Abstract
Purpose
Factors that differentially regulate oocyte and granulosa cell growth within the early preantral follicle and how these factors differ at each stage of follicle growth remain poorly understood. The aim of this study was to isolate and evaluate the effect of recombinant growth and differentiation factor 9 (GDF9) on oocyte and granulosa cell growth at the primary and early secondary stages of preantral follicle growth during in vitro culture.
Methods
Primary stage follicles (diameters of 50–89 μm) and early secondary stage follicles (diameters of 90–120 μm) were isolated from immature mice, and individual, intact follicles were cultured in vitro in the presence and absence of recombinant GDF9. The effects of GDF9 on follicle growth were determined by the assessment of changes in the follicle volume during culture. The growth of the granulosa cell and oocyte compartments of the follicles was evaluated separately at each stage.
Results
GDF9 significantly increased the growth of isolated follicles at both the primary and early secondary follicle stages. Independent evaluation of the granulosa cell and oocyte compartments revealed that, while GDF9 promoted granulosa cell growth at both stages of folliculogenesis, oocyte growth was stage specific. GDF9 promoted growth of the oocyte at the primary, but not the early secondary, follicle stage.
Conclusions
These findings demonstrate a stage-specific role for GDF9 in the regulation of oocyte and granulosa cell growth at the primary and early secondary stages of preantral follicle development.
Keywords: GDF9, Growth and differentiation factor 9, Oocyte, Granulosa cell, In vitro follicle culture
Introduction
Folliculogenesis is a highly dynamic process involving coordinated growth of the oocyte and surrounding granulosa cells by a complex regulatory network of autocrine, paracrine, juxtacrine, and endocrine factors [1–4]. Although the molecular and cellular mechanisms by which antral follicle development is regulated are well documented, the factors and pathways involved in early preantral follicle growth and development remain poorly understood and difficult to study in isolation. It is clear, however, that the oocyte factors play a vital role [4–6].
Growth and differentiation factor 9 (GDF9), a member of the transforming growth factor-β superfamily, is implicated as a local factor with critical roles at multiple stages of follicle development, including promoting follicle growth from the primary to secondary stage [6–11]. GDF9 is expressed by growing oocytes beginning at the primary stage across a range of species, from rodents [12–15] to humans [16], and is required for growth of the primary follicle as knocking GDF9 out in mice results in arrest of folliculogenesis at the primary stage [17]. A similar phenotype was also observed in sheep with homozygous mutations in GDF9 [18–20]. GDF9 might also play a role at the primary stage in human folliculogenesis, as follicles of women with PCOS have decreased GDF9 expression and demonstrate a slowing of the primary to secondary follicle transition [21–23]. Studies to define GDF9 effects on follicle growth in culture have shown mixed results. GDF9 treatment during culture of whole ovaries specifically promoted the growth of primary-staged follicles but had no detectable effect on the growth of secondary-staged follicles [24]. In contrast, studies in which isolated follicles were cultured with recombinant GDF9 reported no effect of GDF9 on primary follicle growth when given in a cocktail of oocyte factors [25] while others observed a significant effect on the growth of secondary follicles [14]. In addition, neither of these studies evaluated the effects of GDF9 on the granulosa cell and oocyte compartments independently. While it has been shown that GDF9 can promote growth of isolated granulosa cells in culture [26–28], studies in GDF9 null mice suggest that it acts to inhibit, rather than promote, growth of the oocyte [29]. The effect of GDF9 alone on the oocyte and granulosa cells during the earliest stages of preantral follicle growth, therefore, remains unclear.
To isolate and investigate the effect of GDF9 on the oocyte and granulosa cells during preantral follicle growth, we adopted an established in vitro follicle culture system for the culture of single, isolated follicles encapsulated within a three-dimensional alginate hydrogel matrix [30–34]. By removing the effects of the surrounding somatic cells and adjacent follicles, these methods of in vitro follicle culture also provide an effective system in which to investigate the role of single factors in early follicular development [30, 33, 35, 36].
The three-dimensional culture system we utilized has been used successfully to grow multilayer secondary follicles from mice, non-human primates, and humans [30, 31, 33, 34, 37–40] and has been shown to support cell-to-cell interactions within the oocyte-granulosa cell complex and maintain the three-dimensional structure of follicles [35, 41, 42]. In mice, the system has been used to successfully grow multilayer secondary follicles to mature eggs that were fertilized and gave rise to viable, fertile pups [30]. Sustained growth of early follicles—specifically, late primary and early secondary follicles—to the antral stage has been achieved in vitro with organ culture [24, 43–45] or coculture of isolated follicles with ovarian stromal cells [46–49], mouse embryonic fibroblasts [50], multiple other follicles [25], or supplemented media [51]. Together, these findings suggest that follicle growth at the primary and early secondary stages is enhanced by, and perhaps dependent upon, factors from the surrounding ovarian environment in addition to the critical role played by the oocyte-derived factor GDF9 [6, 7, 17–23].
