Abstract
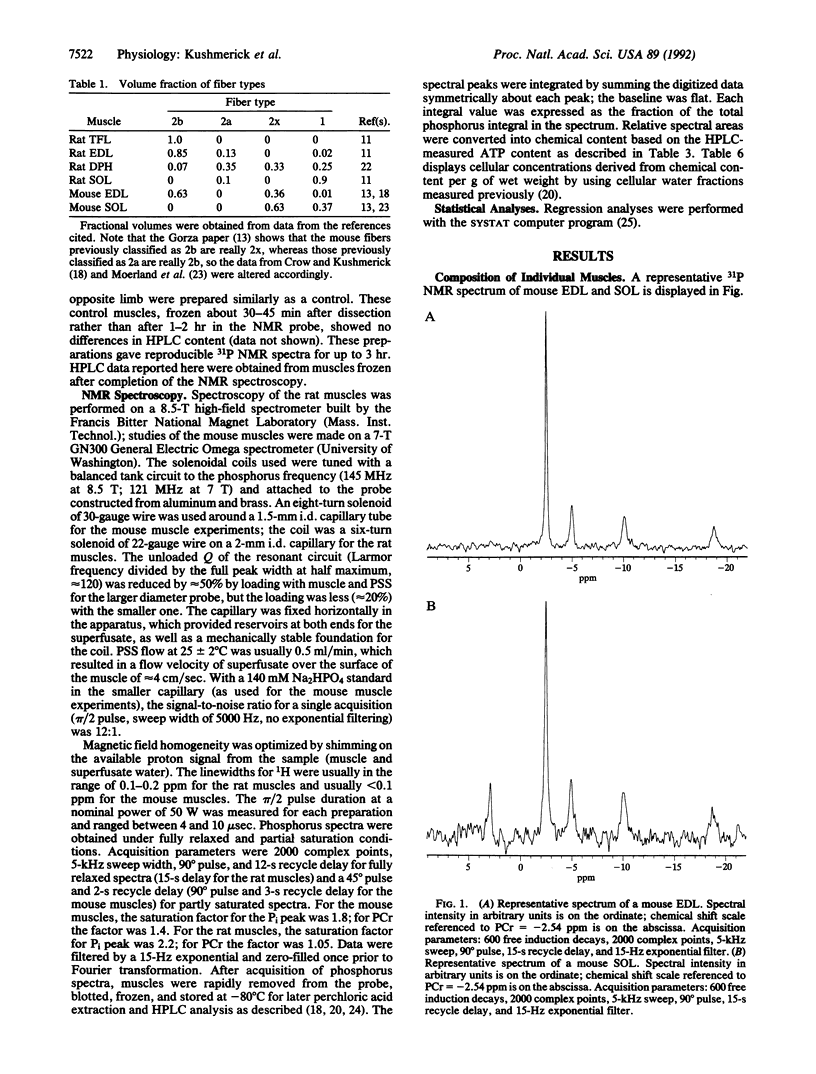
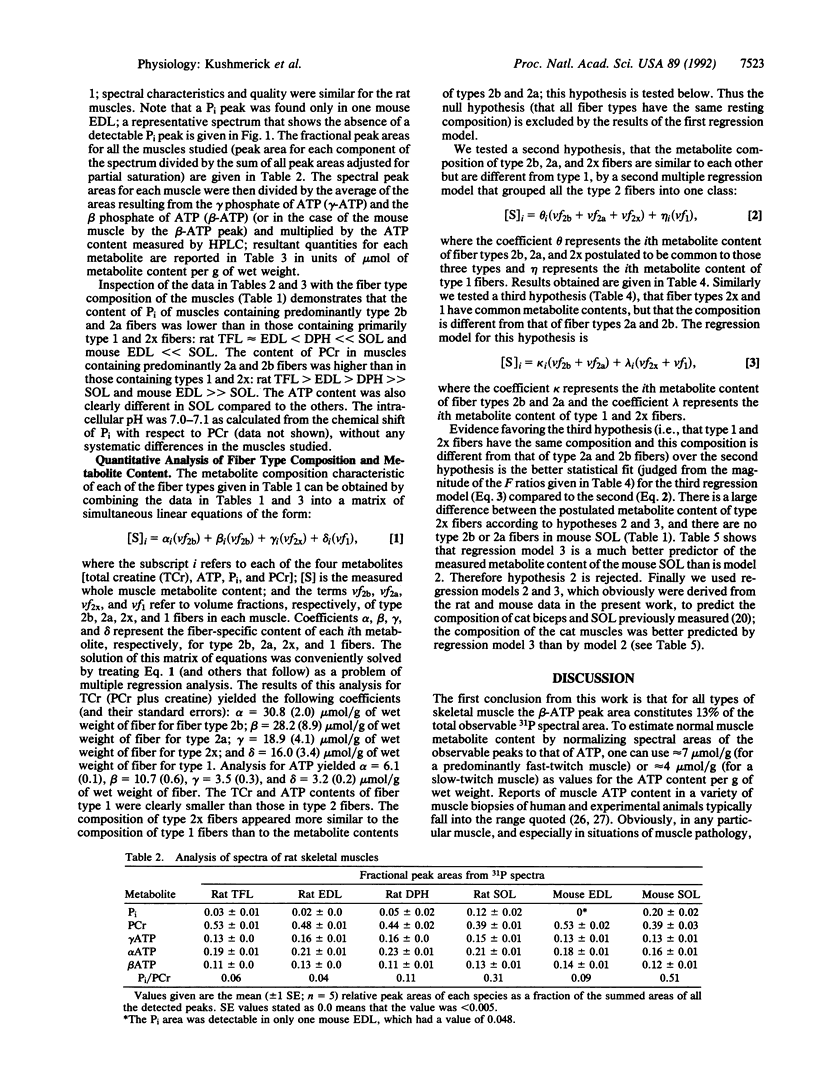
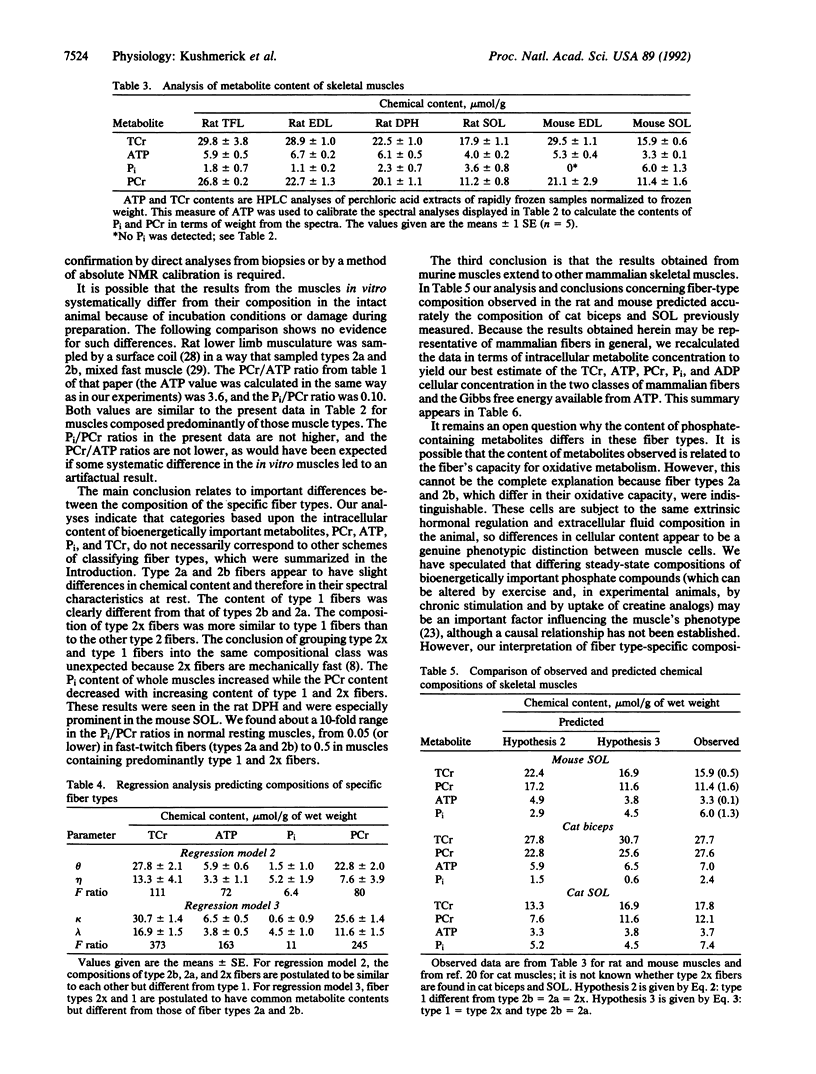
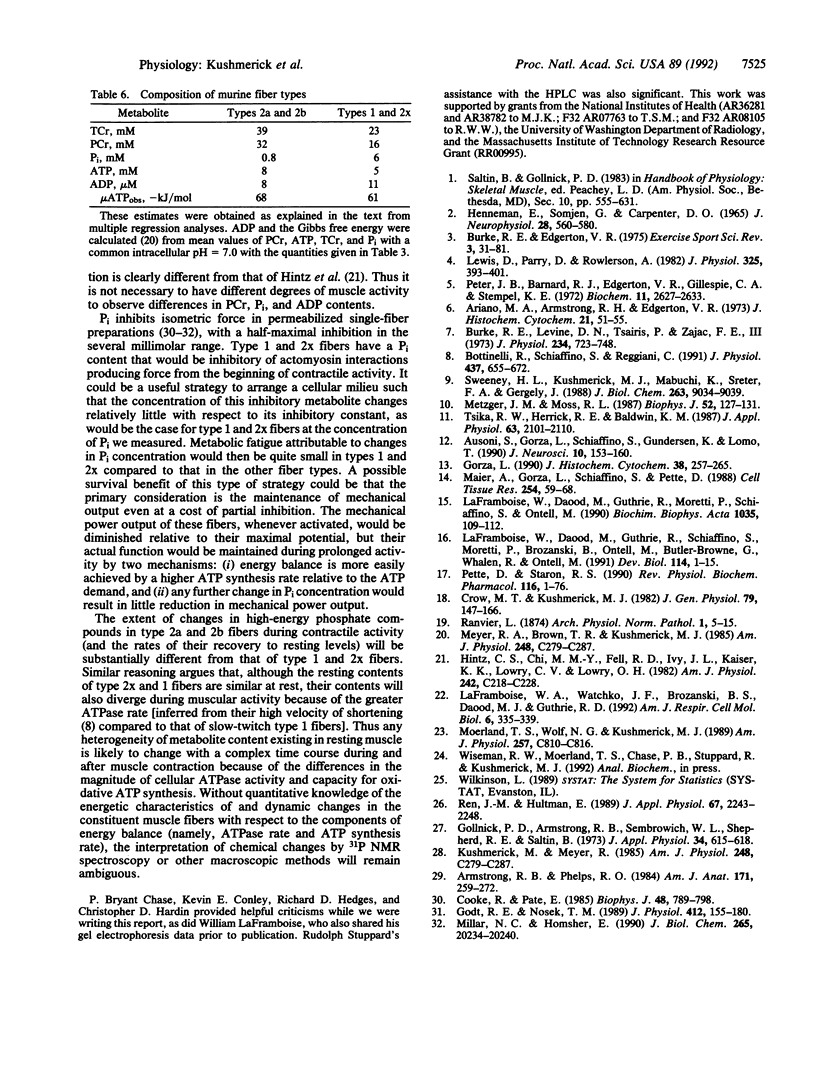
We tested the proposition that muscle cell types have different contents of phosphocreatine (PCr), ATP, and Pi by 31P NMR spectroscopy and HPLC analyses of adult rat and mouse muscles containing various volume fractions of different fiber types. There was a 2-fold difference in the PCr content between muscles with a high volume fraction of fiber types 1 and 2x versus those with fast-twitch (types 2a and 2b) fiber types. Pi content was low, and PCr and ATP contents were high in muscles with large contents of type 2b and 2a fibers; the reverse was true in muscles with a large volume fraction of type 1 and 2x fibers. There is a large range in the Pi/PCr ratios in normal resting muscles, from less than 0.05 in type 2 to 0.51 in type 1 fibers, depending upon the distribution of their component fiber types. In all muscles, the peak area resulting from the beta phosphate of ATP constituted approximately 13% of the sum of all peak areas observable in the 31P spectrum. Fiber types 2a and 2b were not distinguishable, and the content of type 2x fibers was similar to type 1 fibers. From the profile of these