Abstract
Purpose
The purpose of this study was to evaluate whether outcomes are different if controlled ovarian stimulation (COS) is started in the luteal phase rather than the follicular phase.
Methods
A systematic review and meta-analysis was performed. Sixteen studies were included in the qualitative analysis, and eight studies with a total of 338 women were included in the quantitative analysis.
Results
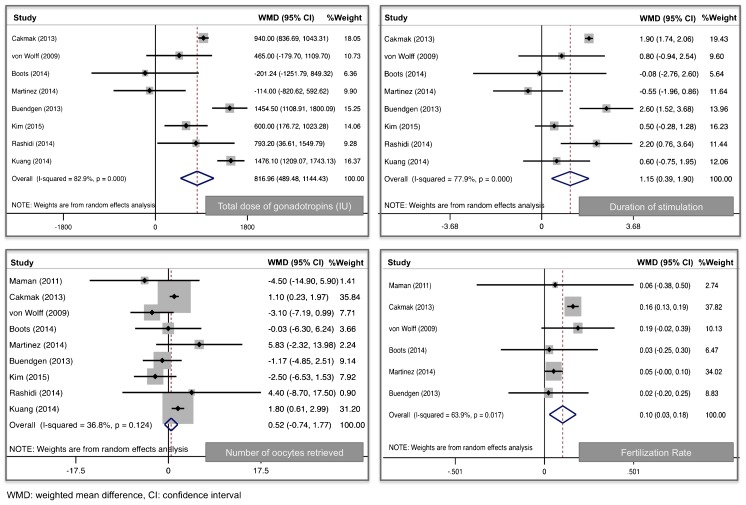
Cycles initiated in the luteal phase were slightly longer (WMD 1.1 days, 95 % CI 0.39–1.9) and utilized more total gonadotropins (WMD 817 IU, 95 % CI 489–1144). However, no differences were noted in peak estradiol levels (WMD −411 pg/ml, 95 % CI −906–84.7) or in the total number of oocytes retrieved (WMD 0.52 oocytes, 95 % CI −0.74–1.7). There were slightly more mature oocytes retrieved in the luteal phase (WMD 0.77 oocytes, 95 % CI 0.21–1.3), and fertilization rates were significantly higher (WMD 10 %, 95 % CI 0.03–0.18). While only three studies reported pregnancy outcomes, no difference was noted in the FET pregnancy rates after COS in the luteal versus follicular phase (RR 0.95, 95 % CI 0.56–1.7).
A post hoc power analysis revealed that a sample of this size was sufficient to detect a clinically meaningful difference of 2 oocytes retrieved with 93 % power.
Conclusion
Although initiating COS in the luteal phase requires a longer stimulation and a higher dose of total gonadotropin, these differences are not clinically significant. Furthermore, COS initiated in the luteal phase does not compromise the quantity or quality of oocytes retrieved compared to outcomes of traditional stimulation in the follicular phase.
Keywords: Luteal phase, Fertility preservation, Oocyte cryopreservation, In vitro fertilization, Controlled ovarian stimulation
Introduction
In 2012, over seven million women had an active diagnosis of cancer, and of those, nearly 95,000 were between the ages of 20 and 40 [1]. As advancements in the efficacy of cancer diagnosis and treatment continue, women facing cancer diagnoses at reproductive ages are confronted with the potential need for gonadotoxic therapy before they have completed their childbearing. Investigation into the opinions of cancer patients faced with potential infertility as a result of their disease treatment reveals that most patients prefer to retain the potential to have genetically related offspring as opposed to using donor gametes or adoption [2]. The American Society of Clinical Oncologists recommends that reproductive-aged patients undergoing cancer treatment be counseled on the possibility of infertility after treatment and offered attempts at fertility preservation [3].
Available fertility preservation strategies include ovarian transposition/shielding, ovarian suppression with gonadotropin- releasing hormone (GnRH) analogues during cytotoxic therapy, cryopreservation of ovarian tissue prior to therapy, or pre-treatment oocyte and embryo cryopreservation [4]. The use of GnRH analogues remains controversial and is not FDA-approved for this indication. Cryopreservation of ovarian tissue does not require stimulation, but is still considered investigational. The strategy most likely to result in pregnancy remains oocyte or embryo cryopreservation [4]. Both methods involve ovarian stimulation as performed during assisted reproductive technology (ART). While new protocols are investigating the retrieval of unstimulated oocytes and maturing them in vitro, these techniques also remain experimental.
Conventional ART protocols require approximately 2 weeks of controlled ovarian stimulation (COS) initiated in the early follicular phase, often after a period of pituitary downregulation with a GnRH agonist. As women may present for fertility preservation consultation at any time during their menstrual cycle, this traditional approach may delay cancer treatments for up to 6 weeks in an effort to optimize oocyte yield. Such a delay in treatment is not ideal and, in some cases, may prevent women from pursuing attempts at fertility preservation in an effort to begin cancer treatments immediately.
These conventional protocols are based on the long-held thought that follicular recruitment occurs once during each menstrual cycle, a theory that has recently been challenged in favor of multiple waves of follicular recruitment [5, 6]. This has led to the hypothesis that ovarian stimulation may be initiated at times other than the early follicular phase, and small studies support this hypothesis.