In this report, our aims were to investigate the role of stimulation with the oocyte factor GDF9 on the growth of early preantral follicles at the primary and early secondary stage and to independently evaluate effects of GDF9 on the granulosa cell and oocyte compartments at each stage. The use of the three-dimensional alginate system for in vitro culture allowed us to isolate and evaluate the effects of recombinant GDF9 in the absence of effects from ovarian stromal factors or adjacent follicles.
Materials and methods
Animals
C57/Bl6 mice used for these experiments were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in a temperature- and light-controlled environment (12 h light/12 h dark) and provided with food and water ad libitum. The University of California, San Diego, Institutional Animal Care and Use Committee approved all animal protocols.
Validation of the in vitro culture system
Preliminary experiments to establish and validate the published in vitro culture system were performed as described by Xu et al. [30]. Specifically, follicles were mechanically isolated from mouse ovaries using insulin-gauge needles in dissection medium (Leibovitz’s L15 medium (Gibco, USA) supplemented with 1 % fetal bovine serum (Gibco, USA) and 0.5 % penicillin-streptomycin (Cellgro, USA)). Primary and early secondary follicles were isolated on postnatal days 10–12. Multilayer secondary follicles were isolated on postnatal days 13–16. Intact follicles at each stage free from surrounding stromal cells were washed and maintained in maintenance medium (Minimum Essential Medium Eagle Alpha Modification (αMEM) (Gibco, USA) supplemented with 1 % fetal bovine serum and 0.5 % penicillin-streptomycin) and individually encapsulated in sodium alginate hydrogel (NovaMatrix, Norway). Each oocyte was washed through two drops of alginate and then transferred into the middle of a single 5-μl 0.5 % sodium alginate droplet. The droplets were immersed in a solution of 50 mM CaCl2/140 mM NaCl for 1 minute to allow cross-linking of the alginate. Each alginate-encapsulated follicle was then washed in a growth medium (αMEM supplemented with 1 mg/ml bovine fetuin (Sigma, USA), 5 μg/ml insulin (Sigma, USA), 5 ng/ml selenium (Sigma, USA), 5 μg/ml transferrin (Sigma, USA), and 3 mg/ml bovine serum albumin (BSA) (MP Biomedicals, USA)) and was transferred to a separate well of a 96-well plate containing 100 μl of growth media with or without the treatments indicated. Sodium alginate and fetuin were prepared as previously described [30]. All follicles were maintained at 37 °C throughout isolation and encapsulation. Encapuslated follicles were cultured at 37 °C in 5 % CO2. Fifty percent of the media was exchanged with fresh growth media with and without the treatments indicated every 2 days. As a test and validation of the in vitro culture conditions, the follicles were cultured in the presence and absence of a recombinant human follicle-stimulating hormone (FSH) (A.F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, USA) at a concentration of 10 mIU/ml (1 ng/ml) as previously published [30] for the times indicated in each figure.
Effect of GDF9 on early preantral follicle growth
To examine the effect of GDF9 on primary and early secondary stage follicles, the follicles were cultured using the protocol as for FSH above except that the follicles were cultured with recombinant mouse GDF9 (R&D Systems, USA) in place of FSH at the concentration indicated in each figure or in media alone.
Assessment of follicle growth and survival
During follicle culture, the follicles were imaged and measured on the days indicated in each figure using an inverted microscope with transmitted light and phase objectives (Leica, Bannockburn, IL, USA). The volume of the oocyte was calculated based on the diameter of the oocyte. Follicle and oocyte diameters were measured from the obtained images using ImageJ 1.33U (National Institutes of Health, Bethesda, Maryland) and a calibrated ocular micrometer. The reported diameters represent the average of the maximum diameter and a second diameter measurement taken perpendicular to the first. The granulosa cell volume was determined by subtracting the volume of the oocyte from the volume of the entire follicle. Oocyte diameters are exclusive of the zona pellucida. The volume of each individual follicle, granulosa cell compartment, or oocyte during culture was normalized to its own starting volume for all determinations of volume fold change. Follicle survival was assessed on each day of imaging by morphological appearance; follicles with fragmented, degenerated, or extruded oocytes and/or detached granulosa cells were considered dead.
Histology
Follicles cultured for 12 days to the antral stage were removed from the alginate beads as described previously by incubation in αMEM medium with alginate lyase at 10 IU/ml for 10 min [30] and fixed for 2 h in 4 % paraformaldehyde in PBS at 4 °C. Follicles were dehydrated in ascending concentrations of ethanol (10–100 %) and embedded in paraffin by an automated tissue processor (Leica, Mannheim, Germany). Serial 5-μm sections were cut and stained with hematoxylin and eosin.
Staging of primary and early secondary follicles
To determine the follicular stage at various follicle diameters, more than 100 individual follicles of 50–120 μm in diameter were mechanically isolated at postnatal days 10–12 and staged using an inverted microscope with a Hoffman Modulation Contrast objective (Nikon, Melville, NY, USA). The reported diameters represent the average of the maximum diameter and a second diameter measurement taken perpendicular to the first.
Statistical analysis
Comparisons of follicle volume fold change between treatment groups were performed using the Mann-Whitney U test, as distributions did not meet assumptions of normality; comparisons of follicle volumes and fold changes to baseline were performed using the Wilcoxon signed-rank test. Comparisons of survival rates between treatment groups were performed by the chi-squared test of proportions. For both, p ≤ 0.05 was considered to be statistically significant. Statistical analyses were performed using Prism GraphPad.
