Abstract
Purpose
We reviewed the influence of dehydroepiandrosterone (DHEA) supplementation in patients with poor ovarian response (POR) undergoing in vitro fertilization or intracytoplasmic sperm injection (IVF/ICSI).
Methods
We searched Embase, MEDLINE, PubMed, and the Cochrane Library (1980–2015) for relevant papers and used the Newcastle–Ottawa Scale scoring system to evaluate study quality. Dichotomous data were expressed as pooled relative risk (RR) estimates with fixed or random effect models. Continuous variables were expressed as the weighted mean difference (WMD). All data were analyzed using Revman Software v. 5 and are shown with 95 % confidence intervals (CI).
Results
Twenty-one studies met the inclusion criteria. DHEA pretreatment increased the clinical pregnancy rate (RR 1.53, 95 % CI 1.25–1.86), live birth rate (RR 1.87, 95 % CI 1.22–2.88), implantation rate (RR 1.56, 95 % CI 1.20–2.01), and antral follicle count (WMD 0.4, 95 % CI 0.14 to 0.66) while reducing miscarriages (RR 0.50, 95 % CI 0.27–0.90). After subgroup analysis, oocyte numbers and anti-Müllerian hormone levels were also enhanced after DHEA treatment. However, the endometrial thickness and estradiol levels on the day of injecting hCG to induce ovulation were similar between the DHEA supplementation groups and controls.
Conclusions
Based on the limited available evidence, DHEA supplementation seems to improve ovarian reserves and IVF/ICSI outcome in patients with POR. Further research is required to clarify the effect of DHEA exposure in assisted reproduction technology.
Keywords: Dehydroepiandrosterone (DHEA), Poor ovarian response, Fertilization in vitro, Intracytoplasmic sperm injection (ICSI), Meta-analysis, Pre-treatment
Introduction
Despite advancements in assisted reproductive technology (ART), poor ovarian response (POR) remains a major problem and presents a challenge in clinical practice [1]. The precise definition of POR has varied over time: advanced female age, poor ovarian response to gonadotropin stimulation, and abnormal markers of ovarian reserve have all been used to diagnose POR [2]. Recently, the Consensus Group for the European Society for Human Reproduction and Embryology (ESHRE) developed the Bologna criteria to help assign more uniform patient groups to clinical trials [3]. According to these criteria, poor responders are diagnosed with at least two of the three following criteria: (1) advanced maternal age (≥40 years) or other risk factors for POR, (2) a previous POR (≤3 oocytes with a conventional ovarian stimulation protocol), and (3) an abnormal ovarian reserve test (antral follicle count, AFC < 5). The reported diagnosis rates of POR ranged from 9 to 24 % among patients undergoing in vitro fertilization or intracytoplasmic sperm injection (IVF/ICSI) treatments [4]. A variety of methods has been applied to improve the ovarian response, including increased gonadotropin dosage, modulation with gonadotropin-releasing hormone (GnRH) flare-up regimes, adjunctive growth hormone treatment, minimal ovarian stimulation with clomiphene citrate, and unstimulated or “natural cycle” IVF. However, the outcomes of these treatments have been less than satisfactory [1, 5].
Dehydroepiandrosterone (DHEA) is an endogenous steroid produced in the zona reticularis of the adrenal cortex and by ovarian theca cells [6]. In the ovary, it promotes follicular development and granulosa cell proliferation by increasing intraovarian androgen concentrations [7]. DHEA can also enhance the level of follicular insulin-like growth factor-1 (IGF-1), which promotes folliculogenesis by enhancing the effect of gonadotropin and reducing follicular arrest [7–9]. Recently, treatment with DHEA has been shown to result in improved ovarian response in patients with POR [2, 6, 10–17]. An international survey showed that 26 % of IVF clinicians add DHEA as an adjuvant to IVF treatment protocols in such women [18]. However, conclusive clinical evidence of DHEA’s influence is limited. Systematic reviews investigated the effect of DHEA in IVF outcomes but their sample size was small [19]. More information is also necessary to evaluate the role of DHEA as an adjuvant to controlled ovarian stimulation in poor-responders and in women with diminished ovarian reserve. During 2014 and 2015, there was an increased number of papers concerning DHEA treatment in patients with POR. Therefore, this review summarizes and discusses recent advances regarding DHEA treatment in such patients.
Methods
Literature search strategy
We performed an extensive literature search of Embase (1980 to July 2015), MEDLINE (1948 to July 2015), PubMed (1946 to July 2015), and the Cochrane Library for all relevant articles under the following Medical Subject Headings (MeSH) terms (https://www.nlm.nih.gov/mesh/MBrowser.html) to generate subsets of studies: (i) “DHEA” or “Dehydroepiandrosterone”, (ii) “Poor ovarian response” or “POR” (because the definition of POR was developed in 2011) or “low response” or “diminished ovarian reserve,” (iii) “IVF” or “ICSI.” We combined these subsets together (subset i with ii and iii) using “AND” to determine citations that were relevant to the following statement: “DHEA treatment in poor ovarian response women undergoing IVF or ICSI”. In addition, all primary papers’ bibliographies were explored to identify any cited publications that had not been identified by the database searches. Only articles written in English were included in the meta-analysis. Two reviewers conducted the searches independently (MZ and YW).
Inclusion/exclusion criteria
We searched specifically for papers that focused on women with POR and diminished ovarian reserve, who were undergoing ovarian stimulation and IVF/ICSI. In all cases, DHEA was given as a supplement before ovarian stimulation and any IVF/ICSI cycle. The main observed outcomes were clinical pregnancy rate, miscarriage rate, live birth rate, implantation rate, oocyte numbers, estradiol (E2) level on the day of administering human chorionic gonadotropin (hCG) for ovulation induction, endometrial thickness, and the ovarian reserve markers anti-Müllerian hormone (AMH) and antral follicle count (AFC). We excluded non-English language papers, overlapping studies, studies with unclear outcomes, and other articles lacking relevant observed factors.
