Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Interplay between myeloma niche stromal cells and myeloid cells generates versikine, a novel damage-associated molecular pattern.
Versikine may promote antigen-presenting cell maturation and CD8+ T-cell activation/recruitment to the tumor bed.
Abstract
Myeloma immunosurveillance remains incompletely understood. We have demonstrated proteolytic processing of the matrix proteoglycan, versican (VCAN), in myeloma tumors. Whereas intact VCAN exerts tolerogenic activities through Toll-like receptor 2 (TLR2) binding, the immunoregulatory consequences of VCAN proteolysis remain unknown. Here we show that human myeloma tumors displaying CD8+ infiltration/aggregates underwent VCAN proteolysis at a site predicted to generate a glycosaminoglycan-bereft N-terminal fragment, versikine. Myeloma-associated macrophages (MAMs), rather than tumor cells, chiefly produced V1-VCAN, the precursor to versikine, whereas stromal cell–derived ADAMTS1 was the most robustly expressed VCAN-degrading protease. Purified versikine induced early expression of inflammatory cytokines interleukin 1β (IL-1β) and IL-6 by human myeloma marrow-derived MAMs. We show that versikine signals through pathways both dependent and independent of Tpl2 kinase, a key regulator of nuclear factor κB1-mediated MAPK activation in macrophages. Unlike intact VCAN, versikine-induced Il-6 production was partially independent of Tlr2. In a model of macrophage-myeloma cell crosstalk, versikine induced components of “T-cell inflammation,” including IRF8-dependent type I interferon transcriptional signatures and T-cell chemoattractant CCL2. Thus the interplay between stromal cells and myeloid cells in the myeloma microenvironment generates versikine, a novel bioactive damage-associated molecular pattern that may facilitate immune sensing of myeloma tumors and modulate the tolerogenic consequences of intact VCAN accumulation. Therapeutic versikine administration may potentiate T-cell–activating immunotherapies.
Introduction
Myeloma is a tumor of plasma cells, which are antibody-producing, terminally differentiated B lymphocytes.1 Myeloma plasma cells typically live within the bone marrow microenvironment (“canonical” myeloma niche) but can often thrive in extramedullary sites and soft tissues (“noncanonical” niches).
We have hypothesized that infiltrating myeloid cells may exert crucial trophic and immunoregulatory functions in both “canonical” and “noncanonical” niches, in part through their regulation of extracellular matrix composition and remodeling.2 We and others have previously demonstrated that versican (VCAN), a large, chondroitin-sulfate matrix proteoglycan, accumulates in myeloma lesions and have hypothesized that VCAN may contribute to myeloma niche immunoregulation.3,4 VCAN has crucial, nonredundant significance in embryonic development5 and emerging roles in cancer inflammation and metastasis.6-9 VCAN promotes tolerogenic polarization of antigen-presenting cells through Toll-like receptor 2 (TLR2).10 VCAN is proteolytically cleaved by ADAMTS-type proteases in a highly regulated manner.5 A cleavage product generated by proteolysis of the Glu441-Ala442 bond within the VCAN V1 isoform, has been termed versikine.5 Versikine has been shown to be bioactive (proapoptotic) during interdigital web regression in the mouse embryo11; however, the roles of VCAN proteolysis and/or versikine in tumor immunomodulation remain unknown.
Study design
Versikine production, purification, and analysis
Methods for expression and purification of recombinant versikine from mammalian cells have been published.12,13 We excluded endotoxin and hyaluronan contamination as detailed in the supplemental Methods, available on the Blood Web site.
Primary bone marrow sample processing and cell culture
Bone marrow aspirates were collected with informed consent under a University of Wisconsin Institutional Review Board–approved protocol (HO07403) and processed as detailed in the supplemental Methods. Cell culture methods are detailed in the supplemental Methods.
Animal experiments
Animal experiments were carried out under an Institutional Animal Care and Use Committee–approved protocol (M2395).
RNA analyses
Cells were lysed in Trizol reagent and RNA extracted per standard methods. Reverse transcriptase polymerase chain reaction (RT-PCR) and RNA sequencing (RNA-seq) were performed as detailed in the supplemental Methods.
Immunohistochemistry
The University of Wisconsin myeloma tissue microarray has been previously reported.3 A second myeloma tissue microarray was purchased from US Biomax (Rockville, MD; catalog no. T291b). Antibodies are listed in the supplemental Methods.
Immunoblot, cytokine detection, and cell-cycle analysis
Antibodies and reagents are listed in the supplemental Methods.