Results
Staging of primary and early secondary follicles
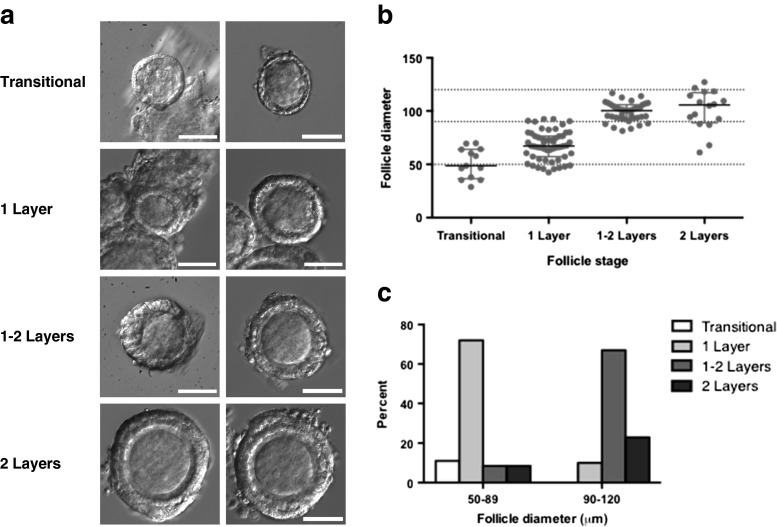
We first determined the range of the starting follicle diameters encompassing the primary and early secondary follicle stages to allow standardization of the early preantral follicle populations for subsequent experiments. We isolated more than 100 follicles, anticipated to be primary or early secondary follicles under the dissecting microscope. The follicles were subsequently imaged and photographed at ×40 for accurate determination of follicle stage and follicle diameter as shown in Fig. 1a. Follicles were categorized as transitional (single layer of granulosa cells with a mix of squamous and cuboidal subtypes), primary (one layer of cuboidal granulosa cells), one to two layers (more than one but less than two full layers of granulosa cells), or secondary (two full layers of granulosa cells) and plotted by stage and diameter. As shown in Fig. 1b, the range and median diameter of the follicles at each stage were as follows: transitional follicles (n = 13) had a median diameter of 49 μm and a range of 29–70 μm; one-layer follicles (n = 55) had a median diameter of 67 μm and a range of 42–92 μm; one- to two-layer follicles (n = 39) had a median diameter of 100 μm and a range of 81–117 μm; and secondary follicles (n = 16) had a median diameter of 106 μm and a range of 61–127 μm. Based on the observed relationship between stage and follicle diameter, we established the cutoffs used for subsequent follicle culture to define primary (50–89 μm) vs. early secondary (90–120 μm) follicles. As shown in Fig. 1c, most of the follicles (83 %) with starting diameters of 50–89 μm were either primary follicles, with one complete layer of cuboidal granulosa cells, or transitional follicles approaching the primary stage, with some squamous and some cuboidal granulosa cells. In contrast, 90 % of the follicles with starting diameters of 90–120 μm were early secondary follicles, with two complete layers of granulosa cells, or approaching the secondary stage, with more than one but less than two full layers of granulosa cells.
Fig. 1.
Follicular staging. More than 100 early preantral follicles of C57Bl6 mice at postnatal days 8–12 were manually isolated. a Follicles were imaged at ×40 for accurate determination of follicle stage and photographed for measurement of follicle diameter. Representative follicles at each stage are shown. Scale bar, 50 μm. b Scatter plot demonstrating the range and median follicle diameter at each follicular stage. Dashed horizontal lines delineate cutoffs chosen at 50–90 μm and 90–120 μm. c Bar graph represents the distribution of follicle stages for each range of follicle diameters selected
Validation of the in vitro culture system
We next validated and tested the alginate hydrogel matrix-enclosed culture system for in vitro follicular culture described by Xu et al. [30]. First, isolated, multilayer secondary follicles (starting diameters of 120–150 μm) were cultured in vitro for 12 days in the presence of 10 mIU/ml FSH. Under these conditions, approximately 75 % of the follicles progressed to the antral follicle stage (Fig. 2a, b), consistent with previously published results [30]. No significant growth was seen in serum-free media alone without the addition of FSH. We next tested the growth of primary and early secondary follicles in the presence or absence of FSH under these in vitro culture conditions (Fig. 2c). Little growth of follicles at these earlier stages was observed, particularly in the absence of FSH, providing an in vitro culture system in which the effect of GDF9 stimulation could be tested in isolation.
Fig. 2.