Quality assessment
Two reviewers (MZ and YW) read the full text of the selected papers and included publications with predefined inclusion criteria independently. Two reviewers (MZ and YW) separately extracted data from each study, including the population, study design, inclusion and exclusion criteria, interventions, and controlled ovarian hyperstimulation (COH) protocols and outcomes. These data were subsequently arranged into a pre-determined form. A third reviewer (WN) arbitrated on article selection and any data extraction-related disagreements. The Newcastle–Ottawa Scale (NOS) scoring system was used to evaluate the quality and methodology of selected articles [20].
Statistical analysis
We calculated relative risk (RR) in fixed and random effect models [21, 22]. The results were combined to create forest plots with Revman Software (Version 5, The Cochrane Collaboration, Oxford, UK, 2003). Dichotomous data for each unit of analysis were calculated as an RR with a 95 % confidence interval (CI). Continuous variables are expressed here as the weighted mean difference (WMD) with 95 % CIs. Heterogeneity was evaluated with I2-index values and reported for each outcome as a P value plus CI range and shown graphically using forest plots (Lewis and Clarke, 2001). For some continuous variables with high heterogeneity, we applied subgroup analysis based on patient age or other influential factors.
Results
Systematic review
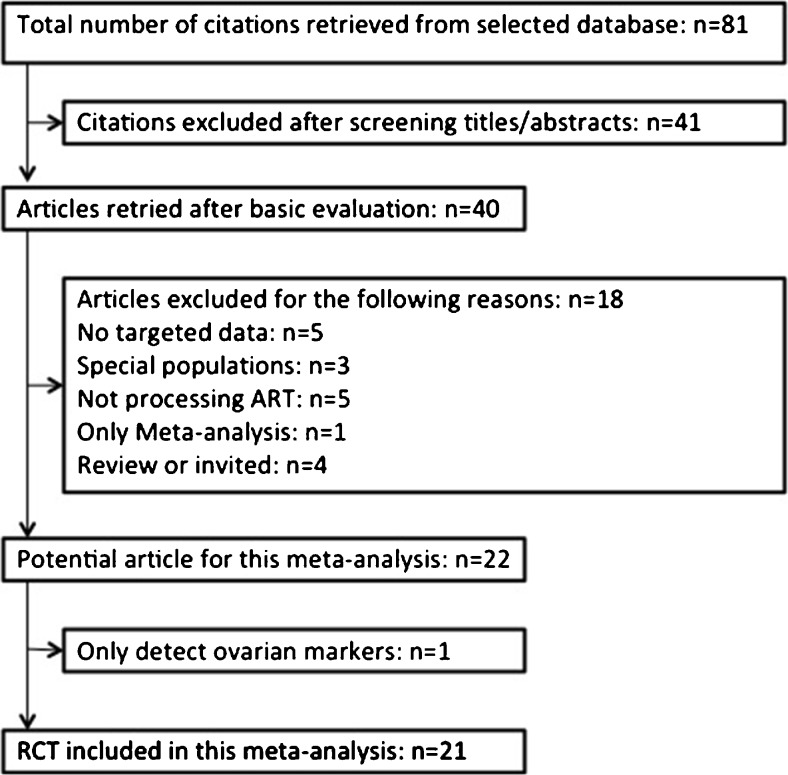
The search strategy yielded a total of 81 publications. Full texts were retrieved for all of these, and 21 fulfilled the inclusion criteria with no disagreement between the reviewers who were responsible for study selection (Fig. 1). Further details on these studies are listed in Table 1. All articles were intended to compare whether pre-treatment with DHEA could improve the IVF/ICSI outcomes of patients with POR. All trials were subjected to meta-analysis to compare IVF/ICSI outcomes, and eight trials were used to compare alternations in hormone levels before and after treatment. The quality control evaluations of all studies are listed in Table 2.
Fig. 1.
Flow diagram detailing selection of studies for inclusion
Table 1.
Methodological characteristic of eligible articles
| Author (Reference) | Number of subjects | Intervention | Study design | Dose of DHEA | Duration |
|---|---|---|---|---|---|
| Barad [13] | 89 cases/101 controls (diminished ovarian reserve) | IVF | Case–control | 75 mg/day | 3.8 ± 0.3 months |
| Barad [12] | 25 self-control (diminished ovarian reserve) | IVF | Prospective cohort study | 75 mg/day | 4.1 ± 0.5 months |
| Sonmezer [23] | 19 self-control (POR) | IVF/ICSI | Prospective cohort study | 75 mg/day | 3-6 months |
| Gleicher [32] | 22 cases/44 controls (diminished ovarian reserve) | None | Case–control | 75 mg/day | 2.4 ± 0.9 months |
| Wiser [14] | 26 cases/25 controls (poor response) | IVF | RCT | 75 mg/day | >2 months |
| Artini [33] | 12 cases/12 controls (POR) | IVF/ICSI | RCT | 75 mg/day | 3 months |
| Moawad [24] | 67 cases/66 controls (poor response) | IVF/ICSI | RCT | 75 mg/day | >3 months |
| Fusi [25] | 38 cases/24 controls and 39 self-controls (poor responses) | IVF | Prospective cohort study | 75 mg/day | >3 months |
| Hyman [26] | 32 self-control (poor responses) | IVF | Prospective cohort study | 75 mg/day | >3 months |
| Singh [27] | 30 self-control (poor responses) | IVF | Prospective cohort study | 75 mg/day | 4 months |
| Yeung [32] | 10 cases/12 control (primary ovarian insufficiency) | IVF | RCT | 75 mg/day | 4 months |
| Yilmaz [36] | 41 self-control (diminished ovarian reserve) | IVF/ICSI | Prospective cohort study | 75 mg/day | >1.5 months |
| Jirge [15] | 25 self-control (POR) | IVF | Prospective cohort study | 75 mg/day | >2 months |
| Kara [28] | 104 cases/104 controls (diminished ovarian reserve) | IVF/ICSI | RCT | 75 mg/day | >3 months |
| Vlahos [2] | 48 cases/113 controls (POR) | IVF | Prospective cohort study | 75 mg/day | >3 months |
| Xu [16] | 189 cases/197 controls (POR) | IVF/ICSI | Case–control | 75 mg/day | 3 months |
| Yeung [6] | 16 cases/16 controls (poor response) | IVF/ICSI | RCT | 75 mg/day | 3 months |
| Zangmo [17] | 50 self-control (poor responses) | IVF | Prospective cohort study | 75 mg/day | 4 months |
| Zhang [29] | 42 cases/42 controls (diminished ovarian reserve) | IVF | RCT | 75 mg/day | 3 months |
| Tartagni [30] | 53 cases/56 controls (poor responses) | IVF | RCT | 75 mg/day | 2 months |
| Tsui [31] | 10 self-control (poor responses) | IVF | Prospective cohort study | 90 mg/day | 3 months |
DHEA dehydroepiandrosterone, POR poor ovarian response, RCT randomized control trials
Table 2.