Statistical Methods
All in vitro experiments were performed in triplicate and statistical significance was assessed using 2-tailed Student t test or 2-way ANOVA analysis where appropriate. Multiple comparisons were made using the Tukey method. A P value <.05 was considered statistically significant.
Results and discussion
To determine whether versikine acts as an endogenous damage-associated molecular pattern (DAMP), we exposed freshly explanted primary myeloma CD14+ cells to recombinant human versikine (1 μM). Versikine rapidly induced inflammatory cytokines interleukin-1β (IL-1β) and IL-6 (Figure 1A-B). However, recombinant versikine had no discernible effects on cell-cycle progression of MM1.S myeloma cells (supplemental Figure 1) or primary CD138+ myeloma cells (not shown).
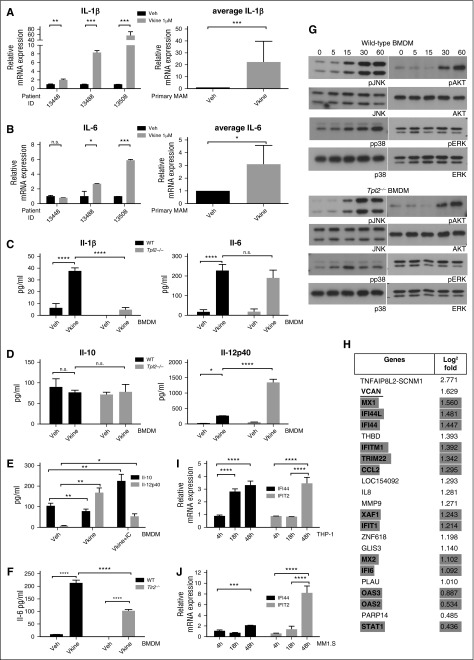
Figure 1.
Versikine, a novel DAMP with immunoregulatory roles in the myeloma microenvironment. (A-B) Freshly explanted MAM from the human myeloma marrow cases indicated were exposed to 1 μM versikine (Vkine) for 12 hours. Relative expression of IL-1β and IL-6 transcripts is shown. Black bars, vehicle; gray bars, versikine-treated; NS, not significant; veh, vehicle. (C-D) Wild-type (WT) and Tpl2−/− BMDMs were treated with 1 μM versikine, and cytokine concentrations were measured in the culture supernatant at 12 hours postexposure. (C) Il-1β (left), Il-6 (right); before Il-1β assay, cells were treated with 5 mM ATP for 20 minutes. (D) Il-10 (left); Il-12p40 (right). (E) Versikine modulates macrophage polarization. BMDMs were exposed to vehicle, versikine alone, or versikine + OVA/anti-OVA immune complexes (IC), as previously described.17 Versikine exposure resulted in M1-like phenotype (Il-12hi, Il-10lo) in the absence of concurrent Fcγ ligation. Versikine + IC promoted macrophage polarization toward an M2b-like, immunoregulatory phenotype (Il-12lo, Il-10hi). (F) WT and Tlr2−/− BMDM were stimulated by versikine for 12 hours and Il-6 protein was measured in the supernatant. (G) Signaling mediators induced by versikine stimulation of WT and Tpl2−/− BMDMs. BMDMs were collected following stimulation with versikine at designed time-points (each number reflects minutes) and subjected to immunoblot analysis with the antibodies shown. (H) RNA-seq analysis of MM1.S myeloma cells exposed to versikine-transduced macrophages for 48 hours. Only 23 genes were differentially expressed and all were upregulated. Thirteen of 23 upregulated genes were ISGs (highlighted in gray). VCAN gene transcription changes are underlined. (I-J) Myeloma cell-macrophage cocultures were exposed to 0.5 μM purified versikine for 4, 18, or 48 hours. Representative ISG transcription is shown for THP-1 (I) and MM1.S cells (J). Relative mRNA transcription is normalized to vehicle-only control at each time point. *P < .05, **P < .01, ***P < .001, ****P < .0001.
