kg/hr infusion (DK group, n = 30) initiating at the end of main procedures of the operation to the time in the PACU. A single dose of intravenous ketorolac (30 mg) was given to all patients at the end of surgery. We respectively evaluated the pain VAS scores, the modified OAA/S scores, the BIS, the vital signs and the perioperative side effects to compare the efficacy of fentanyl, remifentanil and dexmedetomidine. Compared with other groups, the modified OAA/S scores were significantly lower in DK group at 0, 5 and 10 minutes after arrival at the PACU (P < 0.05), whereas the pain VAS and BIS were not significantly different from other groups. The blood pressure and heart rate in the DK group were significantly lower than those of other groups at the PACU (P < 0.05). DK group, at sedative doses, had the better postoperative hemodynamic stability than RK group or FK group and demonstrated a similar effect of pain control as RK group and FK group with patient awareness during sedation in the PACU. (World Health Organization registry, KCT0001524).
Keywords: Dexmedetomidine, Fentanyl, Remifentanil, Surgery, Pain Control, Hemodynamic Stability
Graphical Abstract
INTRODUCTION
Perioperative and postoperative pain control, maintenance of hemodynamic stability, proper sedation and awakening are a major part of anesthetic management. The opioids such as fentanyl and remifentanil have been widely used in operating room and PACU. Dexmedetomidine, recently introduced, is often used as it has both analgesic potency and sedative effect.
Dexmedetomidine is the highly selective α2-adrenergic agonist, which provides sedation, anxiolysis, hypnosis, analgesia, and sympatholysis (1). A loading dose of dexmedetomidine is 1 µg/kg over 10 minutes, and maintenance dose is 0.2-0.7 µg/kg/hr for procedural sedation. It has been used in the operating room and the intensive care unit (ICU) for sedation (2). It is not associated with respiratory depression, despite profound levels of sedation (3). It decreases sympathetic outflow of central nervous system in a dose-dependent manner and has analgesic effects best described as “opioid-sparing” (4). It has been known to reduce opioid requirements by 30% to 50% (5). Consistent with the pharmacological effect, dexmedetomidine decreases heart rate (HR) and arterial pressure. These hemodynamic changes are associated with reduction in norepinephrine plasma levels (6). The analgesic potential of α2-agonists, however, does not approximate the potency of opioids. Nevertheless, in neuropathic pain in which opioid relief is suboptimal, α2-agonists may offer specific advantages (7). These properties (sedation, lack of respiratory depression and analgesia sparing) of dexmedetomidine help reduce the significant postoperative pain and provide proper sedation after major surgical procedures, which can result in hemodynamic stability in PACU.
Although fentanyl and remifentanil have been commonly used for pain control and sedation in perioperative and postoperative period, dexmedetomidine can be an effective alternative as it has both analgesic and sedative effect like fentanyl and remifentanil. The aim of this study is to compare the efficacy of dexmedetomidine and opioids such as fentanyl and remifentanil with respect to hemodynamic stability, sedation and postoperative pain control after completion of total laparoscopic hysterectomy.
MATERIALS AND METHODS
Study subjects
With ethics committee approval and written informed consent, 90 Female patients aged 18-60 years with American Society of Anesthesiologists (ASA) physical status I-II patients scheduled for laparoscopic total hysterectomy with general anesthesia were included in a prospective, randomized, double-blind study.
The exclusion criteria included a history of recent respiratory tract infection, alcohol abuse, diabetes mellitus, hypertension and patients with severe bronchopulmonary disease, cardiovascular, endocrinological, neuropathic, renal or hepatic disorders, an allergy to opioids or current use of analgesics or psychoactive drugs.