Quality of controlled studies passing eligibility criteria presented by stratification of research methodology and Newcastle–Ottawa scale (for non-randomized observational studies)
| Author (Year) | Study design | Randomisation | Blinding | Sample size estimation | Analysis | Newcastle-Ottawa scale | – | |
|---|---|---|---|---|---|---|---|---|
| – | – | – | – | – | – | Selection | Comparablility | Outcome |
| Barad [13] | Case– control | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Barad [12] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Sonmezer [23] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Gleicher [32] | Case–control | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Wiser [14] | RCT | |||||||
| Artini [33] | RCT | |||||||
| Moawad [24] | RCT | |||||||
| Fusi [25] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Hyman [26] | Prospective cohort study | None | None | N/A | Intention to treat analysis | ** | * | ** |
| Singh [27] | Prospective cohort study | None | None | N/A | Intention to treat analysis | ** | * | ** |
| Yeung [32] | RCT | |||||||
| Yilmaz [36] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Jirge [15] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
| Kara [28] | RCT | |||||||
| Vlahos [2] | Prospective cohort study | None | None | N/A | Intention to treat analysis | **** | * | ** |
| Xu [16] | Case–control | None | None | N/A | Intention to treat analysis | ** | * | ** |
| Yeung [6] | RCT | |||||||
| Zangmo [17] | Prospective cohort study | None | None | N/A | Intention to treat analysis | ** | * | ** |
| Zhang [29] | RCT | |||||||
| Tartagni [30] | RCT | |||||||
| Tsui [31] | Prospective cohort study | None | None | N/A | Intention to treat analysis | *** | * | ** |
Primary outcomes
Clinical pregnancy rates
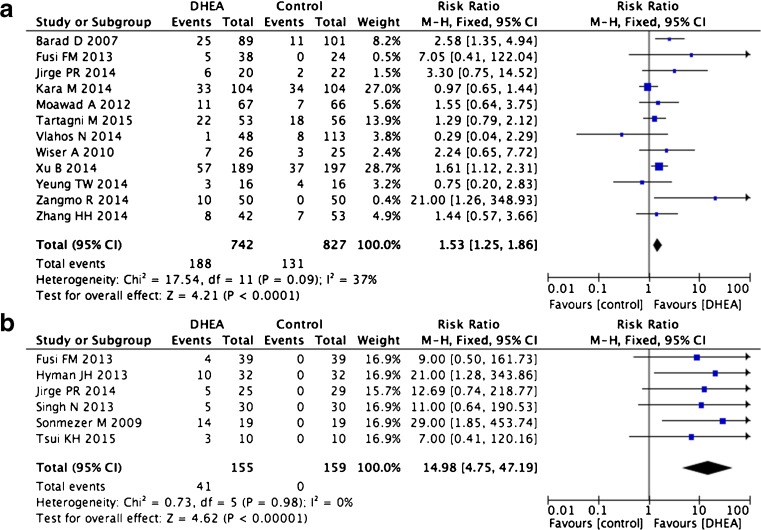
In all, 17 studies based on 1883 patients and 18 trials (one study included two separate trials) were used to calculate clinical pregnancy rates [2, 13–15, 17, 23–32]. Eight of these were randomized control trials (RCTs), 10 were cohort studies, and three were case–control studies. There was no indication of statistical heterogeneity in the case–control and RCT studies (I2 = 37 %). Our pooled analysis of these two types of studies showed statistically significant increases in the pregnancy rates of patients treated with DHEA compared with untreated control groups (12 trials RR 1.53, 95 % CI 1.25–1.86, p < 0.0001, Fig. 2a). Self-controlled trials are less informative for clinical pregnancy rate because patients only enter such studies if a first cycle of IVF/ICSF fails. Therefore, the pregnancy rate of the “control” group will always be 0 %, and DHEA treatment can only result in increased pregnancy rates. Thus, a high overall effect was observed in these types of studies (six trials, RR 14.98, 95 % CI 4.75–47.19, p < 0.0001, Fig. 2b).
Fig. 2.