In vitro follicle culture system. a Multilayer secondary follicles (120–150 μm in diameter) of C57/Bl6 mice at postnatal days 13–16 were encapsulated in 0.5 % alginate and in vitro cultured in the presence or absence of 10 mIU/ml (1–3 ng/ml) recombinant human FSH to the antral follicle stage. The follicles were isolated from 6 different mice in at least 3 different experiments with more than 60 follicles evaluated. b–c Box and whisker plots indicate the median, interquartile range, and minimum and maximum values for the follicle volume fold change during culture. Dashed horizontal lines indicate baseline at start of culture. b Growth observed in late multilayer secondary follicles progressing to the antral stage in the presence and absence of 10 mIU/ml recombinant human FSH (120–150 μm in diameter, n = 18 in a representative experiment). Imaging of the follicles cultured without FSH was discontinued after 6 days since they did not appear to grow significantly. c Growth observed in primary follicles (50–89 μm in diameter, n = 15 in two separate experiments) and early secondary follicles (90–120 μm in diameter, n = 17 in two separate experiments) in the presence and absence of 10 mIU/ml recombinant human FSH
Effect of GDF9 on early preantral follicle growth
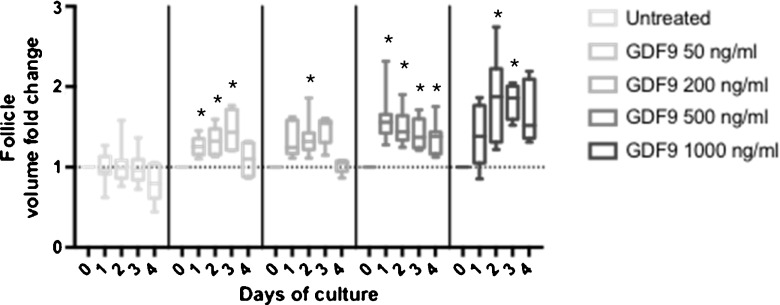
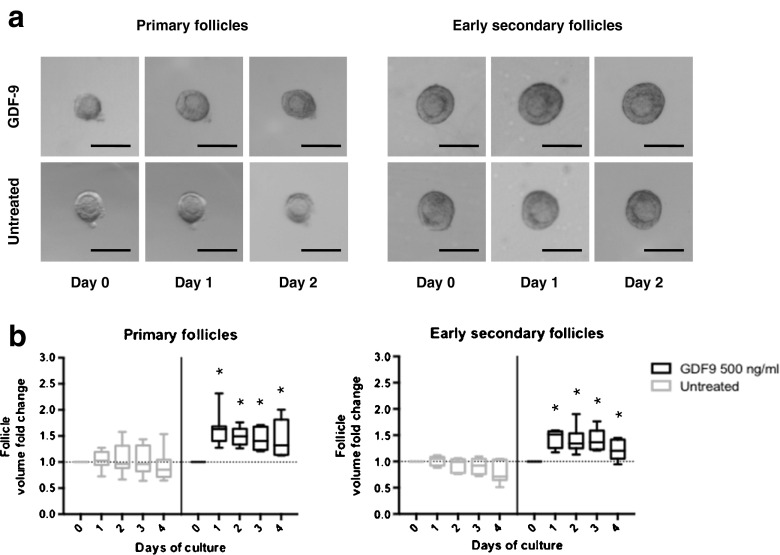
We next investigated the effect of GDF9 on the growth of isolated primary follicles and early secondary follicles in vitro using the conditions established above. We first used a mixture of primary and early secondary follicles to determine a dose-response effect of GDF9 on follicle growth (Fig. 3). While follicle growth was observed at all doses tested, subsequent experiments were performed with GDF9 500 ng/ml as this represented the lowest dose with the most sustained response and was within the range of doses reported in the literature [28, 52–54]. We then cultured isolated primary follicles (50–89 μm in diameter, median diameter 77.9 μm at the start of culture) and early secondary follicles (90–120 μm in diameter, median diameter 101.4 μm at the start of culture) in the presence or absence of GDF9 at 500 ng/ml in serum-free media (Fig. 4a). Measuring the volume of the primary follicles revealed an increase in the volume with GDF9 treatment (peak median increase of 63 %) compared with serum-free media alone (peak median increase of 0.5 %) (Fig. 4b, left panel). A similar increase in follicle volume was seen with GDF9 treatment of early secondary follicles (peak median increase of 52 %) compared with serum-free media alone (peak median increase of 5 %) (Fig. 4b, right panel). The increase in follicle volume at each stage due to GDF9 was rapid and greatest over the first 24 h of culture. No further growth in the volume of the follicle was observed between days 1–4. A small cohort of follicles were cultured up to 12 days with no additional growth observed; therefore, culture was performed for only 4 days for all subsequent experiments. Additionally, no increase in either follicle volume or survival was seen with costimulation with GDF9 and FSH beyond that observed with FSH or GDF9 alone in a small cohort of follicles (data not shown) and was not further tested. Follicle survival during 4 days of culture in the presence and absence of GDF9 was 69 and 75 % (p = 0.7), respectively, for primary follicles, and 75 and 73 % (p = 0.9), respectively, for early secondary follicles. These findings suggest a role for GDF9 in the growth of the early preantral follicle at both the primary and early secondary stages.
Fig. 3.
Effect of GDF9 stimulation at varied doses on early follicle growth in vitro. Box and whisker plots indicate the median, interquartile range, and minimum and maximum values for the follicle volume fold change observed in primary and early secondary follicles (50–120 μm in diameter) in response to stimulation with GDF9 at concentrations of 0, 50, 200, 500, and 1000 ng/ml. Dashed horizontal line indicates baseline at start of culture. *p < 0.05 in Wilcoxon signed-rank test compared to baseline
Fig. 4.