Before surgery, the patients were randomly divided into three groups using a computer-generated random number table and sealed envelope method (Table 1). Randomization was performed by an anesthesiologist who was not involved in the anesthetic management of the patients or data collection. In every case, study drugs were administered by anesthetic nurses while the anesthesiologist in charge did not know what they were. All patients were premedicated with an intramuscular injection of glycopyrrolate (0.2 mg), 30 minutes before their arrival in the operating room. In the operating room, routine monitoring included three lead electro-cardiogram (EKG), non-invasive systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, heart rate (HR), respiratory rate, pulse oximetry and end-tidal CO2.
Table 1. Demographic findings of subjects by the group.
| Parameters | FK group (n = 30) | RK group (n = 30) | DK group (n = 30) |
|---|---|---|---|
| Age, yr | 49.7 ± 5.1 | 46.6 ± 3.2 | 45.1 ± 3.9 |
| Weight, kg | 66.6 ± 7.1 | 63.8 ± 6.1 | 61.7 ± 9.3 |
| ASA (I/II) | 28/2 | 24/6 | 27/3 |
| Height, cm | 159.1 ± 6.3 | 161.8 ± 5.5 | 160.6 ± 4.9 |
| Duration of operation, min | 108.0 ± 21.2 | 99.8 ± 21.6 | 97.3 ± 24.9 |
| Duration of anesthesia, min | 137.1 ± 13.6 | 126.9 ± 30.4 | 132.1 ± 18.1 |
Data are expressed as mean ± SD.
FK group, fentanyl group; RK group, remifentanil group; DK group, dexmedetomidine group; ASA, American Society of Anesthesiologists.
Electrodes for monitoring the Bispectral Index (BISTM, model A-2000®; Aspect Medical Systems, USA) were attached to the head on arrival to the operating room. Visual analogue scales (VAS) scores for pre- or post-operative pain were measured and a modified observer's assessment of alertness (OAA/S) score of sedation and hypnosis were measured in the postanesthetic care unit (PACU) (Table 2).
Table 2. Observer's assessment of alertness/sedation score.
| Assessment | Score |
|---|---|
| Responds readily to name spoken in normal tone | 5 (Alert) |
| Lethargic response to name spoken in normal tone | 4 |
| Responds only after name is called loudly and/or repeatedly | 3 |
| Responds only after mild prodding or shaking | 2 |
| Responds only after painful trapezius squeeze | 1 |
| Does not respond to painful trapezius squeeze | 0 |
Induction was accomplished with full preoxygenation, 1% lidocaine (40 mg) and 1% propofol (2 mg/kg), followed by rocuronium (0.6 mg/kg). Anesthesia was maintained with O2 at 2 L/min, N2O at 3 L/min, 6–7 vol% of desflurane, and rocuronium was given if required. The end-tidal CO2 was maintained at 35–40 mmHg.
We started administration of the study drugs (fentanyl, remifentanil, dexmedetomidine) at a point when pain is began to reduce, i.e. at 60 minutes intraoperatively (t5-op-60). A single dose of intravenous ketorolac (30 mg) was given to all patients at the end of surgery and additional study drugs were administered according to group designation. The total dose of fentanyl, remifentanil and dexmedetomidine administered during surgery and in the PACU was recorded.
Group FK (n = 30) was given a loading dose of fentanyl (1.0 µg/kg) over 1 minute followed by a continuous infusion of 0.4 µg/kg/hr and group RK (n = 30) was given a loading dose of remifentanil (1.0 µg/kg) over 1 minute followed by continuous infusion of 0.08 µg/kg/min. For group DK (n = 30), a loading dose of dexmedetomidine (1 µg/kg) was given over 10 minutes followed by a continuous infusion of 0.5 µg/kg/hr at the end of main procedures of the operation to the time in the PACU. The doses used in three groups were considered sedative doses. HR below 50 beats/min was treated with atropine (10 µg/kg) intravenously. SBP below 80 mmHg was treated with ephedrine (5 mg) intravenously.
Anesthetic gases were turned off 5 minutes before the completion of surgery and at the end of surgery, the patients were given 10 mg of pyridostigmine and 0.2 mg of glycopyrrolate intravenously. The patients were ventilated with 100% oxygen at 5 L/min until the patient was fully awake and had recovered from the muscle relaxation.