Von Wolff and colleagues were some of the first to demonstrate that ovarian stimulation could safely and effectively be initiated outside of the early follicular phase [7]. Sonmezer et al. followed with a case series documenting success with random-start ovarian stimulation in three women with newly diagnosed breast cancer [8]. Likewise, Nayak and Wakim demonstrated that with luteal phase stimulation, four women were able to complete ovarian stimulation and embryo cryopreservation and begin cancer treatments within 17 days of presentation [9]. Ozkaya and colleagues published a case report in early 2012 detailing the successful luteal start of a woman with recently diagnosed Hodgkin lymphoma [10], and Cakmak et al. showed there was no difference between conventional and random-start IVF cycles in a retrospective cohort of 125 women desiring fertility preservation prior to radiation or chemotherapy [11].
The opportunity to effectively stimulate follicular development and generate mature oocytes or embryos for cryopreservation at any point during the menstrual cycle is a promising advancement in fertility preservation for reproductive-aged women undergoing gonadotoxic therapy. However, the current literature includes only observational and mostly small studies. Thus, we undertook a systematic review of the literature and meta-analysis of the currently published cases to evaluate the success of ovarian stimulation for fertility preservation initiated during the luteal phase. We also included cases from our institution.
Materials and methods
The conduct and reporting of this systematic review closely adhered to guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [12].
Search strategy
Our librarian (AH) created search strategies for the concepts of cancer (neoplasms), in vitro fertilization (IVF), and random, immediate, luteal phase, and follicular phase ovarian stimulation for fertility preservation. She used a combination of standardized index terms and plain language keywords, harvested from indices, dictionaries, and on-topic articles. To exclude animals, she used the Human filter for PubMed recommended in Cochrane Handbook for Systematic Reviews of Interventions and used that as a model to create similar filters for the other databases searched [13]. The search strategies were launched in PubMed 1946-, Embase 1947-, Scopus 1823-, CENTRAL (Cochrane Central Register of Controlled Trials), Clinicaltrials.gov, the Cochrane Library, Proquest Dissertations and Theses, FirstSearch Proceedings, and Google Scholar. Searches were limited to English using database supplied limits. All publication years were considered. Searches were completed in October 2013, and the searches were executed again in July 2015 to check for relevant new citations. The full, detailed search strategies for each of the databases are available in the supplemental Appendix. All results were exported to EndNote. The automatic duplicate finder was applied, and 93 duplicates were assumed to be accurately identified and removed, resulting in a total of 544 unique citations, with an additional 136 results added after the updated literature search. Additional studies were identified by reviewing the references of included studies.
Study selection criteria
All studies that evaluated women who underwent ovarian stimulation initiated in the luteal phase and subsequent oocyte retrieval were included in the systematic review and qualitative analysis. Only original research published in English were included, and data in the abstract form only were excluded. Because of the limited published data, study design, length of follow-up, and publication year were not restricted. To be included in the quantitative meta-analysis, studies were required to compare women who initiated their ovarian stimulation in the luteal phase to a control group of women who initiated their stimulation in the follicular phase. Studies that reported one or less women in either cohort were excluded from analysis. The primary outcome was number of oocytes retrieved. Secondary outcomes included total dose of gonadotropins, duration of stimulation, peak estradiol level, and pregnancy rate.
Study selection and data abstraction
The results of the systematic search were thoroughly reviewed independently by two authors (CEB, MM). Data from included studies were then extracted for study design, study location, and year of publication. Patient characteristics including age and ovarian reserve parameters, specifically antral follicle count, follicle stimulating hormone, and anti-Müllerian hormone, were collected. ART characteristics, such as total dose of gonadotropins, duration of stimulation in days, peak estradiol level, number of oocytes retrieved, fertilization rate, number of embryos transferred, number of embryos cryopreserved, and pregnancy outcomes, were also extracted.
Primary data collection
Primary data from Washington University’s IVF program from January 2001 through December 2013 was also analyzed for inclusion. ART cycles performed for fertility preservation were identified in the institution’s Society of Assisted Reproductive Technologies (SART) database. Stimulations begun in the luteal phase were compared to those begun in the follicular phase. Demographics, ovarian reserve parameters, as well as stimulation characteristics, were abstracted from patient charts.
Statistical methods
Statistical analyses were performed in STATA 14 (Statacorp, College Station, TX, USA). Meta-analysis for weighted mean differences (WMD) and 95 % confidence intervals (CI) for study outcome data were quantified using a DerSimonion-Laird random effects model [14]. Cochran’s Q statistic (x2) was used to assess the presence of heterogeneity, and I2 quantified the magnitude of heterogeneity [(I2 = Q − degrees of freedom) × 100 / Q], where degrees of freedom = k − I, Q is Cochran’s Q statistic, and k is the number of studies [15].