GDF9 enhances the growth of both primary and early secondary follicles in vitro. a Follicles of C57Bl6 mice at postnatal days 8–12 were manually isolated and encapsulated in 0.5 % alginate and cultured in serum-free media in the presence or absence of 500 ng/ml GDF9. Scale bar, 100 μm. b Box and whisker plots indicate the median, interquartile range, and minimum and maximum values for the follicle volume fold change observed in primary (50–89 μm in diameter) and early secondary follicles (90–120 μm in diameter) in response to stimulation with GDF9 at 500 ng/ml. Follicles were isolated from 6 different mice in at least 3 different experiments with more than 60 follicles evaluated. Dashed horizontal lines indicate baseline at start of culture. *p < 0.05 in Mann-Whitney U test compared to the untreated population on the same day of culture
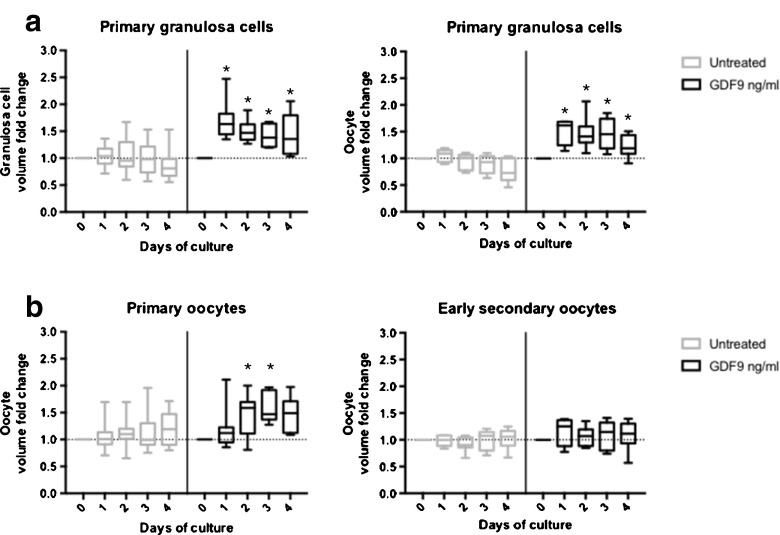
We next asked whether the growth induced by GDF9 in primary and early secondary follicles represented growth of the granulosa cells, oocyte, or both follicular compartments. As shown in Fig. 5a, the growth of the granulosa cells within the follicles follows the same pattern as that of the follicles themselves, with rapid growth with GDF9 treatment over the first 24 h of culture. The same growth pattern is seen for both primary (peak median increase of 63 % with GDF9 vs. median increase 9 % with serum-free media alone) and early secondary (peak median increase 62 % with GDF9 vs. median increase 9 % with serum-free media alone) follicles. These data demonstrate that GDF9 promotes growth of the granulosa cell compartment of both primary and early secondary follicles.
Fig. 5.
GDF9 enhances oocyte growth in a stage-dependent manner. For primary (50–89 μm) and early secondary (90–120 μm) follicles cultured in the presence and absence of GDF9, changes in the granulosa cell, and oocyte follicular compartments were calculated and graphed separately. a Box and whisker plots indicate the median, interquartile range, and minimum and maximum values for the granulosa volume fold change observed in response to stimulation with GDF9 at 500 ng/ml. b Box and whisker plots indicate the median, interquartile range, and minimum and maximum values for the oocyte volume fold change observed in response to stimulation with GDF9 at 500 ng/ml. The follicles were isolated from 6 different mice in at least 3 different experiments with more than 60 follicles evaluated. Dashed horizontal lines indicate baseline at start of culture. *p < 0.05 in Mann-Whitney U test compared to the untreated population on the same day of culture
We also looked at the effect of GDF9 treatment on growth of the oocyte in primary and early secondary follicles. Interestingly, we observed an increase in oocyte volume in the primary follicle in response to treatment with GDF9 (peak median increase 58 % with GDF9), which was significantly greater than the increase seen without GDF9 (peak median increase 19 % with serum-free media alone), as shown in Fig. 5b (p = <0.01 on day 3 of culture). In contrast, no oocyte growth was apparent with or without GDF9 treatment in the early secondary follicles (peak median increase 25 % with GDF9 vs. peak median increase of 7 % with serum-free media alone). The maximum oocyte diameter did not approach the maximum average diameter of 70 μm expected at the late secondary follicle stage [55] in either group (median 45.2 μm for primary follicles; median 57.9 μm for early secondary follicles). The observed effect on the oocyte at the primary stage was delayed relative to the response seen in granulosa cells, with the maximal difference in response to GDF9 treatment observed on day 3 of culture. Together, these data suggest that different factors promote oocyte growth at the primary relative to the secondary stage and demonstrate that GDF9 plays a role in growth of the oocyte at the primary but not the early secondary stage of folliculogenesis.