Blood pressure and HR were measured every 5 minutes and were recorded for this study at t0 (before induction), t1 (after induction), t2 (after incision), t3 (operation for 15 minutes), t9 (operation for 90 minutes) and R0 (arrival at recovery room) and R30 (30 minutes after arrival at recovery room).
The incidences of postoperative side effects (nausea, vomiting, dry mouth, shivering, hypotension, bradycardia, desaturation, etc.) in three groups were recorded in the PACU. The BIS, VAS scores, modified OAA/S scores of sedation, vital signs, respiratory rate and end-tidal CO2 were also measured simultaneously (8,9).
Statistical analysis
The number of patients required in each group was determined using a power calculation based on data from our preliminary study of dexmedetomidine and its influence on the haemodynamic responses (unpublished). This calculation established that 30 patients were required in each group for a type I error of 0.05, a type II error of 0.2, Δ = 10 and SD = 12 for the SBP parameter. Recruitment was increased by 10% to compensate for unexpected loss.
Statistical analyses were performed using GraphPad Prism (GraphPad Software, USA, 5.0). A one-way ANOVA with Tukey's post hoc multiple comparison test was used for comparison between groups. The results are expressed as the mean ± SD or absolute number. A P value of < 0.05 was considered to be statistically significant.
Ethics statement
The study protocol was approved by our institutional review board of St. Vincent's Hospital, The Catholic University of Korea (VC13MISI0080). Informed consent was submitted by the recruited subjects. This study was listed on a World Health Organization recognized registry (KCT0001524).
RESULTS
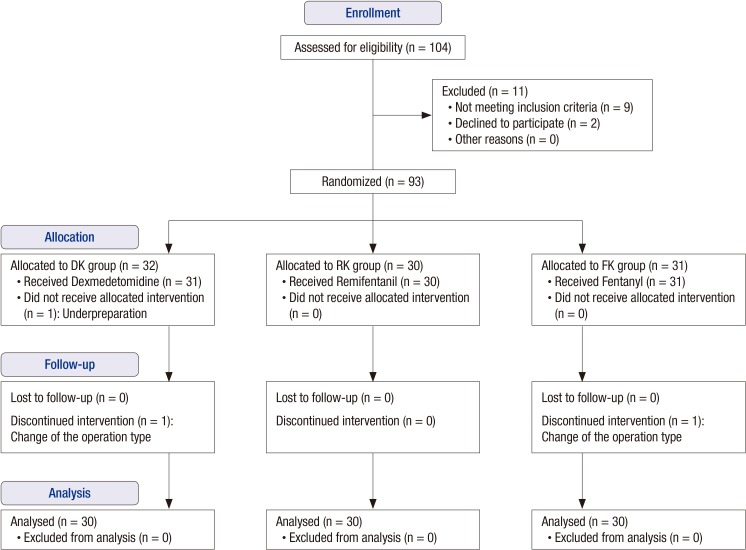
A total 93 patients were randomized into the study. One patient was excluded due to underpreparation, and 92 patients were allocated to intervention groups and received the allocated interventions. Of these, two patients were lost to follow-up due to change to open abdominal hysterectomy (Fig. 1).
Fig. 1.
Flow diagram of progress through the study in Comparison of an intraoperative infusion of dexmedetomidine, fentanyl and remifentanil on perioperative hemodynamics, sedation quality and postoperative pain control.
The three groups had similar age, weight, height, ASA status, duration of the operation time and duration of the anesthetic time (Table 1). The patients in each group received a total of 137.7 ± 40.2 µg of DEX, 108.5 ± 29 µg of fentanyl, and 416.2 ± 37.2 µg of remifentanil.