VCAN, the precursor to versikine, has been variably reported to be expressed by carcinoma tumor cells or components of the carcinoma microenvironment; however, its cellular origin in the myeloma niche is unknown. We carried out RT-PCR using VCAN isoform-specific primers in paired myeloma CD138+ plasma cells, CD14+ monocytes/macrophages, and CD14/CD138 double-negative fractions from 3 newly diagnosed patients. All 4 VCAN isoforms were predominantly expressed by CD14+ monocytes/macrophages (supplemental Figure 2A). Known versicanases include ADAMTS-1, -4, -5, -9, -15, and -20.5 We readily detected ADAMTS1, ADAMTS4, and ADAMTS5 messenger RNA (mRNA) expression in bone marrow mononuclear cell lysate whereas the rest were undetectable (data not shown). ADAMTS expression has been reported in bone marrow–derived mesenchymal stromal cells (BM-MSC).14 We found that ADAMTS1 was expressed at higher levels by myeloma-derived BM-MSC compared with normal donor BM-MSC (supplemental Figure 2B). BM-MSC expressed much higher levels of ADAMTS1 message than either CD138+ plasma cells or CD14+ monocytes/macrophages (supplemental Figure 2C).
Inflammatory signaling in macrophages engages the MAP3K TPL2 (COT, MAP3K8).3,15 Tpl2 loss in murine bone marrow–derived macrophages (BMDM) abrogated Il-1β production in response to versikine, but had little impact on Il-6 production (Figure 1C). Interestingly, versikine induced Il-12p40 but not Il-10 (Figure 1D-E). Tpl2 negatively regulated Il-12p40 production in response to versikine (Figure 1D), in a manner analogous to TLR agonists.16 Whereas versikine induced Il-12p40 in isolation, concurrent Fcγ receptor ligation through addition of ovalbumin (OVA)/anti-OVA immune complexes promoted Il-10 production and skewed macrophage polarization toward an immunoregulatory M2b phenotype (Il-12lo-Il-10hi)17 (Figure 1E).
Intact VCAN is thought to signal through TLR2.10 However, Tlr2−/− BMDM showed only a partial Il-6 production defect (approximately 50%) in response to recombinant versikine (Figure 1F).
Versikine stimulation of wild-type BMDM rapidly induced JNK, p38-MAPK, and AKT phosphorylation, but Tpl2 loss only affected p38-MAPK phosphorylation (Figure 1G).
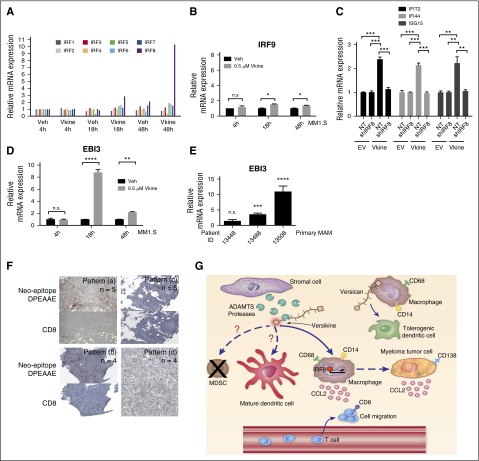
Human THP-1 monocytic cells can be induced to generate macrophages that provide a functional model to study macrophage regulation, including polarization.18 THP-1 macrophages transduced with versikine-plasmid or empty-vector control were cocultured with human myeloma MM1.S cells for 48 hours and RNA-seq analysis was performed on each cell type. Remarkably, only 23 genes were differentially expressed in MM1.S cells exposed to versikine-secreting macrophages and all were upregulated: among these, 13 genes defined a type I interferon (IFN) signature (Figure 1H). Interestingly, VCAN transcription was itself induced, suggesting a positive autoregulatory loop responding to cleaved VCAN. THP-1 macrophages expressing versikine demonstrated differential regulation (>2-fold) of 39 genes (4 downregulated, 35 upregulated): 13 upregulated genes defined a type I IFN signature (supplemental Table 1). Recombinant versikine induced upregulation of IFN-stimulated genes (ISGs) in THP-1 macrophages as well as in cocultured MM1.S myeloma cells (Figure 1I-J). IRF8 was dramatically induced at 18 and 48 hours in MM1.S cells, but induction of other IFN-regulatory factors (IRFs) was minimal (Figure 2A-B). Interestingly, IRF8 was upregulated in MM1.S cells only in the presence of versikine-producing macrophages. Knockdown of IRF8 expression impaired ISG induction in THP-1 macrophages (Figure 2C). Irf8 expression in transplanted tumor cells has been shown to be inducible through an Il-27–dependent mechanism.19 We observed upregulation of the IL-27 subunit, EBI3, in tumor cells and in primary myeloma-associated macrophages (MAMs) treated with versikine (Figures 2D-E).
Figure 2.