Discussion
We have found that stimulation with the oocyte factor GDF9 promotes the growth of isolated follicles at both the primary and early secondary stages during in vitro culture and that it does so in a stage-specific manner. Unexpectedly, while a similar effect on granulosa cell growth was observed at both stages, GDF9 enhanced growth of the oocyte in a stage-specific manner, stimulating the growth of the oocyte only within the primary follicle. Our hypothesis that GDF9 would stimulate growth of primary stage follicles was based on published findings demonstrating complete arrest of folliculogenesis at the primary follicle stage in mice lacking GDF9 [17]. There are at least two possible explanations for the arrest of primary follicle growth observed in the GDF9 knockout mouse. GDF9 might act (i) directly on granulosa cells and/or the oocyte to stimulate primary follicle growth and/or (ii) indirectly by suppressing potential inhibitory effects of the surrounding stromal cells within the ovary. In our in vitro culture system, the isolated primary follicles demonstrated very little growth in the absence of any stimulation, but significant growth with the addition of GDF9. A similar pattern of follicle growth was seen in early secondary follicles in response to GDF9 treatment but not in the absence of GDF9. Together, our findings suggest that GDF9 can act on isolated primary and early secondary follicles to stimulate follicle growth.
While the oocytes of the isolated primary and early secondary follicles would be expected to secrete endogenous GDF9 [56], local concentrations might be diluted and less effective with in vitro culture of isolated follicles. Given that GDF9 acts in an autocrine and/or paracrine manner, the degree to which such dilution might occur cannot be determined using current technology, as it is not possible to quantitate endogenous GDF9 activity. Our findings, however, demonstrate that increasing GDF9 levels with the addition of exogenous GDF9 can stimulate growth of the early preantal follicle during in vitro culture. It is possible that we would have observed larger effects of GDF9 stimulation in the absence of endogenous levels of GDF9. An interesting question for future study will be the degree to which stimulation with recombinant GDF9 during in vitro culture can rescue the block in folliculogenesis at the primary follicle stage observed in GDF9 null mice [17].
Growth stimulated by GDF9 in both primary and early secondary follicles is rapid and transient, with the effect on growth of the follicle overall seen over the first 24 h. A significant effect on granulosa cell growth was seen at both the primary and early secondary stage. This effect is consistent with multiple previous studies in which GDF9 has been shown to stimulate the proliferation of isolated granulosa cells in vitro [26–28]. However, no significant further growth of the granulosa cell compartment is seen after 24 h, whereas the oocyte in the primary follicles continues to grow through day 3. Whether the additional growth of the granulosa cell compartment with GDF9 treatment represented cell proliferation, hypertrophy, or a combination of these processes is not known. The follicles remained healthy in appearance in culture for 12 days despite no further growth. That the failure of GDF9 stimulation to achieve a sustained effect on growth of the follicle and granulosa cell growth could potentially be explained by downregulation of receptor levels after 24 h of culture is an interesting possibility that could not be properly tested due to technical limitations. An alternative possibility is that, while GDF9 is required for progression past the primary stage in vivo and can stimulate growth at both the primary and early secondary stages in vitro, the addition of GDF9 alone is not sufficient to advance follicles from the primary to the secondary stage or from the early to the multilayer secondary stage. This would suggest that additional factors from either the follicle itself or from the surrounding stromal cells within the ovary are necessary for sustained growth. This idea is supported by publications that have demonstrated enhanced growth of primary and early preantral follicles in the presence of other follicles or ovarian stromal cells [24, 25, 43–49]. Whether any of these stromal factors might be induced by GDF9 has not been investigated.
Whether GDF9 acts directly on the oocyte to induce follicle growth, indirectly through the granulosa cell or both, is not clear, given that both granulosa cells and oocytes in primary follicles express ALK5 [57–61] and BMPR2 [58, 62], the reported type I [63, 64] and II receptors for GDF9 [65], respectively. However, it is controversial whether ALK5 is the only type I receptor for GDF9 as Alk5 conditional knockout mice have no defects in follicular development and cumulus expansion [62], which is a distinct phenotype from Gdf9 null mice [17]. Interestingly, the current data showed that while the maximal effect of GDF9 treatment on granulosa cell growth was seen at 24 h of culture, the maximal effect of GDF9 on the oocyte was relatively delayed at 72 h of culture. This delayed effect is more consistent with an indirect effect. Such an indirect effect could be due to the altered expression of granulosa cell and/or oocyte factors. The determination of genes and pathways in the follicle regulated by GDF9, using recently developed approaches to transcriptome analysis in a low number of cells, will be an interesting line of future investigation and provide insight into the mechanism by which GDF9 stimulates early preantral follicle growth.
Our findings in isolated follicles, where the effect of GDF9 alone could be examined, demonstrate that GDF9 can stimulate oocyte growth at the primary stage. This finding was surprising as the absence of GDF9 in knockout mice resulted in isolated growth of the oocyte without synchronous growth of granulosa cells, suggesting that GDF9 acts to limit oocyte growth in the primary follicle [17, 29]. Based on the finding of elevated production of KIT-ligand from granulosa cells of GDF9 knockout mice [66], it has been proposed that GDF9 limits, rather than promotes, oocyte growth at the primary follicle stage by suppressing granulosa cell production of KIT-ligand, a factor known to stimulate oocyte growth [67]. However, others have shown increased KIT-ligand expression with GDF9 treatment of isolated granulosa cells [24]. These results suggest the interplay of factors that ultimately regulate oocyte growth in vivo is more complex than previously recognized.