Table 2 summarizes the adverse events during the study. There were no significant differences in the incidence of side effects among the three groups (Table 3). These adverse events were observed mainly during the immediate postoperative period. Dry mouth was reported in three patients in Group DK. Nausea, vomiting, shivering and bradycardia were reported in Group FK and Group RK.
Table 3. Side effect profiles for each group.
| Side effects | No. (%) of patients | ||
|---|---|---|---|
| FK group (n = 30) | RK group (n = 30) | DK group (n = 30) | |
| Nausea | 3 (10) | 3 (10) | 0 (0) |
| Vomiting | 3 (10) | 2 (6.7) | 0 (0) |
| Dry mouth | 0 (0) | 0 (0) | 3 (10) |
| Shivering | 3 (10) | 3 (10) | 0 (0) |
| Hypotension (SBP < 80 mmHg) | 1 (3.3) | 2 (6.7) | 0 (0) |
| Bradycardia (< 40 rate/min) | 1 (3.3) | 3 (10) | 1 (3.3) |
Hypotension: SBP < 80 mmHg or DBP < 50 mmHg or < 30% level before drug injection.
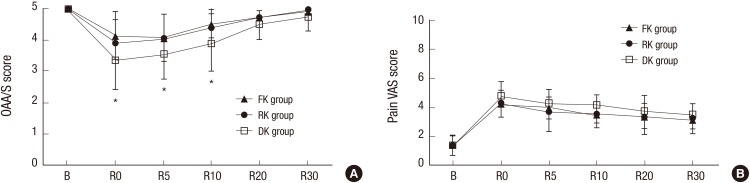
Modified OAA/S scores of sedation were significantly lower in Group DK compared with other Groups at R0 (arrival at recovery room), and at R5 and R10 (Fig. 2A). VAS scores of postoperative pain were not significantly different among the three groups (Fig. 2B).
Fig. 2.
Changes in (A) modified observer's assessment of alertness /sedation (OAA/S) scores and (B) pain visual analogue scale (VAS) scores for the three groups.
FK group, fentanyl-ketorolac group; RK group, remifentanil-ketorolac group; DK group, dexmedetomidine-ketorolac group. B, arrival at operation; R0, arrival at recovery room, R5, 5 minutes after arrival at recovery room; R10, 10 minutes after arrival at recovery room; R20, 20 minutes after arrival at recovery room; R30, 30 minutes after arrival at recovery room.
*DK group Indicates significantly different than other groups (P < 0.05).
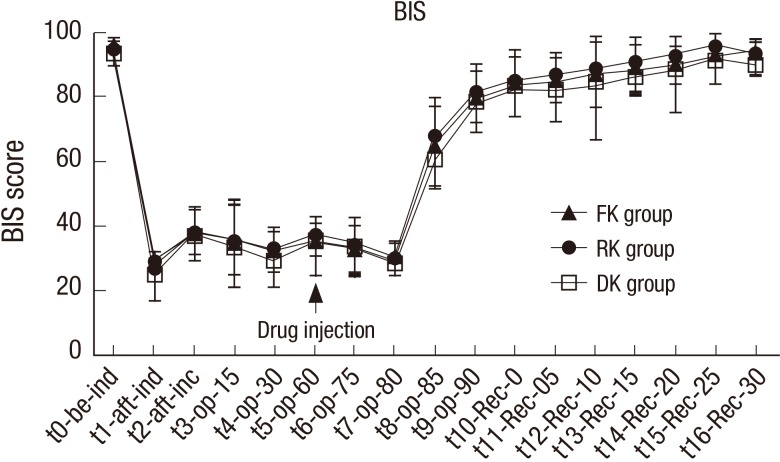
Changes in the BIS were not significantly different among the three groups at all times from t0 (before induction time) to t16-Rec-30 (30 minutes after arrival at recovery room; Fig. 3).
Fig. 3.
Changes in bispectral index (BIS) for the three groups. FK group, fentanyl-ketorolac group.