Results
Systematic review
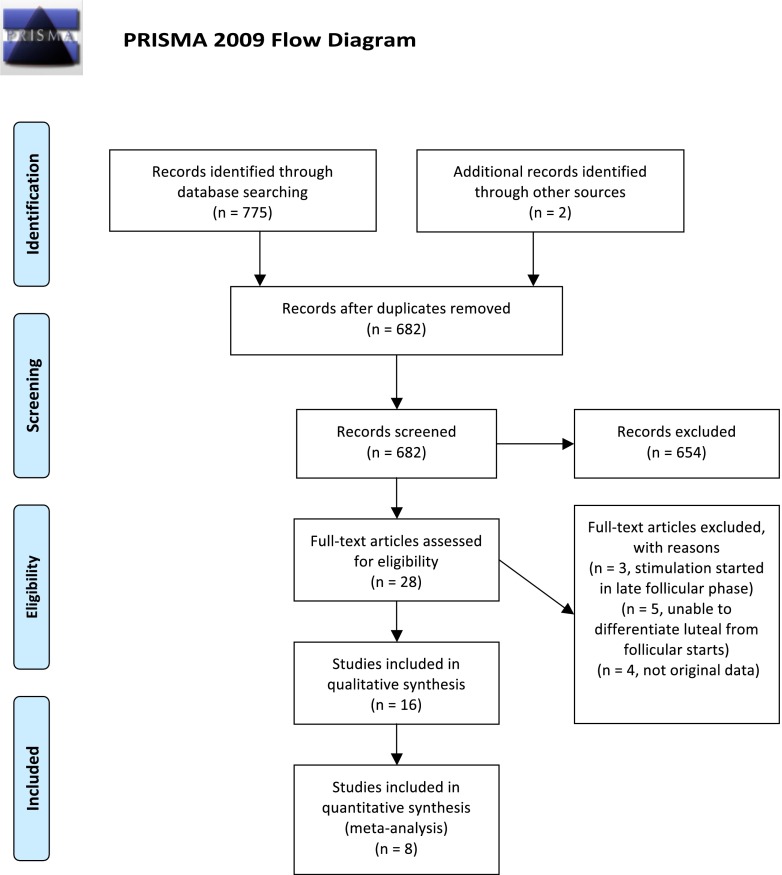
As shown in Fig. 1, the systematic review produced 682 unique studies. Two additional studies were included during review of the literature. Twenty-eight articles were then closely reviewed. One article described random starts for fertility preservation, but stimulation was initiated only in the follicular phase [16]. Two articles by the same author described one patient who ovulated during but not prior to stimulation and were therefore excluded [17, 18]. Five studies did not differentiate luteal from follicular phase starts [9, 19–22]. Three studies did not report original data [23–25]. One study was excluded as the authors’ most current publication was included and previous works were presumed to include the same patients [26]. Sixteen studies met all the inclusion criteria for qualitative analysis (Tables 1 and 2).
Fig. 1.
PRISMA four-phase flow diagram of search yield, screening, and inclusion steps. Adapted from [40]. For more information, visit www.prisma-statement.org
Table 1.
Qualitative analysis of included studies
| Authors | Year | Location | Study design | Study population | Luteal phase (n) | Follicular phase (n) | Results |
|---|---|---|---|---|---|---|---|
| Bedoschi et al. | 2010 | Brazil | Retrospective case series | Fertility preservation | 2 | 0 | No comparison |
| Buendgen et al. | 2013 | Germany | Prospective case control | Infertility | 10 | 30 | Luteal required higher doses of gonadotropin and resulted in lower estradiol levels. |
| Cakmak et al. | 2013 | San Francisco, CA | Retrospective cohort | Fertility preservation | 22 | 102 | Luteal required higher doses of gonadotropin and longer duration of stimulation. |
| Dittrich et al. | 2013 | Germany | Retrospective case series | Fertility preservation | 1 | 13 | No comparison |
| Keskin et al. | 2015 | Turkey | Retrospective case report | Fertility preservation | 1 | 2 | No comparison |
| Kim et al. | 2015 | Korea | Retrospective case control | Fertility preservation | 5 | 17 | Luteal required higher doses of gonadotropin. |
| Kuang et al.1 | 2014 | China | Prospective paired cohort | Infertility | 30 | 38 | Luteal required higher doses of gonadotropins but retrieved more oocytes. |
| Kuang et al.2 | 2014 | China | Prospective cohort | Infertility | 242 | 0 | No comparison |
| Maman et al. | 2011 | Israel | Retrospective cohort | Fertility preservation | 5 | 13 | No significant differences |
| Martinez et al. | 2014 | Spain | Prospective paired cohort | Oocyte donors | 9 | 9 | No significant differences |
| Ozkaya et al. | 2012 | Valhalla, NY | Retrospective case report | Fertility preservation | 1 | 0 | No comparison |
| Rashidi et al. | 2014 | Iran | Prospective cohort | Fertility preservation | 3 | 11 | No comparison |
| Schuffner et al. | 2012 | Brazil | Retrospective case series | Fertility preservation | 1 | 2 | No comparison |
| Sonmezer et al. | 2011 | Valhalla, NY | Retrospective case series | Fertility preservation | 1 | 2 | No comparison |
| Suikkari et al. | 2000 | Finland | Prospective case series | Infertility | 12 | 0 | No comparison |
| von Wolff et al. | 2009 | Germany | Prospective cohort | Fertility preservation | 12 | 28 | No significant differences |
| Washington University | 2013 | St. Louis, MO | Retrospective cohort | Fertility preservation | 5 | 38 | No significant differences |
Table 2.
Meta-analysis comparing ART cycles initiated in the luteal versus follicular phase
| Luteal | Follicular | P value | |
|---|---|---|---|
| Age (years) | 30.9 | 31.2 | NS |
| Duration of stimulation (days) | 11.0 | 9.7 | 0.003 |
| Total dose of gonadotropins (IU) | 2820.4 | 2522.1 | <0.001 |
| Peak estradiol (pg/nL) | 1334.9 | 1620.6 | NS |
| Number of oocytes retrieved | 10.6 | 11.9 | NS |
| Number of mature oocytes | 7.6 | 8.4 | 0.007 |
| Fertilization rate | 77.2 % | 68.9 % | 0.008 |
| Number of embryos cryopreserved | 4.7 | 6.2 | NS |
Weighted means
NS not significant
Qualitative analysis
Study Design
Seven studies were prospectively designed, while seven were retrospective case reports or case series. The majority of the included subjects were pursuing fertility preservation. However, four studies evaluated infertile patients with indications for ART and one study analyzed oocyte donors and their recipients. All but one of the authors utilized GnRH antagonist protocols [27]. Human chorionic gonadotropin (hCG) was the predominant medication used to induce oocyte maturation. Although four authors induced maturation with a GnRH agonist [10, 27–29], three authors used either hCG or GnRH agonist depending on the clinical scenario [11, 30, 31], and one author performed in vitro maturation following neither hCG nor a GnRH agonist [32]. All of the included studies reported the primary outcome of number of oocytes retrieved.