That the effect of GDF9 on oocyte growth is stage specific, stimulating oocyte growth at the primary, but not early secondary stage, was also unexpected. Oocyte growth did not occur with granulosa cell growth in the early secondary stage, despite the fact that the oocytes were not near their maximal expected diameters typically reached during the multilayered secondary stage [55]. Why oocytes in the primary and early secondary follicles demonstrate different responses to GDF9 stimulation is unknown. It is, however, conceivable that germ/somatic cell communication in response to GDF9 diverges between the primary and early secondary stages of folliculogenesis.
One of several possible explanations for our findings is that GDF9 plays a role in stimulating growth of the oocyte in the primary follicle but that a different factor takes over at the secondary follicle stage to drive oocyte growth. A potential candidate for such a factor is KIT ligand. Consistent with this model, KIT-ligand expression is limited in the primary follicle [66, 68] but significantly higher at the secondary and subsequent stages of folliculogenesis [68]. Further, findings in GDF9 null mice suggested GDF9 might suppress KIT-ligand expression at the primary stage [66], which suggests a potential mechanism for the observed stage-specific effects. The relationship between GDF9 and KIT-ligand expression and activity will be an interesting avenue for future study in this in vitro culture system.
In summary, the factors coordinating the growth of the oocyte and surrounding granulosa cells in early preantral stage follicles are not fully known, and uncovering the effect of single factors at these early stages is challenging. Taking advantage of an in vitro culture system developed for isolated follicles, we have dissected the effect of GDF9 on folliculogenesis at the primary and early secondary follicle stages. Our findings demonstrate a stage-specific role for GDF9 in the autocrine and/or paracrine regulation of early preantral folliculogenesis, promoting granulosa cell growth at both stages of early follicle development, but promoting growth of the oocyte only at the primary stage. These findings are further evidence that different factors may be required for oocyte growth at each stage of early preantral follicle development.
Acknowledgments
We thank Dr. Teresa Woodruff and Dr. Min Xu for their advice regarding follicle isolation, encapsulation, and culture and for their gifts of alginate and fetuin reagents.
Compliance with ethical standards
The University of California, San Diego, Institutional Animal Care and Use Committee approved all animal protocols.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research was funded by a Pilot Study Project from the Oncofertility Consortium: Fertility Preservation for Women (U54 RR024347), the National Institutes of Health (NIH) grant R01 HD41494 and the National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. Dr. Cook-Andersen was supported by the Women’s Reproductive Health Research grant K12 HD001259.
Footnotes
Capsule
These findings demonstrate a stage-specific role for GDF9 in the regulation of oocyte and granulosa cell growth at the primary and early secondary stages of preantral follicle development.
Contributor Information
Heidi Cook-Andersen, Email: hcookandersen@ucsd.edu.
Shunichi Shimasaki, Email: sshimasaki@ucsd.edu.
References
- 1.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–38. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–46. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod (Oxford, England) 2010;25(12):2944–54. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 4.Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online. 2007;14(6):758–64. doi: 10.1016/S1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- 5.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99(5):2890–4. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson GF, Shimasaki S. The role of the oocyte in folliculogenesis. Trends Endocrinol Metab TEM. 2000;11(5):193–8. doi: 10.1016/S1043-2760(00)00249-6. [DOI] [PubMed] [Google Scholar]
- 7.Shimasaki S, Moore RK, Erickson GF, Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reprod Suppl. 2003;61:323–37. [PubMed] [Google Scholar]
- 8.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, et al. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110(8):E776–85. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol Endocrinol. 2010;24(12):2303–14. doi: 10.1210/me.2010-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–21. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 11.Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24(6):1230–9. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–6. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 13.Jaatinen R, Laitinen MP, Vuojolainen K, Aaltonen J, Louhio H, Heikinheimo K, et al. Localization of growth differentiation factor-9 (GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9B. Mol Cell Endocrinol. 1999;156(1–2):189–93. doi: 10.1016/S0303-7207(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, et al. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140(3):1236–44. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- 15.McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444–9. [PubMed] [Google Scholar]
- 16.Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84(8):2744–50. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 17.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–5. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 18.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70(4):900–9. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 19.Nicol L, Bishop SC, Pong-Wong R, Bendixen C, Holm LE, Rhind SM, et al. Homozygosity for a single base-pair mutation in the oocyte-specific GDF9 gene results in sterility in Thoka sheep. Reproduction. 2009;138(6):921–33. doi: 10.1530/REP-09-0193. [DOI] [PubMed] [Google Scholar]
- 20.Souza CJ, McNeilly AS, Benavides MV, Melo EO, Moraes JC. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim Genet. 2014;45(5):732–9. doi: 10.1111/age.12190. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1337–44. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- 22.Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321–7. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 23.Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014 doi: 10.1007/s10815-014-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod. 2002;67(3):1018–24. doi: 10.1095/biolreprod.101.002527. [DOI] [PubMed] [Google Scholar]