Risk difference for clinical pregnancy rate in patients treated with DHEA or not
Miscarriage rate
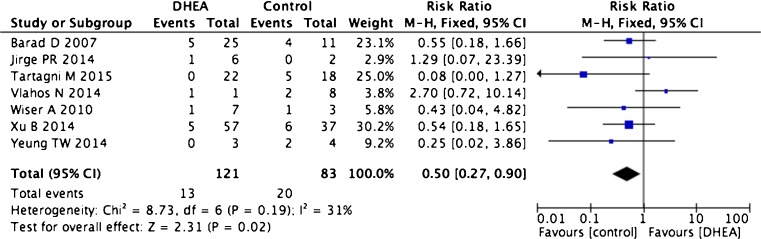
Seven trials including 204 patients were used to calculate miscarriage rates. These consisted of three RCTs, two cohort trials, and two case–control trials [2, 13–16, 30, 32]. There was no indication of statistical heterogeneity (I2 = 31 %). Women undergoing DHEA treatment had lower miscarriage rates after treatment than the controls (seven trials, RR 0.50, 95 % CI 0.27–0.90, p = 0.02, Fig. 3).
Fig. 3.
Risk difference for miscarriage rate in patients treated with DHEA or not
Live birth rate
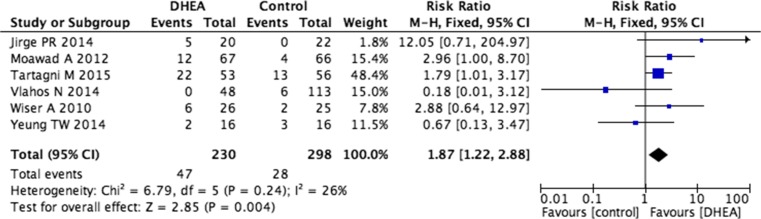
Six trials including 528 patients were used to calculate live birth rates: four were RCT trials and two were prospective cohort trials [2, 14, 15, 24, 30, 32]. There were significantly higher live birth rates in the DHEA treatment groups compared with controls (RR 1.87, 95 % CI 1.22–2.88, p = 0.004) with no indication of statistical heterogeneity (I2 = 26 %, Fig. 4).
Fig. 4.
Risk difference for live birth rate in patients treated with DHEA or not
Secondary outcomes
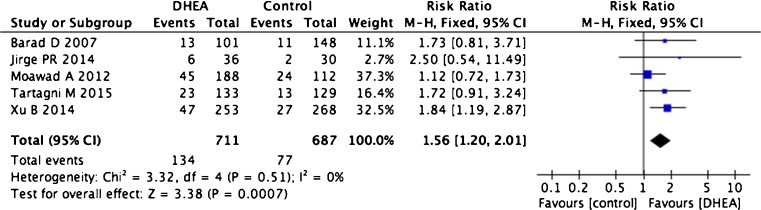
Implantation rate
Three case–control trials, two RCTs, and a prospective cohort study covering 1398 patients were utilized to determine implantation rates [13, 15, 16, 24, 30]. The implantation rate in patients treated with DHEA was significantly increased compared with untreated control patients (five trials, RR 1.56, 95 % CI 1.20–2.01, p = 0.0007) with no indication of statistical heterogeneity (I2 = 0 %, Fig. 5).
Fig. 5.
Risk difference for implantation rate in patients treated with DHEA or not
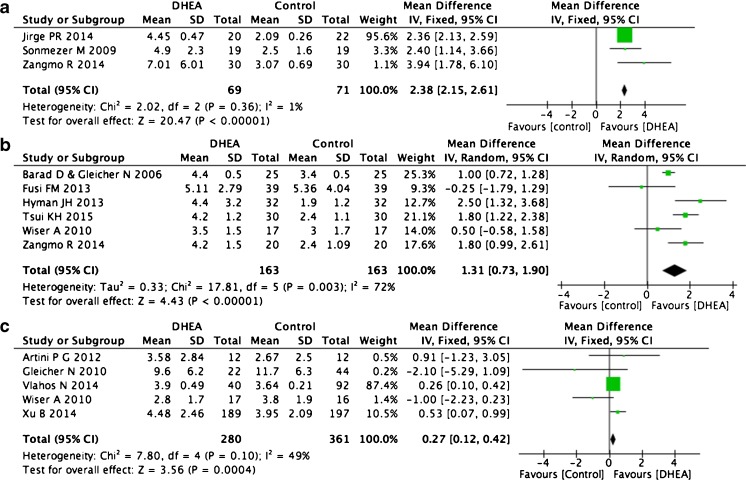
Oocyte numbers
Nine of the 18 trials that collected oocyte number data were self-control studies and were used for this analysis. Because ovarian reserves decrease sharply in women aged over 36 years [17] and will influence the retrieved oocyte number, we divided the studies into distinct categories based on patient age over or under 36 years old and analyzed them separately. Three trials including 69 patients that studied women younger than 36 years showed significant increases in oocyte number after DHEA treatment (WMD 2.38, 95 % CI 2.15–2.61, p < 0.0001, I2 = 1 %, Fig. 6a) [15, 17, 23]. Six trials including 163 patients that studied women older than 36 also indicated increased oocyte numbers after DHEA treatment (WMD 1.31, 95 % CI 0.73–1.90, p < 0.0001, I2 = 72 %, Fig. 6b) [12, 14, 17, 25, 26, 31]. We also compared the results from case–control trials. Of nine such trials, we selected five (total 280 patients) and discarded four when controlling for age, DHEA treatment time, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) doses for COH [2, 14, 16, 33, 34]. This analysis also indicated a larger retrieved oocyte number after DHEA treatment (WMD 0.27, 95 % CI 0.12–0.42, p = 0.0004, I2 = 49 %, Fig. 6c).
Fig. 6.