metabolites, we could distinguish only two classes of fibers. For type 2a and 2b fibers, the intracellular concentrations were 8 mM ATP, 39 mM total creatine, 32 mM PCr, 0.8 mM Pi, and 8 microM ADP. For type 1 and 2x fibers, these quantities were 5 mM ATP, 23 mM total creatine, 16 mM PCr, 6 mM Pi, and 11 microM ADP. Thus our results establish an additional criterion upon which to distinguish skeletal muscle cells, one based on the resting content of bioenergetically important metabolites. These results also provide the basis for estimating skeletal muscle fiber-type composition from noninvasive NMR spectroscopic data.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Phelps R. O. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984 Nov;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Ausoni S., Gorza L., Schiaffino S., Gundersen K., Lømo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. J Neurosci. 1990 Jan;10(1):153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit properties and selective involvement in movement. Exerc Sport Sci Rev. 1975;3:31–81. [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Sembrowich W. L., Shepherd R. E., Saltin B. Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. J Appl Physiol. 1973 May;34(5):615–618. doi: 10.1152/jappl.1973.34.5.615. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990 Feb;38(2):257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Chi M. M., Fell R. D., Ivy J. L., Kaiser K. K., Lowry C. V., Lowry O. H. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol. 1982 Mar;242(3):C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- LaFramboise W. A., Daood M. J., Guthrie R. D., Moretti P., Schiaffino S., Ontell M. Electrophoretic separation and immunological identification of type 2X myosin heavy chain in rat skeletal muscle. Biochim Biophys Acta. 1990 Jul 20;1035(1):109–112. doi: 10.1016/0304-4165(90)90181-u. [DOI] [PubMed] [Google Scholar]
- LaFramboise W. A., Daood M. J., Guthrie R. D., Schiaffino S., Moretti P., Brozanski B., Ontell M. P., Butler-Browne G. S., Whalen R. G., Ontell M. Emergence of the mature myosin phenotype in the rat diaphragm muscle. Dev Biol. 1991 Mar;144(1):1–15. doi: 10.1016/0012-1606(91)90473-g. [DOI] [PubMed] [Google Scholar]
- LaFramboise W. A., Watchko J. F., Brozanski B. S., Daood M. J., Guthrie R. D. Myosin heavy chain expression in respiratory muscles of the rat. Am J Respir Cell Mol Biol. 1992 Mar;6(3):335–339. doi: 10.1165/ajrcmb/6.3.335. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Parry D. J., Rowlerson A. Isometric contractions of motor units and immunohistochemistry of mouse soleus muscle. J Physiol. 1982 Apr;325:393–401. doi: 10.1113/jphysiol.1982.sp014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A., Gorza L., Schiaffino S., Pette D. A combined histochemical and immunohistochemical study on the dynamics of fast-to-slow fiber transformation in chronically stimulated rabbit muscle. Cell Tissue Res. 1988 Oct;254(1):59–68. doi: 10.1007/BF00220017. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Moerland T. S., Wolf N. G., Kushmerick M. J. Administration of a creatine analogue induces isomyosin transitions in muscle. Am J Physiol. 1989 Oct;257(4 Pt 1):C810–C816. doi: 10.1152/ajpcell.1989.257.4.C810. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R. S. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Ren J. M., Hultman E. Regulation of glycogenolysis in human skeletal muscle. J Appl Physiol (1985) 1989 Dec;67(6):2243–2248. doi: 10.1152/jappl.1989.67.6.2243. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Tsika R. W., Herrick R. E., Baldwin K. M. Subunit composition of rodent isomyosins and their distribution in hindlimb skeletal muscles. J Appl Physiol (1985) 1987 Nov;63(5):2101–2110. doi: 10.1152/jappl.1987.63.5.2101. [DOI] [PubMed] [Google Scholar]