- 25.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145(1):19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119(Pt 18):3811–21. doi: 10.1242/jcs.03105. [DOI] [PubMed] [Google Scholar]
- 27.Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62(2):370–7. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 28.Liao WX, Moore RK, Shimasaki S. Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors bone morphogenetic protein-15 and growth and differentiation factor-9. J Biol Chem. 2004;279(17):17391–6. doi: 10.1074/jbc.M401050200. [DOI] [PubMed] [Google Scholar]
- 29.Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204(2):373–84. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12(10):2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9(5):1013–21. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 32.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 33.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–48. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30(29):5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73(5):942–50. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, et al. Primate follicular development and oocyte maturation in vitro. Adv Exp Med Biol. 2013;761:43–67. doi: 10.1007/978-1-4614-8214-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81(3):587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod (Oxford, England) 2009;24(10):2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod (Oxford, England) 2011;26(5):1061–72. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod (Oxford, England) 2012;27(6):1801–10. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–6. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 45.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93(8):2633–9. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141(6):809–20. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu MF, Huang WT, Tsay C, Hsu HF, Liu BT, Chiou CM, et al. The stage-dependent inhibitory effect of porcine follicular cells on the development of preantral follicles. Anim Reprod Sci. 2002;73(1–2):73–88. doi: 10.1016/S0378-4320(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 48.Itoh T, Hoshi H. Efficient isolation and long-term viability of bovine small preantral follicles in vitro. In Vitro Cell Dev Biol Anim. 2000;36(4):235–40. doi: 10.1290/1071-2690(2000)036<0235:EIALTV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31(8):1013–28. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng A. 2012;18(11–12):1229–38. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD. Supplemented alphaMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2013;110(12):3258–68. doi: 10.1002/bit.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spicer LJ, Aad PY, Allen D, Mazerbourg S, Hsueh AJ. Growth differentiation factor-9 has divergent effects on proliferation and steroidogenesis of bovine granulosa cells. J Endocrinol. 2006;189(2):329–39. doi: 10.1677/joe.1.06503. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330(6002):366–9. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson LN, Mottershead DG, Dunning KR, Robker RL, Gilchrist RB, Russell DL. Heparan sulfate proteoglycans regulate responses to oocyte paracrine signals in ovarian follicle morphogenesis. Endocrinology. 2012;153(9):4544–55. doi: 10.1210/en.2012-1181. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–7. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 56.Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, et al. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol Reprod. 2004;71(3):732–9. doi: 10.1095/biolreprod.104.028852. [DOI] [PubMed] [Google Scholar]
- 57.Gueripel X, Benahmed M, Gougeon A. Sequential gonadotropin treatment of immature mice leads to amplification of transforming growth factor beta action, via upregulation of receptor-type 1, Smad 2 and 4, and downregulation of Smad 6. Biol Reprod. 2004;70(3):640–8. doi: 10.1095/biolreprod.103.021162. [DOI] [PubMed] [Google Scholar]
- 58.Sun RZ, Lei L, Cheng L, Jin ZF, Zu SJ, Shan ZY, et al. Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles. J Mol Histol. 2010;41(6):325–32. doi: 10.1007/s10735-010-9294-2. [DOI] [PubMed] [Google Scholar]
- 59.Feary ES, Juengel JL, Smith P, French MC, O’Connell AR, Lawrence SB, et al. Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the Woodlands FecX2W mutation. Biol Reprod. 2007;77(6):990–8. doi: 10.1095/biolreprod.107.062752. [DOI] [PubMed] [Google Scholar]
- 60.Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction. 2009;138(1):115–29. doi: 10.1530/REP-08-0538. [DOI] [PubMed] [Google Scholar]
- 61.Oron G, Fisch B, Ao A, Zhang XY, Farhi J, Ben-Haroush A, et al. Expression of growth-differentiating factor 9 and its type 1 receptor in human ovaries. Reprod Biomed Online. 2010;21(1):109–17. doi: 10.1016/j.rbmo.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Li Q, Agno JE, Edson MA, Nagaraja AK, Nagashima T, Matzuk MM. Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011;7(10):e1002320. doi: 10.1371/journal.pgen.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, et al. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol. 2004;18(3):653–65. doi: 10.1210/me.2003-0393. [DOI] [PubMed] [Google Scholar]
- 64.Kaivo-Oja N, Mottershead DG, Mazerbourg S, Myllymaa S, Duprat S, Gilchrist RB, et al. Adenoviral gene transfer allows Smad-responsive gene promoter analyses and delineation of type I receptor usage of transforming growth factor-beta family ligands in cultured human granulosa luteal cells. J Clin Endocrinol Metab. 2005;90(1):271–8. doi: 10.1210/jc.2004-1288. [DOI] [PubMed] [Google Scholar]
- 65.Vitt UA, Mazerbourg S, Klein C, Hsueh AJ. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67(2):473–80. doi: 10.1095/biolreprod67.2.473. [DOI] [PubMed] [Google Scholar]
- 66.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13(6):1018–34. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 67.Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161(1):194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- 68.Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, et al. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157(1):85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]