RK group, remifentanil-ketorolac group; DK group, dexmedetomidine-ketorolac group. t0, before induction time; t1, after induction time; t2, after incision time; t3, operation 15 minutes; t4, operation 30 minutes; t5, operation 60 minutes: t6, operation 75 minutes; t7, operation 80 minutes; t8, operation 85 minutes; t9, operation 90 minutes; t10-Rec-0, arrival at recovery room; t11-Rec-05, 5 minutes after arrival at recovery room; t12-Rec-10, 10 minutes after arrival at recovery room; t13-Rec-15, 15 minutes after arrival at recovery room; t14-Rec-20, 20 minutes after arrival at recovery room; t15-Rec-25, 25 minutes after arrival at recovery room; t16-Rec-30, 30 minutes after arrival at recovery room.
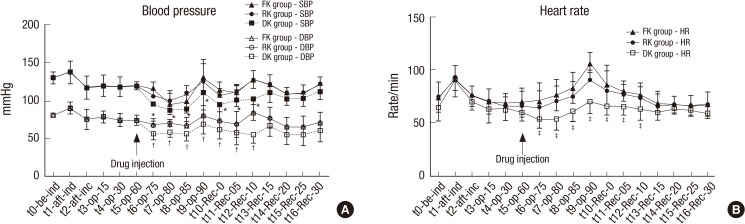
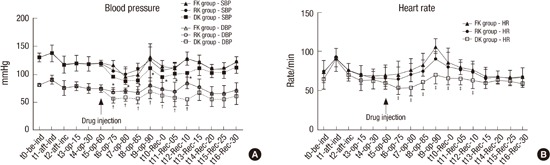
After starting the injection of study drugs in the three groups, SBP, DBP and HR were significantly lower in Group DK compared with other Groups from t6 (operation for 75 minutes) to t12-Rec-10 (10 minutes after arrival at recovery room; Fig. 4A and 4B).
Fig. 4.
Changes in (A) blood pressure and (B) heart rate for the three groups.
FK group, fentanyl-ketorolac group; RK group: remifentanil-ketorolac group, DK group: dexmedetomidine-ketorolac group. t0, before induction time; t1, after induction time; t2, after incision time; t3, operation 15 minutes; t4, operation 30 minutes; t5, operation 60 minutes: t6, operation 75 minutes; t7, operation 80 minutes; t8, operation 85 minutes; t9, operation 90 minutes; t10-Rec-0, arrival at recovery room; t11-Rec-05, 5 minutes after arrival at recovery room; t12-Rec-10, 10 minutes after arrival at recovery room; t13-Rec-15, 15 minutes after arrival at recovery room; t14-Rec-20, 20 minutes after arrival at recovery room; t15-Rec-25, 25 minutes after arrival at recovery room; t16-rec-30, 30 minutes after arrival at recovery room.
*DK group indicates significantly lower than other groups for systolic blood pressure (P < 0.05); †DK group indicates significantly lower than other groups for diastolic blood pressure (P < 0.05); ‡DK group indicates significantly lower than other groups for heart rate (P < 0.05).
DISCUSSION
After its approval by the Food and Drug Administration (FDA) in 1999, dexmedetomidine has become well-established as a sedative-hypnotic agent. Dexmedetomidine is chemically related to clonidine but its affinity for α2-receptors is eight times higher (1,620:1) when it is compared with clonidine (200:1). It is now being used off-label outside of the ICU, in various settings such as maintaining airway, providing hemodynamic stability and adjunctive analgesia in the operating room, sedation in diagnostic and procedure units without the need for tracheal intubation, and prevention of withdrawal following the prolonged administration of opioids and benzodiazepines (9). It was also used for sedation in monitored anesthesia care (MAC) (10). Unlike other sedatives, dexmedetomidine-induced sedation allows the patients to open the eyes for response to verbal stimulation and communication, showing normal cognitive abilities. And after surgery or other procedures, it can be used without restrictions during physical examinations to identify the patient's general condition and neurological status (11).