Pregnancy
Only four studies reported pregnancy rates following frozen embryo transfers (FET). Kuang et al. initiated ovarian stimulation after spontaneous ovulation in 242 women, of which 229 had embryos cryopreserved and subsequently transferred with an ongoing pregnancy rate of 48.9 % [28]. However, the authors did not include a comparison cohort. In another study by Kuang et al., the authors performed a double stimulation and retrieval in one cycle [27]. Stimulation was initiated in the early follicular phase with letrozole, clomiphene, and human menopausal gonadotropin (hMG). Oocyte maturation was triggered with a GnRH agonist and transvaginal follicular aspiration was performed. HMG and letrozole were then re-initiated after the retrieval for a second stage of stimulation followed by trigger with a GnRH agonist and a second oocyte retrieval. Pregnancy rates from oocytes obtained in the follicular stimulation were compared to those retrieved in the luteal stimulation. Similar implantation and ongoing pregnancy rates were noted. Buendgen et al. prospectively compared ten women whose stimulation started in the luteal phase to 30 women matched by age, BMI, and duration of infertility who were stimulated in the early follicular phase [33]. Only one of the ten luteal starts had an ongoing pregnancy >12 weeks gestation, while six of the 30 follicular starts had ongoing pregnancies. This difference did not reach statistical significance. Finally, Martinez et al. compared the cycles of nine oocyte donors who each underwent one luteal start COS and one follicular start COS [29]. There was no difference in pregnancy rates between recipients receiving oocytes retrieved after a luteal start COS (58.3 %, 7/12) and recipients of oocytes from a COS initiated in the early follicular phase (62.5 %, 5/8). None of the studies described pregnancy outcomes from patients undergoing ART for fertility preservation.
Washington University Primary Data
A total of 43 women completed ovarian stimulation and oocyte retrieval for fertility preservation. Thirty-eight women were stimulated in the follicular phase, while five were stimulated in the luteal phase. There were no differences in the total dose of gonadotropin, duration of stimulation, number of total oocytes or mature oocytes retrieved, or fertilization rate. There was a significant difference in the estradiol levels on day of trigger. However, three of the five luteal start patients received an aromatase inhibitor throughout the stimulation and all of them received a GnRH antagonist at initiation of stimulation. Only 12 of the 38 follicular starts received an aromatase inhibitor and most only received the GnRH antagonist after rise in estradiol and evidence of follicular growth.
Quantitative analysis
The final quantitative meta-analysis included eight published studies, plus the primary data from Washington University for a total of 338 women [7, 11, 29, 33, 34]. Eight of the qualitative studies were excluded due to the lack of a control group or due to a sample size of only one subject [8, 10, 28, 32, 35–38].
In comparing the COS characteristics of cycles initiated in the luteal and follicular phases, age was not statistically different between the two cohorts and no significant heterogeneity was noted (WMD 0.22, 95 % CI −0.87–1.32, I2 0 %) (Table 2) . As illustrated in Fig. 2, cycles initiated in the luteal phase were slightly longer (WMD 1.1 days, 95 % CI 0.39–1.9, I2 78 %) and utilized more total gonadotropins (WMD 817 IU, 95 % CI 489–1144, I2 83 %). However, no differences were noted in peak estradiol levels (WMD −411 pg/mL, 95 % CI −906–84.7, I2 90 %) or in the total number of oocytes retrieved (WMD 0.52, 95 % CI −0.74–1.7, I2 37 %). There were slightly more mature oocytes retrieved in the luteal phase (WMD 0.77, 95 % CI 0.21–1.3, I2 0 %). A post hoc power analysis revealed that a sample of this size would be able to detect a clinically meaningful difference of 2 oocytes retrieved with 93 % power.
Fig. 2.
Forest plots comparing ovarian stimulation initiated in the luteal versus follicular phase. WMD weighted mean difference, CI confidence interval
Fertilization rates were significantly higher in women initiating COS in the luteal phase (WMD 0.10, 95 % CI 0.03–0.18, I2 64 %). Four studies described the number of embryos cryopreserved and when pooled, showed no statistical difference (WMD −0.01, 95 % CI −2–2, I2 0 %). While only three studies reported pregnancy outcomes, no differences were noted in the FET pregnancy rates after COS in the luteal versus follicular phase (RR 0.95, 95 % CI 0.56–1.7, I2 0 %).
A sub-analysis was performed including only studies describing patients undergoing fertility preservation (7, 11, 30, 31, 34, WU primary data). The COS of 251 women were analyzed, and when comparing luteal starts to follicular starts, there was no difference in age (WMD 0.54 years, 95 % CI −0.94–2.0, I2 0 %), peak estradiol (WMD −337 pg/mL, −849–175, I2 91.4 %), or number of oocytes (WMD −0.6 oocytes, 95 % CI −2.8–1.6, I2 37 %). Again, luteal phase starts utilized more total gonadotropins (WMD 683 IU, 95 % CI 369–997, I2 53 %), were stimulated longer (WMD 1.3 days, 95 % CI 0.37–2.1, I2 76 %), and the oocytes fertilized more efficiently (WMD 0.16, 95 % CI 0.13–0.19, I2 0 %).