Weighted mean difference for oocyte numbers in patients treated with DHEA or not
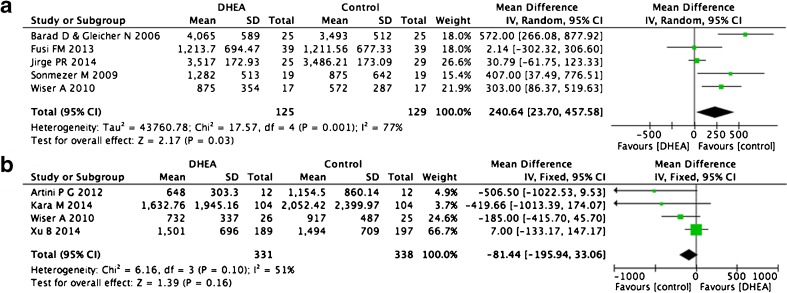
E2 level on the day of hCG administration
Eleven trials included information about the E2 level on the day of hCG administration. Six of these were self-control comparisons [12, 14, 15, 23, 25, 27]. We excluded one because the study population of Singh et al. [27] was divided according to age, but also had positive results for DHEA treatment. These studies indicated that DHEA increased peak E2 levels on the day of hCG administration (five trials, WMD 240.64, 95 % CI 23.7–457.58, p = 0.03; Fig. 7a) [12, 14, 15, 23, 25]. A random effect was used instead of a fixed effect because of the higher heterogeneity between trials (I2 = 77 %). For case–control studies, after normalizing for age, DHEA treatment time, FSH, and LH dose for COH, we selected four out of five trials, but the result did not display significant differences between two groups (WMD −81.44, 95 % CI −195.94 to +33.46, p = 0.16, I2 = 51 %), and the controls were even higher (Fig. 7b) [14, 16, 28, 34].
Fig. 7.
Weighted mean difference for E2 on hCG day in patients treated with DHEA or not
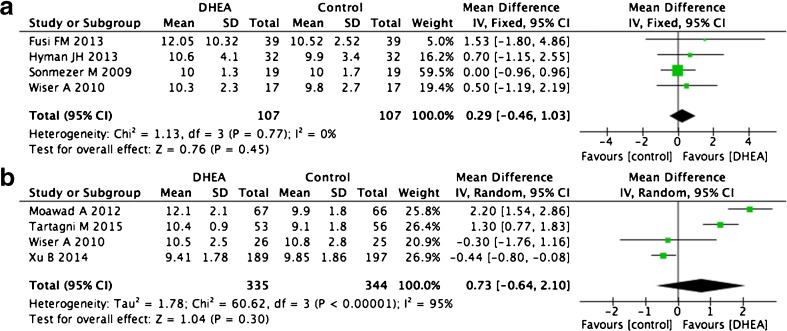
Endometrial thickness
Seven studies including eight trials compared endometrial thickness on the day of administering hCG. Four self-control studies showed no difference before and after DHEA supplementation (WMD 0.29, 95 % CI −0.16 to +1.03, p = 0.45, I2 = 0 %) [14, 23, 25, 26]. Four articles that compared DHEA pre-treatment groups with control groups also showed no differences in this factor, although they did display high levels of heterogeneity (WMD 0.73, 95 % CI −0.64 to +2.10, p = 0.30, I2 = 95 %, Fig. 8) [14, 16, 24, 30].
Fig. 8.
Weighted mean difference for endometrial thickness in patients treated with DHEA or not
Ovarian reserve markers
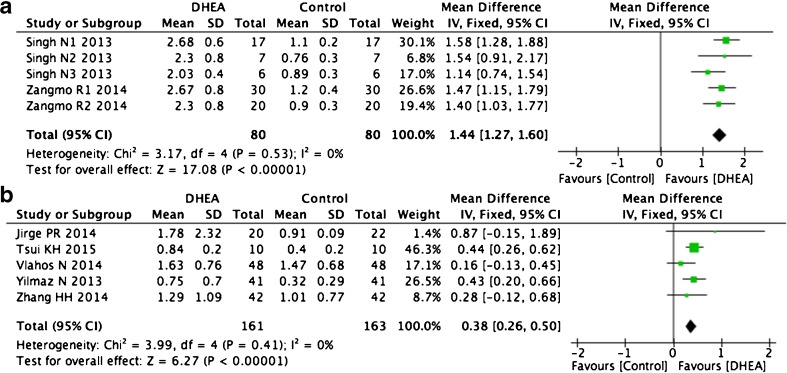
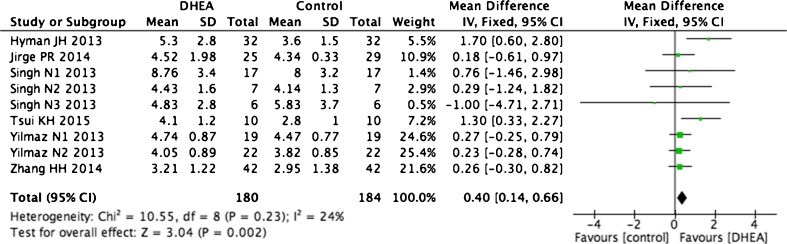
We also investigated the AMH levels and AFC in the same patients before and after DHEA supplementation. Because decreases in ovarian reserve markers are correlated with increased age [35], some articles divided women into groups according to age. Therefore, our meta-analysis divided these studies into subgroups to reduce heterogeneity. Two articles with different population groups showed a significant increase in AMH level for all age groups after DHEA treatment (WMD 1.44, 95 % CI 1.27–1.6, p < 0.0001, I2 = 0 %, Fig. 9a) [17, 27]. The other five articles [2, 15, 29, 31, 36] also showed similar results (WMD 0.38, 95 % CI 0.26–0.5, P < 0.0001, I2 = 0 %; Fig. 9b). Six self-control studies also indicated that DHEA treatment increased the AFC, with no significant heterogeneity observed (WMD 0.4, 95 % CI 0.14–0.66, p = 0.002, I2 = 24 %, Fig. 10) [15, 26, 27, 29, 31, 36].