Dexmedetomidine has sedative and analgesia-sparing effects via central actions in the locus coeruleus (LC) and in the dorsal horn of the spinal cord. The primary action of all α2-adrenergic agonists is an inhibition of norepinephrine release causing an attenuation of excitation in the central nervous system, especially in the LC (12). To our knowledge, the α2-adrenergic agonists have significant analgesic effects. Even though the opioids have more analgesic effects than dexmedetomidine, it is reported that dexmedetomidine can reduce opioid requirements by 30% to 50% and is a safer drug for sedation (13). Several studies have documented that it provides stable hemodynamics and has no effect on respiratory function (3,9).
The sedation produced by α2-adrenoreceptor agonists, unlike the one by traditional sedatives, such as benzodiazepines, propofol, barbiturates and etomidate does not depend primarily on activation of the gamma-aminobutyric acid (GABA) system. Furthermore, the primary site of α2-agonist action does not seem to be the cerebral cortex as would be the case with GABA-mimetic drugs. The α2-adrenoreceptor agonists seem to target a different type of sedation compared with GABA-mimetic drugs (14).
A BIS monitor is a neurophysiological monitoring device which continually analyses a patient's electroencephalograms (EEG) during general anesthesia to assess the level of consciousness. The BIS monitor provides a single dimensionless number, the BIS value, ranging from 0 to 99 (15). We routinely use an intraoperative BIS monitor, and its target score is 40 to 60 during general anesthesia. In this study, we chose desflurane, which has very low partition coefficient, because it has little influence on the BIS score during and after surgery. As discussed earlier, the LC is the predominant noradrenergic nucleus in the brain which has a number of efferent connections, particularly to the frontal lobes. Thus, the LC plays a role as an important modulator of wakefulness. Farber et al. (16) reported that dexmedetomidine (20 µg/kg) and halothane (1%–2%) produced quantitatively similar EEG changes in chronically-instrumented cats. According to this study, halothane resulted in unconsciousness and a lack of response to tail clamping, whereas dexmedetomidine produced profound sedation with preservation of the tail-clamp response. Other study demonstrated that the infusion of dexmedetomidine at 0.6 µg/kg/hr produces EEG changes that correspond to a BIS of 60 (moderate to deep sedation) (2). These findings suggest that the BIS score based on EEG parameters may be influenced by the presence of α2-agonists. As a result, the BIS score for dexmedetomidine and other opioids appear to show similar results in sedative doses.
Dexmedetomidine has also been used in adult patients having a high risk for coronary heart disease and it provides perioperative hemodynamic stability. There were reductions in total cost/patient, total length of stay in the hospital and days in the ICU with shorter duration of mechanical ventilation. The use of dexmedetomidine may also contribute to a lower incidence of shivering and morbidity (17).
Fentanyl and remifentanil are commonly used opioids not only for intraoperative analgesia but for postoperative pain control. In our institution, after surgery and arriving at the recovery room, all patients receive titrated doses of IV fentanyl with a loading dose of 25-100 µg, a continuous dose of 10-60 µg/hr, an intermittent dose of 10-50 ug and a lockout interval dose 6–8 minutes (range: 3-10 minutes) with PCA for postoperative pain control. After administration of an intravenous dose of 100 μg, its onset time of action is 1–2 minutes and its duration is 30–60 minutes.
Remifentanil is a selective, ultra-short-acting µ-opioid receptor agonist with an analgesic potency similar to that of fentanyl and a blood–brain equilibration time similar to that of alfentanil. Remifentanil is unique among the opioids as it undergoes metabolism via non-specific plasma and tissue esterases to inactive metabolites. Its pharmacokinetic properties are characterized by a small Vss of 0.3–0.4 L/kg, rapid clearance of 40–60 mL/min/kg and low variability compared with other i.v. anesthetic drugs. T1/2α and t1/2β are 1–2 and 8–20 minutes, respectively. Context-sensitive half-time is independent of the duration of infusion and is estimated to be about 4 minutes (18).