Discussion
Our data suggest that ovarian stimulation may safely and effectively be initiated at any point in the menstrual cycle. Despite an increase in the duration of stimulation and total dose of gonadotropins, the quantity and quality of oocytes (as evidenced by fertilization rates) are not different between COS initiated in the luteal phase and the follicular phase. This supports the evolving theory that follicular recruitment does not occur once during the menstrual cycle but instead in multiple waves [6]. Currently, the most effective and accessible method of fertility preservation remains oocyte or embryo cryopreservation [4], and by initiating COS upon presentation, providers can expedite the process of fertility preservation and facilitate prompt initiation of cancer therapy.
Fertility preservation with oocyte or embryo cryopreservation is relatively new. As a result, the majority of the available data on outcomes involve rates of oocyte/embryo yield, and in some cases, oocyte fertilization rates. Our finding of significantly increased rates of oocyte fertilization in women initiating COS in the luteal phase is intriguing and one that has not been published previously. Further investigation into the impact of the timing of COS on fertilization outcomes is warranted. One hypothetical explanation and also a potential risk of bias is that women who initiated their stimulation in the luteal phase represent only women who are regularly cycling, whereas the cohort of women in the follicular phase could include anovulatory women whose disease state is inhibiting normal hormonal pulsatility or women with other conditions, such as polycystic ovarian syndrome, hypothalamic amenorrhea, or premature ovarian insufficiency, who are theoretically, subfertile. This bias is addressed by one of the included studies. Martinez et al. prospectively analyzed the stimulation of nine oocyte donors in the follicular phase and the same nine donors when stimulation was initiated in the luteal phase [29]. Although using a small sample size, there were no differences in stimulation parameters or in recipient pregnancy rates.
Rates of clinical pregnancies and live births, outcomes that are highly important to patients and potentially influential in their decision to pursue fertility preservation, are lacking. Only four studies reported pregnancy outcomes and none of them included women undergoing fertility preservation [27–29, 33]. The limited duration of follow-up and the novelty of fertility preservation make data on pregnancy outcomes limited as few patients have progressed in their treatment course to be ready to attempt pregnancy. As more patients undergo COS for fertility preservation and recover from their cancer diagnosis and treatment, we will have the opportunity to investigate pregnancy, birth, and long-term outcomes in the context of randomly timed COS.
Strengths of this study include the rigorous adherence to the protocol for systematic reviews and meta-analyses. Oocyte yield was chosen as the primary outcome as it is a reflection of ovarian reserve and responsiveness, and it can be used as a predictor of pregnancy and live birth rates, which are clinically useful and of interest to both providers and patients. A post hoc power analysis revealed that a sample of this size would be able to detect a clinically meaningful difference of 2 oocytes retrieved with 93 % power. Lastly, the conclusion of this meta-analysis is in agreement with the results of the majority of individually included studies.
Meta-analyses are inherently limited by the quality of the individual studies. Of the studies included in our analysis, ten of 13 were retrospective designs and sample sizes ranged from 1 to 242. There were no level I data available on this topic. The included observational studies were performed at varying centers all over the world where clinical practice inevitably differs. In addition, the patient population presumably differs by type and severity of cancer, treatment age, urgency of COS and oncologic treatment, and time to COS. As a result, there was significant heterogeneity among several of the stimulation outcomes. I2 quantified the magnitude of heterogeneity [15]. Duration of stimulation, total dose of gonadotropin, and estradiol levels were all considered to have severe heterogeneity (78, 83, 90 %, respectively), and, therefore, these results should be interpreted with caution. However, the heterogeneity of some of the variables improved in the sub-analysis of only fertility preservation patients (76, 53, 92 %). As expected in fertility preservation cycles, the range of estradiol levels was wide due to variable use of aromatase inhibitors to minimize exposure of estrogen sensitive tumors. Importantly, the primary outcome of the study, number of oocytes retrieved, demonstrated only mild heterogeneity (37 %).
This systematic review and meta-analysis is a representation of the current literature. The objective of the systematic review was to summarize the available evidence and encourage continued research on this important and timely topic. Dissemination of such information is critical as our group has demonstrated that fertility preservation practices are variable among Reproductive Endocrinologists [39]. Although the meta-analysis contains significant clinical and statistical heterogeneity, this method of quantitative analysis is reasonable when only observational data exists. Randomized controlled trials have not been performed and would be arguably unethical in fertility preservation cycles where a delay in therapy could jeopardize oncologic therapy. Therefore, the collective conclusion of this combined systematic review and meta-analysis reassures the continued use of random-start ovarian stimulation in the setting of fertility preservation.
In summary, COS for fertility preservation can safely and effectively be initiated in the luteal phase without compromising oocyte number or quality and should be discussed as an option with all reproductive-aged women facing gonadotoxic therapies. Dissemination of this information is important as application of fertility preservation strategies vary widely [39]. More work is needed before luteal phase starts are applied broadly in the general infertility population, but they do have potential in improving convenience of ART.
Acknowledgments
C.E.B. received support from the National Research Training Program in Reproductive Medicine sponsored by the National Institute of Health (T32 HD040135-13) and the Scientific Advisory Board of Vivere Health. E.S.J. received support from the Women’s Reproductive Health Research (WRHR) Program sponsored by the National Institute of Health (K12 HD063086), the Institute of Clinical and Translational Sciences (ICTS) at Washington University (UL1 TR000448), the Barnes Jewish Hospital Foundation, and the March of Dimes. A.R.C also received funding from the WRHR and ICTS programs.