Fig. 9.
Weighted mean difference for AMH in patients treated with DHEA or not
Fig. 10.
Weighted mean difference for AFC in patients treated with DHEA or not
Discussion
The purpose of this meta-analysis was to evaluate whether DHEA supplementation would increase ovarian response as well as IVF/ICSI outcome. Our results suggested that it improves both outcomes. In total, 21 publications were analyzed, which included eight RCTs, 10 cohort studies, and three case–control studies. All included observational studies were either RCT or fulfilled the NOS scoring system. Unlike previous meta-analyses, we accepted self-controlled studies. These types of trials accounted for a large proportion of the total studies performed, especially for comparisons of AMH levels and AFC. Self-controlled studies are typically thought to have a bias towards more ideal outcomes because of how they are performed; thus, we analyzed these groups separately from two-group studies [19]. When facing high heterogeneity, we used subgroup analysis wherever possible to avoid comparing different populations with potentially different responses to DHEA treatment or gonadotropin doses [17, 27]. A paucity of high-quality studies remains the main limitation to establish the effectiveness of DHEA treatment in ovarian response and IVF/ICSI outcomes in clinical settings.
Another challenge was to evaluate the various criteria that different studies used to assess POR. Many papers were published before the ESHRE consensus and included incomplete POR definitions. However, we chose to include their contributions to the analysis of DHEA treatment in patients with POR. In addition, trials focusing on patients with low functional ovarian (age-specific) reserve were included in this analysis. Because our aim was to study the influence of DHEA on ovarian response, the inclusion of these studies was relevant. When faced with limited information on how POR was defined, all authors discussed each case individually to form a consensus about whether to include individual studies for this meta-analysis.
The most direct criteria used to assess the influence of DHEA pre-treatment were clinical pregnancy, miscarriage, and live birth rates. Our data showed a consistent effect of DHEA in increasing clinical pregnancy and live birth rates, while decreasing the miscarriage rate. These results emphasize the positive influence of DHEA for patients with POR. DHEA treatment has also been shown to improve outcomes in women who do not display POR but still pursued IVF/ICSI treatments. Like Tartagni et al. reported that DHEA supplementation in infertile women who underwent IVF treatment led to a significantly higher live birth rate and lower miscarriage rate [37]. Artini et al. suggested that DHEA might decrease the level of hypoxic inducible factor1, and there were significantly more mature oocytes retrieved from selected follicles in the DHEA treatment group compared with controls (0.50 ± 0.52 vs. 0.08 ± 0.29, p = 0.018) [34]. Combined with our data, these studies illustrate the positive influence of DHEA as an adjuvant in women undergoing IVF/ICSI cycles.
The secondary criteria we applied included oocyte numbers, endometrial thickness, implantation rate, and E2 level on the day of hCG administration, as well as other ovarian reserve markers. The number of retrieved oocytes was calculated in part to evaluate any direct effect of DHEA on follicle generation. After matching treatment duration, age, and gonadotropin dose, we separated the retrieved oocyte numbers into three subgroups. As expected, both self-controlled and case–control (including RCT) subgroups showed increased numbers of retrieved oocytes after DHEA treatment. However, our meta-analysis showed that DHEA supplementation promoted the implantation rate but did not improve endometrial thickness. This outcome might have reflected better embryo quality [17]. However, this factor was difficult to assess because it is a subjective indicator with differing criteria and scoring systems. In addition, few publications provided data on embryo quality, which made it difficult to perform any meta-analysis. In addition, as a number of studies that tracked this indicator displayed highly heterogeneous results, so were excluded from our meta-analysis [13, 24, 28, 30]. Intriguingly, Barad et al. [11, 13] found that DHEA treatment resulted in fewer oocytes, fewer normal, and transferred embryos, but higher clinical pregnancy rates. The results presented here partly agree with those findings concerning DHEA’s positive effects on clinical pregnancy rates. However, our analysis indicates that DHEA treatment leads to increased oocyte numbers. Based on these apparently conflicting results, further study on the relationship between DHEA treatment and oocyte numbers is necessary.
Clinicians have usually performed self-controlled studies for AMH levels and AFC before and after DHEA treatment. Usually, both of these parameters have been shown to decrease with aging [35]. But we confirmed significant improvements in these parameters after DHEA supplementation. A previous study on follicular developmental indicated that DHEA exposure stimulated the initiation of growth in primordial follicles, improves gonadotrophin-response, promotes granulosa cell proliferation, enhances AMH expression, and delays the effect of ovary aging [38]. The same group also observed increased primordial follicle initiation and prenatal follicle development in both cortical grafts and the remaining ovarian tissue, which supports our analysis that indicates higher AMH and AFC levels after DHEA treatment. Tsui et al. used genetic methods to explain the function of DHEA. A significant difference in the expression of genes in women with POR before and after DHEA supplementation (all p < 0.05) was observed [31]. Gene ontology (GO) analysis showed that genes related to extracellular matrix formation were upregulated, including HAS2, VCAN, and THBS1. By contrast, genes related to cell development, differentiation, and apoptosis regulation were downregulated. This evidence highlights the function of DHEA at the transcriptional level. However, evidence opposing the view of DHEA as a beneficial adjuvant to increase AMH/AFC levels in IVF/ICSI has been presented. For example, Hyman et al. recruited 32 women into a self-controlled trail and found that DHEA did not influence the recruitment of preantral or tiny antral follicles (there were no differences in AMH or inhibin B levels). However, DHEA treatment did rescue small antral follicles from atresia (increased AFC) [26]. Again, more studies are needed to address the mechanism of DHEA in IVF/ICSI cycles. Besides, genetic factors might be reasons behind the varying effects of DHEA in different individuals. For example, Weghofer et al. found that free testosterone significantly affected clinical pregnancy potential (β = 1.101 ± 0.508, p = 0.03) unless women possessed abnormal FMR1 genotypes [39].