Remifentanil infusion can provide critically ill patients with analgesia and sedation (19). Low doses of remifentanil (up to 0.05 μg/kg/min) help critically-ill patients achieve calmness and sedation. But increasing remifentanil doses can cause respiratory depression and require controlled mechanical ventilation. Depending on clinical needs, sedation can be titrated to different targets, such as obtaining calmness, loss of consciousness (LOC), decreasing sympathetic tone or even inhibiting respiratory rate (20).
As the doses used in group FK and group RK were sedative ones, it may be insufficient to reduce severe pain. The analgesic potency of group DK was not significantly different from other opioid group. Furthermore, the patients in group DK had the ability to communicate with normal cognition and had reduced postoperative nausea & vomiting, as well as hemodynamic stability.
In conclusion, dexmedetomidine, at sedative doses, had the better postoperative hemodynamic stability than fentanyl or remifentanil and demonstrated a similar effect of pain control as fentanyl and remifentanil with patient awareness during sedation in the PACU.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Choi JW, Jung HS. Data collection: Choi JW, Joo JD, Kim DW, In JH, Kwon SY, Seo K, Han D, Cheon GY. Writing the draft: Jung HS. Revision of manuscript: Choi JW, Jung HS. Final approval: all authors.
References
- 1.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–308. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery. 2005;57:1–10. doi: 10.1227/01.neu.0000163476.42034.a1. [DOI] [PubMed] [Google Scholar]
- 6.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Yaksh TL, Pogrel JW, Lee YW, Chaplan SR. Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther. 1995;272:207–214. [PubMed] [Google Scholar]
- 8.Busick T, Kussman M, Scheidt T, Tobias JD. Preliminary experience with dexmedetomidine for monitored anesthesia care during ENT surgical procedures. Am J Ther. 2008;15:520–527. doi: 10.1097/MJT.0b013e31815ae755. [DOI] [PubMed] [Google Scholar]
- 9.Precedex (dexmedetomidine hydrochloride) [package insert] Lake Forest, IL: Hospira, Inc.; 2008. [Google Scholar]
- 10.Mester R, Easley RB, Brady KM, Chilson K, Tobias JD. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during cardiac catheterization. Am J Ther. 2008;15:24–30. doi: 10.1097/MJT.0b013e3180a72255. [DOI] [PubMed] [Google Scholar]
- 11.Joo HS, Kapoor S, Rose DK, Naik VN. The intubating laryngeal mask airway after induction of general anesthesia versus awake fiberoptic intubation in patients with difficult airways. Anesth Analg. 2001;92:1342–1346. doi: 10.1097/00000539-200105000-00050. [DOI] [PubMed] [Google Scholar]
- 12.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–158. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol. 2008;508:648–662. doi: 10.1002/cne.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Farber NE, Poterack KA, Schmeling WT. Dexmedetomidine and halothane produce similar alterations in electroencephalographic and electromyographic activity in cats. Brain Res. 1997;774:131–141. doi: 10.1016/s0006-8993(97)81696-4. [DOI] [PubMed] [Google Scholar]
- 17.Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4:619–627. doi: 10.1517/17425255.4.5.619. [DOI] [PubMed] [Google Scholar]
- 18.Bürkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. 1996;83:646–651. doi: 10.1097/00000539-199609000-00038. [DOI] [PubMed] [Google Scholar]
- 19.Tipps LB, Coplin WM, Murry KR, Rhoney DH. Safety and feasibility of continuous infusion of remifentanil in the neurosurgical intensive care unit. Neurosurgery. 2000;46:596–601. doi: 10.1097/00006123-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Cavaliere F, Antonelli M, Arcangeli A, Conti G, Costa R, Pennisi MA, Proietti R. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Can J Anaesth. 2002;49:1088–1094. doi: 10.1007/BF03017909. [DOI] [PubMed] [Google Scholar]