Appendix: Systematic review search strategies
PubMed
(“Neoplasms” [Mesh] OR “neoplasm” OR “neoplasms” OR “cancer” OR “cancers” OR “neoplasia” OR “neoplasias” OR “carcinoma” OR “sarcoma” OR “sarcomas” OR “neoplastic disease”) AND (“Fertilization in Vitro” [Mesh] OR “Reproductive Techniques, Assisted” [Mesh] OR “Fertilization in Vitro” OR “In Vitro Fertilization” OR “IVF” OR “In Vitro Fertilizations” OR “Test-Tube Fertilization” OR “Test Tube Fertilization” OR “Test-Tube Fertilizations” [tiab] OR “Fertilizations in Vitro” OR “Test-Tube Babies” OR “Test Tube Babies” OR “Test-Tube Baby” OR “extracorporeal fertilization” OR “in vitro fertilization” OR “testtube baby” [tiab]) AND (“Luteal Phase” [Mesh] OR “Follicular Phase” [Mesh] OR “Luteal Phase” OR “random” OR “immediate” OR “Menstrual Secretory Phase” [tiab] OR “Postovulatory Phase” OR “Follicular Phase” OR “Menstrual Proliferative Phase” OR “Preovulatory Phase”) NOT ((“Animals” [Mesh]) NOT (“Animals” [Mesh] AND “Humans” [Mesh]))
=203 results, 187 after limiting to English on 9/27/2013
Updated results = 39 results, 37 after limiting to English between 9/28/2013–7/13/2015
Embase
“neoplasm”/exp OR neoplasm OR neoplasms OR cancer OR cancers OR neoplasia OR neoplasias OR carcinoma OR carcinomas OR sarcoma OR sarcomas OR tumor OR tumors OR tumour OR tumours AND (“fertilization in vitro”/exp OR “infertility therapy”/exp OR “Fertilization in Vitro” OR “In Vitro Fertilization” OR “IVF” OR “In Vitro Fertilizations” OR “Test-Tube Fertilization” OR “Test Tube Fertilization” OR “Test-Tube Fertilizations” OR “Fertilizations in Vitro” OR “Test-Tube Babies” OR “Test Tube Babies” OR “Test-Tube Baby” OR “extracorporeal fertilization” OR “in vitro fertilization” OR “testtube baby”) AND (“random” OR “immediate” OR “luteal phase”/exp OR “Luteal Phase” OR “Menstrual Secretory Phase” OR “Postovulatory Phase” OR “follicular phase”/exp OR “Follicular Phase” OR “Menstrual Proliferative Phase” OR “Preovulatory Phase”) NOT ([animals]/lim NOT [humans]/lim)
=217 results, 205 after limiting to English on 9/27/2013
Updated results = 63 results (same number after limiting to English) after limiting to 9/28/2013–7/13/2015
Scopus
(TITLE-ABS-KEY (neoplasm OR neoplasms OR cancer* OR neoplasia* OR carcinoma* OR sarcoma* OR “neoplastic disease”)) AND (TITLE-ABS-KEY (“Fertilization in Vitro” OR “In Vitro Fertilization” OR “IVF” OR “In Vitro Fertilizations” OR “Test-Tube Fertilization” OR “Test Tube Fertilization” OR “Test-Tube Fertilizations” OR “Fertilizations in Vitro” OR “Test-Tube Babies” OR “Test Tube Babies” OR “Test-Tube Baby” OR “extracorporeal fertilization” OR “in vitro fertilization” OR “testtube baby”)) AND (“random start” OR “immediate start” OR “Luteal Phase” OR “Menstrual Secretory Phase” OR “Postovulatory Phase” OR “Follicular Phase” OR “Menstrual Proliferative Phase” OR “Preovulatory Phase”) AND (LIMIT-TO(LANGUAGE, “English”))
=156 results, 140 after limiting to English on 10/2/2013
Updated results
*Note: You cannot limit a Scopus search to a day/month/year date. You can only limit by year. I have limited from 2013–present, so there may be some repeat articles from the initial search.
=46 results, 45 after limiting to English on 7/13/2015
Cochrane Library
“Neoplasms” [Mesh] OR “neoplasm” OR “neoplasms” OR “cancer” OR “cancers” OR “neoplasia” OR “neoplasias” OR “carcinoma” OR “sarcoma” OR “sarcomas” OR “neoplastic disease”
AND (“Fertilization in Vitro” [Mesh] OR “Reproductive Techniques, Assisted” [Mesh] OR “Fertilization in Vitro” OR “In Vitro Fertilization” OR “IVF” OR “In Vitro Fertilizations” OR “Test-Tube Fertilization” OR “Test Tube Fertilization” OR “Test-Tube Fertilizations” OR “Fertilizations in Vitro” OR “Test-Tube Babies” OR “Test Tube Babies” OR “Test-Tube Baby” OR “extracorporeal fertilization” OR “in vitro fertilization” OR “testtube baby”)
AND (“random” OR “immediate” OR “Luteal Phase” [Mesh] OR “Luteal Phase” OR “Menstrual Secretory Phase” OR “Postovulatory Phase” OR “Follicular Phase” [Mesh] OR “Follicular Phase” OR “Menstrual Proliferative Phase” OR “Preovulatory Phase”)
Cochrane results = 88 citations (46 Cochrane Reviews, 10 other reviews, 30 trials, 1 economic evaluation, and 1 Cochrane group. Cochrane Group info won’t be included in Endnote library) on 10/2/13
Updated results
Note: There is no way to limit by date in the Cochrane Library. I have included all citations from 10/2013 to the present.