Versikine acts through IRF8 to promote transcription of ISGs. (A) IRF transcription in MM1.S cells, following 48 hours of coculture with THP-1 macrophages in the presence of 0.5 μM versikine (Vkine) or vehicle (veh). Expression is normalized to veh-only levels at 4 hours. (B) IRF9 mRNA levels in MM1.S cells cocultured with THP-1 macrophages in the presence of 0.5 μM versikine (gray bars) or vehicle (black bars) for indicated time lengths. (C) THP-1 cells expressing Vkine or an empty-vector (EV) control were transduced with control lentivirus (NT) or lentivirus expressing short hairpin (shRNA) targeting IRF8 (shIRF8) (see supplemental Figure 3 for validation of IRF8 knockdown at the protein level). Versikine-mediated induction of 3 ISGs shown was measured in the presence and absence of IRF8. (D) EBI3 transcription in MM1.S cells cocultured with THP-1 macrophages and treated with 0.5 μM versikine (gray bars) or vehicle (black bars) for indicated time lengths. (E) RT-PCR analysis for EBI3 transcripts in patient-derived, freshly explanted MAM treated with 1 μM versikine for 12 hours. Relative expression is normalized to vehicle-only control (= 1). (F) Staining of human myeloma bone marrow core biopsy consecutive sections with antibodies against neoepitope DPEAAE generated by V1-VCAN cleavage at Glu441-Ala442 and T-cell marker CD8. DPEAAE constitutes the C terminus of versikine. Four patterns of staining were observed in 19 informative punches: Pattern (a): intense/moderate VCAN proteolysis-CD8 infiltration/aggregates (>5 CD8+ cells in cluster). Pattern (b): intense/moderate VCAN proteolysis-CD8 poor (single/doublet CD8+ cells sparsely distributed within tumor). Pattern (c): weak/focal VCAN proteolysis-CD8 poor (single/doublet CD8+ cells sparsely distributed within tumor). Pattern (d): absent VCAN proteolysis-CD8 poor (single/doublet CD8+ cells sparsely distributed within tumor). (G) Proposed immunomodulatory roles of VCAN proteolysis in the myeloma microenvironment. Whereas intact VCAN is thought to exert tolerogenic activities through TLR2 binding on antigen-presenting cells, its proteolytic product, versikine, may promote immunosurveillance through IRF8-mediated effects on antigen-presenting cells and tumor cells. Currently untested hypotheses are represented by broken lines. *P < .05, **P < .01, ***P < .001, ****P < .0001.
We stained myeloma bone marrow biopsy sections with antibodies against a VCAN neoepitope (DPEAAE441) generated by Glu441-Ala442 cleavage of V1-VCAN.5 DPEAAE441 constitutes the C terminus of versikine. Consecutive sections were stained for T-cell marker CD8. We observed 4 staining patterns in 19 core biopsies (Figure 2F). Myeloma tumors displaying CD8+ infiltration/aggregates20 (n = 5 of 19) demonstrated intense/moderate VCAN proteolysis, as detected by the anti-DPEAAE antibody.
We hypothesized that the regulated degradation of VCAN by ADAMTS-type versicanases may modulate its tolerogenic potential by controlling VCAN bioavailability, disrupting its extracellular matrix networks and/or by generating novel bioactive fragments. We report that versikine, a product of VCAN proteolysis, possesses immunoregulatory activities that may promote innate myeloma sensing and “T-cell inflammation” through induction of type I IFN signatures, macrophage activation (IL-1β, IL-6, IL-12), T-cell chemotactic mediators (CCL2),21,22 and IRF8 induction, the latter known to be critical for dendritic and myeloid-derived suppressor cell maturation and homeostasis, respectively,23,24 as well as macrophage inflammatory responses25 (Figure 2G). Taken together, our results suggest that versikine may antagonize the tolerogenic actions of intact VCAN and thus, may provide a novel antitumor strategy. The findings also suggest that, in addition to small leucine-rich proteoglycans previously shown to act as DAMPs,26 fragments of large aggregating proteoglycans may have the capacity to stimulate innate immunity and provide a bridge to adaptive immunity.
Acknowledgments
The authors thank the Emery Bresnick and Lixin Rui laboratories at University of Wisconsin (UW)–Madison for help with experiments and equipment, Sandra Splinter-BonDurant and Xiao-yu Liu (both at UW-Madison) for help with generation and analysis of RNA-seq data, and Carol Dizack for help with medical illustration. The authors also thank Constantine Mitsiades (Dana-Farber Cancer Institute) for providing MM1.S cells and advice; Valbona Cali and the Cleveland Clinic, National Heart, Lung, and Blood Institute (NHLBI)-supported, Programs of Excellence in Glycosciences/Hyaluronan Core for fluorescence-assisted carbohydrate electrophoresis analysis of versikine; and Paul Sondel (UW-Madison), Michael Johnson (UW-Madison), and Christopher Koch (Cleveland Clinic) for critical review of the manuscript.