Traditionally, based on the effect DHEA has on the reproductive system, it has been used as an androgen replacement for a number of years [40–43]. In IVF/ICSI cycles, the use of DHEA supplementation was initially based on the clinical experience that increased intraovarian androgen levels would increase retrieved oocyte numbers and reduce the required gonadotropins dose [44]. This clinical concept has been supported through a number of studies, which have begun to uncover the molecular mechanism behind DHEA treatment. Similar to ovarian follicles, DHEA levels decrease with age [45]. Because DHEA is a precursor of estradiol and testosterone, it can affect ovarian follicle generation through the activation of relative receptors and via steroid production [45]. IGF-1 is also suspected to be one of the molecular pathways enhanced by DHEA treatment [12]. Shoae-Hassani et al. suggested that DHEA might provide a suitable microenvironment for endometrial innervation [46]. It not only enhanced the survival rates of dissociated neurons in culture but also could activate the AKT protein kinase pathway as well as nerve growth factor, which enhanced neuronal activity. DHEA might indirectly increase survival rate of neural cells by stimulating the production of brain-derived neurotropic factor, which is known to promote the differentiation and survival of neurons.
Conclusions
We included data from more than 1000 patients in this meta-analysis. DHEA supplementation appears to have improved IVF/ICSI outcomes and ovarian response in patients with POR. However, because very few RCT trials analyzing DHEA adjuvant therapy in IVF/ICSI cycles have been performed for women with POT, some questions remain about whether this treatment improves outcomes. Thus, until additional high-quality clinical trials (particularly RCTs) can confirm or reject this hypothesis, the beneficial role of DHEA cannot be conclusively proven in patients with POR.
Authors’ roles
YS was responsible for designing and coordinating the study. All authors were responsible for data collection, data analysis, and data interpretation. MZ and JX were responsible for the statistical analysis and for writing the manuscript and contributed equally to this study. WN was responsible for reviewing the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31271605 to Yingpu Sun) and by the Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou University (to Wenbin Niu).
Footnotes
Capsule Summary
A meta-analysis of more than 1,000 patients suggests that dehydroepiandrosterone as an adjuvant to IVF/ICSI treatment protocols improved the outcomes for women with a poor ovarian response.
Meixiang Zhang and Wenbin Niu contributed equally to this work.
References
- 1.Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9:61–76. doi: 10.1093/humupd/dmg007. [DOI] [PubMed] [Google Scholar]
- 2.Vlahos N, Papalouka M, Triantafyllidou O, Vlachos A, Vakas P, Grimbizis G, et al. Dehydroepiandrosterone administration before IVF in poor responders: a prospective cohort study. Reprod Biomed Online. 2014. [DOI] [PubMed]
- 3.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–24. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 4.Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997;104:521–7. doi: 10.1111/j.1471-0528.1997.tb11525.x. [DOI] [PubMed] [Google Scholar]
- 5.Loutradis D, Vomvolaki E, Drakakis P. Poor responder protocols for in-vitro fertilization: options and results. Curr Opin Obstet Gynecol. 2008;20:374–8. doi: 10.1097/GCO.0b013e328305b9b8. [DOI] [PubMed] [Google Scholar]
- 6.Yeung TW, Chai J, Li RH, Lee VC, Ho PC, Ng EH. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil Steril. 2014;102:108–15. doi: 10.1016/j.fertnstert.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–9. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129–32. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 9.Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70:107–10. doi: 10.1016/S0015-0282(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 11.Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84:756. doi: 10.1016/j.fertnstert.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21:2845–9. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- 13.Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24:629–34. doi: 10.1007/s10815-007-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496–500. doi: 10.1093/humrep/deq220. [DOI] [PubMed] [Google Scholar]
- 15.Jirge PR, Chougule SM, Gavali VG, Bhomkar DA. Impact of dehydroepiandrosterone on clinical outcome in poor responders: a pilot study in women undergoing in vitro fertilization, using bologna criteria. J Hum Reprod Sci. 2014;7:175–80. doi: 10.4103/0974-1208.142477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Li Z, Yue J, Jin L, Li Y, Ai J, et al. Effect of dehydroepiandrosterone administration in patients with poor ovarian response according to the Bologna criteria. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0099858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zangmo R, Singh N, Kumar S, Vanamail P, Tiwari A. Role of dehydroepiandrosterone in improving oocyte and embryo quality in IVF cycles. Reprod Biomed Online. 2014;28:743–7. doi: 10.1016/j.rbmo.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 18.VF Worldwide Survey. Poor responders: how to define, diagnose and treat? http://www.IVF-Worldwide.com. [DOI] [PubMed]
- 19.Narkwichean A, Maalouf W, Campbell BK, Jayaprakasan K. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol. 2013;11:44. doi: 10.1186/1477-7827-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells GA SB, O’connell D, Peterson J, Welch V, Losos MPT: The Newcastle-Ottawa Scale (NOS) for assessing thequality if nonrandomized studies in meta-analyses. Ottawa; 2004. http://www.ohri.ca/programs/ clinical_epidemiology/oxford.asp.