=19 Cochrane reviews and 5 CENTRAL clinical trials on 7/13/2015
ClinicalTrials.gov
(cancer OR neoplasms OR carcinoma OR sarcoma) AND (“IVF” OR “In Vitro Fertilization” OR “Fertilization in Vitro”) AND (“random” OR “immediate” OR “Luteal Phase” OR “Follicular Phase”)
=37 studies on 10/2/13, only 1 of the 37 studies is relevant to your research question (only 1 study addresses cancer and fertility) on 10/2/2013
Updated search: I did not find any new results since the initial search.
ProQuest dissertations and theses
(AB, TI (cancer OR neoplasms OR carcinoma OR sarcoma)) AND (AB, TI (“IVF” OR “In Vitro Fertilization” OR “Fertilization in Vitro”)) AND (AB, TI (“random” OR “immediate” OR “Luteal Phase” OR “Follicular Phase”))
=15 results on 10/2/13
Proceedings
kw: Cancer and (kw: In w Vitro w Fertilization OR kw: IVF) and (kw: random OR kw: immediate OR kw: luteal w phase OR kw: follicular w phase) = 57 results on 10/2/2013
In each of the results I searched for the term “Cancer” within the proceedings to find relevant results. Below you will find the most relevant proceedings abstracts. I can also send you a complete list of results if you would like to review them all. I do not include these in the EndNote library because they don’t format well for the library.
Society for Gynecologic Investigation; Scientific program & abstracts
Conference: Annual meeting; 60th (2013; Mar: Orlando, FL)
Sponsor: Society for Gynecologic Investigation
Publication: Thousand Oaks, Calif.; Sage; [2013]
Language: English
Series: REPRODUCTIVE SCIENCES; 2013; VOL 20; NUMB 3; SUPP;
CN084316501 Author(s): Pavone, M.E.; Hirshfeld-Cytron, J.; Lawson, A.; Smith, K.; Klock, S. Title: Fertility preservation outcomes may differ by cancer diagnosis page(s): p. S-036
ASRM 2010: scientific abstracts to be presented at the Annual Meeting of the American Society for Reproductive Medicine
Conference: Annual meeting (2010; Oct: Denver, CO)
Sponsor: American Society for Reproductive Medicine
Publication: Elsevier Science; 2010
Language: English
Series: FERTILITY AND STERILITY -INTERNATIONAL EDITION-; 2010; VOL 94; NUMB 4; SUPP;
CN080327315 Author(s): Oktay, K.; Lee, S.; Kim, J. Y.; Moy, F. Title: Long-term outcomes and safety of letrozole-FSH protocol in women with breast cancer undergoing fertility preservation: a prospective-controlled study Page(s): p. S11
CN080332413 Author(s): Oktay, K.; Lee, S. Title: Does higher starting dose of FSH stimulation improve fertility preservation cycle outcomes in women with breast cancer? Page(s): p. S160
American Society for Reproductive Medicine
Conference: Annual meeting; 63rd (2007; Oct: Washington, DC)
Sponsor: American Society for Reproductive Medicine
Publication: Elsevier; 2007
Language: English
Series: FERTILITY AND STERILITY -INTERNATIONAL EDITION-; 2007; VOL 88; SUPPL 1;
CN065945755 Author(s): Azim, A. M.; Oktay, K. Title: Safety of ovarian stimulation with letrozole and gonadotropins in breast cancer patients undergoing embryo or oocyte cryopreservation: a prospective controlled study Page(s): p. S33
CN065954823 Author(s): Elizur, S. E.; Holzer, H. E.; Demirtas, E.; Chian, R. C.; Son, W. Y.; Tan, S. L. Title: In vitro maturation (IVM) and vitrification of oocytes retrieved at the luteal phase in cancer patients facing urgent gonadotoxic treatment Page(s): p. S340
Innovative techniques in human embryo viability assessment
Conference: Symposium (2008)
Publication: Cambridge; RBM Online; 2008
Language: English
Series: REPRODUCTIVE BIOMEDICINE ONLINE -PRINT EDITION-; 2008; VOL 17; NUMB 4;
CN069933165 Author(s): Demirtas, E.; Elizur, S.E.; Holzer, H.; Gidoni, Y.; Son, W.-Y.; Chian, R.-C.; Tan, S.L. Title: Case report: Immature oocyte retrieval in the luteal phase to preserve fertility in cancer patients Page(s): p. 520–523
American Society for Reproductive Medicine; ASRM 2003
Conference: Annual meeting; 59th (2003; Oct: San Antonio, TX)
Sponsor: American Society for Reproductive Medicine
Publication: American Society for Reproductive Medicine; 2003
Language: English
Series: FERTILITY AND STERILITY -INTERNATIONAL EDITION-; 2003; VOL 80; SUPPL 3;
CN050026279 Author(s): Meirow, D.; Maman, E.; Farber, B.; Kaufman, B.; Dor, J. Title: fertility preservation using art and embryo cryopreservation prior to chemotherapy in breast cancer patients. new and safe protocol for ovarian stimulation Page(s): p. P-15
Google Scholar
(Cancer) AND (“IVF” OR “in vitro fertilization”) AND (“random start” OR “immediate start” OR “luteal phase” OR “follicular phase”)
=8, 190 results. I reviewed around 20 pages of results (200 citations) before the results were no longer relevant. I found 2 relevant citations that were not already in the EndNote library and added those to the citation list.
Compliance with ethical standards
Institutional review board approval was obtained from Washington University prior to the chart review and data extraction.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Capsule
Ovarian stimulation may safely and effectively be initiated in the luteal phase without compromising oocyte number or quality.
References
- 1.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission posted to the SEER website. 2015.