This work was supported by an American Cancer Society Research Scholar Grant (127508-RSG-15-045-01-LIB) and an American Society of Hematology Bridge Grant (F.A.); the Wisconsin Alumni Research Foundation through the UW Graduate School and a Kirschstein National Research Service Award (grant T32HL007899) (C.H.); funding from the UW Carbone Cancer Center Trillium Fund for Multiple Myeloma Research, the UW Department of Medicine, the UW Carbone Cancer Center (Core Grant P30 CA014520), the UW-Madison School of Medicine and Public Health, and the National Institutes of Health NHLBI-funded Programs of Excellence in Glycosciences (grant HL107147).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.H., S.F., J.J., S.X.C., J..L.J., S.P., and F.A. performed experiments; C.L., I.M., N.C., S.M., and P.H. provided reagents and/or advice on study design; S.S.A. provided crucial expertise, advice, and reagents throughout the study; F.A. was responsible overall for study design; C.H. and F.A. wrote the manuscript; and all coauthors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: C.H. and F.A. are listed as inventors in U.S. Provisional Patent Application No. 62/305 761 (March 9, 2016). The remaining authors declare no competing financial interests.
Correspondence: Fotis Asimakopoulos, University of Wisconsin-Madison School of Medicine and Public Health, 1111 Highland Ave, WIMR 4031, Madison, WI 53705; e-mail: fasimako@medicine.wisc.edu.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Asimakopoulos F, Kim J, Denu RA, et al. Macrophages in multiple myeloma: emerging concepts and therapeutic implications. Leuk Lymphoma. 2013;54(10):2112–2121. doi: 10.3109/10428194.2013.778409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hope C, Ollar SJ, Heninger E, et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood. 2014;123(21):3305–3315. doi: 10.1182/blood-2014-02-554071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N, Khan R, Kumar R, Kumar L, Sharma A. Versican and its associated molecules: potential diagnostic markers for multiple myeloma. Clin Chim Acta. 2015;442:119–124. doi: 10.1016/j.cca.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28(1-2):233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao D, Joshi N, Choi H, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Reports. 2015;13(12):2851–2864. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 11.McCulloch DR, Nelson CM, Dixon LJ, et al. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17(5):687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulcer SJ, Day AJ, Apte SS. Isolation and purification of versican and analysis of versican proteolysis. Methods Mol Biol. 2015;1229:587–604. doi: 10.1007/978-1-4939-1714-3_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulcer SJ, Nelson CM, Quintero MV, et al. Determinants of versican-V1 proteoglycan processing by the metalloproteinase ADAMTS5. J Biol Chem. 2014;289(40):27859–27873. doi: 10.1074/jbc.M114.573287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bret C, Hose D, Reme T, et al. Gene expression profile of ADAMs and ADAMTSs metalloproteinases in normal and malignant plasma cells and in the bone marrow environment. Exp Hematol. 2011;39(5):546–557.e8. doi: 10.1016/j.exphem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Vougioukalaki M, Kanellis DC, Gkouskou K, Eliopoulos AG. Tpl2 kinase signal transduction in inflammation and cancer. Cancer Lett. 2011;304(2):80–89. doi: 10.1016/j.canlet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Jensen JL, Rakhmilevich A, Heninger E, et al. Tumoricidal effects of macrophage-activating immunotherapy in a murine model of relapsed/refractory multiple myeloma. Cancer Immunol Res. 2015;3(8):881–890. doi: 10.1158/2326-6066.CIR-15-0025-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80(6):1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577. doi: 10.1186/s12885-015-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattei F, Schiavoni G, Sestili P, et al. IRF-8 controls melanoma progression by regulating the cross talk between cancer and immune cells within the tumor microenvironment. Neoplasia. 2012;14(12):1223–1235. doi: 10.1593/neo.121444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gérard A, Khan O, Beemiller P, et al. Secondary T cell-T cell synaptic interactions drive the differentiation of protective CD8+ T cells. Nat Immunol. 2013;14(4):356–363. doi: 10.1038/ni.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non-t-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42(4):663–671. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 23.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waight JD, Netherby C, Hensen ML, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancino A, Termanini A, Barozzi I, et al. A dual cis-regulatory code links IRF8 to constitutive and inducible gene expression in macrophages. Genes Dev. 2015;29(4):394–408. doi: 10.1101/gad.257592.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289(51):35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]