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 23.Sonmezer M, Ozmen B, Cil AP, Ozkavukcu S, Tasci T, Olmus H, et al. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod Biomed Online. 2009;19:508–13. doi: 10.1016/j.rbmo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Moawad A, Shaeer M. Long-term androgen priming by use of dehydroepiandrosterone (DHEA) improves IVF outcome in poor-responder patients. A randomized controlled study. Middle East Fertility Society Journal. 2012;17:268–74. doi: 10.1016/j.mefs.2012.11.002. [DOI] [Google Scholar]
- 25.Fusi FM, Ferrario M, Bosisio C, Arnoldi M, Zanga L. DHEA supplementation positively affects spontaneous pregnancies in women with diminished ovarian function. Gynecol Endocrinol. 2013;29:940–3. doi: 10.3109/09513590.2013.819087. [DOI] [PubMed] [Google Scholar]
- 26.Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Gal M, Alerhand S, et al. DHEA supplementation may improve IVF outcome in poor responders: a proposed mechanism. Eur J Obstet Gynecol Reprod Biol. 2013;168:49–53. doi: 10.1016/j.ejogrb.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Zangmo R, Kumar S, Roy KK, Sharma JB, Malhotra N, et al. A prospective study on role of dehydroepiandrosterone (DHEA) on improving the ovarian reserve markers in infertile patients with poor ovarian reserve. Gynecol Endocrinol. 2013;29:989–92. doi: 10.3109/09513590.2013.824957. [DOI] [PubMed] [Google Scholar]
- 28.Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Does dehydroepiandrosterone supplementation really affect IVF-ICSI outcome in women with poor ovarian reserve? Eur J Obstet Gynecol Reprod Biol. 2014;173:63–5. doi: 10.1016/j.ejogrb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HH, Xu PY, Wu J, Zou WW, Xu XM, Cao XY, et al. Dehydroepiandrosterone improves follicular fluid bone morphogenetic protein-15 and accumulated embryo score of infertility patients with diminished ovarian reserve undergoing in vitro fertilization: a randomized controlled trial. J Ovarian Res. 2014;7:93. doi: 10.1186/s13048-014-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tartagni M, Cicinelli MV, Baldini D, Tartagni MV, Alrasheed H, DeSalvia MA, et al. Dehydroepiandrosterone decreases the age-related decline of the in vitro fertilization outcome in women younger than 40 years old. Reprod Biol Endocrinol. 2015;13:18. doi: 10.1186/s12958-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsui KH, Lin LT, Chang R, Huang BS, Cheng JT, Wang PH. Effects of dehydroepiandrosterone supplementation on women with poor ovarian response: A preliminary report and review. Taiwan J Obstet Gynecol. 2015;54:131–6. doi: 10.1016/j.tjog.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98:380–8. doi: 10.1210/jc.2012-3071. [DOI] [PubMed] [Google Scholar]
- 33.Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010;21:360–5. doi: 10.1016/j.rbmo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Artini PG, Simi G, Ruggiero M, Pinelli S, Di Berardino OM, Papini F, et al. DHEA supplementation improves follicular microenviroment in poor responder patients. Gynecol Endocrinol. 2012;28:669–73. doi: 10.3109/09513590.2012.705386. [DOI] [PubMed] [Google Scholar]
- 35.Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Mullerian hormone. Reprod Biomed Online. 2015;31:486–96. doi: 10.1016/j.rbmo.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz N, Uygur D, Inal H, Gorkem U, Cicek N, Mollamahmutoglu L. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: serum AMH, inhibin B and antral follicle count. Eur J Obstet Gynecol Reprod Biol. 2013;169:257–60. doi: 10.1016/j.ejogrb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Tartagni M, De Pergola G, Damiani GR, Pellegrino A, Baldini D, Tartagni MV, et al. Potential benefit of Dehydroepiandrosterone supplementation for infertile but not poor responder patients in a IVF program. Minerva Ginecol 2014. [PubMed]
- 38.Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2014;29:146–54. doi: 10.1093/humrep/det408. [DOI] [PubMed] [Google Scholar]
- 39.Weghofer A, Kim A, Barad DH, Gleicher N. The impact of androgen metabolism and FMR1 genotypes on pregnancy potential in women with dehydroepiandrosterone (DHEA) supplementation. Hum Reprod. 2012;27:3287–93. doi: 10.1093/humrep/des265. [DOI] [PubMed] [Google Scholar]
- 40.Saltzman E, Guay A. Dehydroepiandrosterone therapy as female androgen replacement. Semin Reprod Med. 2006;24:97–105. doi: 10.1055/s-2006-939568. [DOI] [PubMed] [Google Scholar]
- 41.Genazzani AR, Inglese S, Lombardi I, Pieri M, Bernardi F, Genazzani AD, et al. Long-term low-dose dehydroepiandrosterone replacement therapy in aging males with partial androgen deficiency. Aging Male. 2004;7:133–43. doi: 10.1080/13685530412331284669. [DOI] [PubMed] [Google Scholar]
- 42.Buvat J. Androgen therapy with dehydroepiandrosterone. World J Urol. 2003;21:346–55. doi: 10.1007/s00345-003-0367-7. [DOI] [PubMed] [Google Scholar]
- 43.Munarriz R, Talakoub L, Flaherty E, Gioia M, Hoag L, Kim NN, et al. Androgen replacement therapy with dehydroepiandrosterone for androgen insufficiency and female sexual dysfunction: androgen and questionnaire results. J Sex Marital Ther. 2002;28(Suppl 1):165–73. doi: 10.1080/00926230252851285. [DOI] [PubMed] [Google Scholar]
- 44.Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–85. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- 45.Harper AJ, Buster JE, Casson PR. Changes in adrenocortical function with aging and therapeutic implications. Semin Reprod Endocrinol. 1999;17:327–38. doi: 10.1055/s-2007-1016242. [DOI] [PubMed] [Google Scholar]
- 46.Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Rezaei-Khaligh H, Verdi J. DHEA provides a microenvironment for endometrial stem cells neurogenesis. Med Hypotheses. 2011;76:843–6. doi: 10.1016/j.mehy.2011.02.033. [DOI] [PubMed] [Google Scholar]