- 2.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 3.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2013;100(5):1214–23. [DOI] [PubMed]
- 5.Cakmak H, Rosen MP. Ovarian stimulation in cancer patients. Fertil Steril. 2013;99(6):1476–84. doi: 10.1016/j.fertnstert.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80(1):116–22. doi: 10.1016/S0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- 7.von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92(4):1360–5. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril 2011;95(6). [DOI] [PubMed]
- 9.Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril. 2011;96(1):e51–4. doi: 10.1016/j.fertnstert.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 10.Ozkaya E, San Roman G, Oktay K. Luteal phase GnRHa trigger in random start fertility preservation cycles. J Assist Reprod Genet. 2012;29(6):503–5. doi: 10.1007/s10815-012-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–80. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J, Green S (ed). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J, Thompson S. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Hussein G, Lood M. Random-start IVF treatment: an emergent fertility preservation technique between cytotoxic treatment courses and stem-cell transplantation in acute myelocytic leukemia. Middle East Fertil Soc J 2015;20:297–300.
- 17.Friedman BE, Pao S, Westphal LM, Lathi RB. Oocyte retrieval following continued stimulation five days beyond ovulation yields live birth after frozen embryo transfer. J Assist Reprod Genet. 2012;29(5):433–5. doi: 10.1007/s10815-012-9721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman BE, Pao S, Westphal LM, Lathi RB. Successful oocyte retrieval and fertilization following continued stimulation 5 days beyond ovulation. Fertil Steril. 2011;95(4):S13. doi: 10.1016/j.fertnstert.2011.01.069. [DOI] [Google Scholar]
- 19.Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R. Ovarian response to stimulation for fertility preservation in women with malignant disease: a systematic review and meta-analysis. Fertil Steril. 2012;97(1):125–33. doi: 10.1016/j.fertnstert.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Huober-Zeeb C, Lawrenz B, Popovici RM, Strowitzki T, Germeyer A, Stute P, et al. Improving fertility preservation in cancer: ovarian tissue cryobanking followed by ovarian stimulation can be efficiently combined. Fertil Steril. 2011;95(1):342–4. doi: 10.1016/j.fertnstert.2010.07.1074. [DOI] [PubMed] [Google Scholar]
- 21.Lawrenz B, Jauckus J, Kupka MS, Strowitzki T, von Wolff M. Fertility preservation in >1,000 patients: patient’s characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. 2011;283(3):651–6. doi: 10.1007/s00404-010-1772-y. [DOI] [PubMed] [Google Scholar]
- 22.Lawrenz B, Neunhoeffer E, Henes M, Lessmann-Bechle S, Kramer B, Fehm T. Management of fertility preservation in young breast cancer patients in a large breast cancer centre. Arch Gynecol Obstet. 2010;282(5):547–51. doi: 10.1007/s00404-010-1523-0. [DOI] [PubMed] [Google Scholar]
- 23.El-Toukhy T, Taranissi M. Which is more counterproductive: a brief delay or “start anyway”? Hum Reprod Oxf Engl. 2006;21(9):2456–7. doi: 10.1093/humrep/del243. [DOI] [PubMed] [Google Scholar]
- 24.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy—a technique in its infancy but already successful in fertility preservation. Eur J Cancer Oxf Engl 1990. 2009;45(9):1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Shalom-Paz E, Holzer HEG. Fertility preservation for cancer patients. Current Options Minerva Ginecol. 2011;63(6):517–30. [PubMed] [Google Scholar]
- 26.von Wolff M, Zeeb C, Lawrenz B, Germeyer A, Neunhoeffer E, Strowitzki T. Cryopreservation of ovarian tissue and cryopreservation of oocytes can be efficiently combined and performed within 2 weeks before chemotherapy. Mol Hum Reprod. 2009;24:i157. doi: 10.1093/humrep/dep783. [DOI] [Google Scholar]
- 27.Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod Biomed Online. 2014;29(6):684–91. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–11. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Martinez F, Clua E, Devesa M, Rodriguez I, Arroyo G, Gonzalez C, et al. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril. 2014;102(5):1307–11. doi: 10.1016/j.fertnstert.2014.07.741. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim SK, Lee HJ, Lee JR, Jee BC, Suh CS, et al. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci. 2015;30(3):290. doi: 10.3346/jkms.2015.30.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashidi BH, Tehrani ES, Ghaffari F. Ovarian stimulation for emergency fertility preservation in cancer patients: a case series study. Gynecol Oncol Rep. 2014;10:19–21. doi: 10.1016/j.gore.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suikkari AM, Tulppala M, Tuuri T, Hovatta O, Barnes F. Luteal phase start of low-dose FSH priming of follicles results in an efficient recovery, maturation and fertilization of immature human oocytes. Hum Reprod Oxf Engl. 2000;15(4):747–51. doi: 10.1093/humrep/15.4.747. [DOI] [PubMed] [Google Scholar]
- 33.Buendgen NK, Schultze-Mosgau A, Cordes T, Diedrich K, Griesinger G. Initiation of ovarian stimulation independent of the menstrual cycle: a case-control study. Arch Gynecol Obstet. 2013;288(4):901–4. doi: 10.1007/s00404-013-2794-z. [DOI] [PubMed] [Google Scholar]
- 34.Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–7. doi: 10.1016/j.fertnstert.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 35.Bedoschi GM, de Albuquerque FO, Ferriani RA, Navarro PA. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet. 2010;27(8):491–4. doi: 10.1007/s10815-010-9429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dittrich R, Lotz L, Mueller A, Hoffmann I, Wachter DL, Amann KU, et al. Oncofertility: combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod Biol Endocrinol. 2013;11:19. doi: 10.1186/1477-7827-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keskin U, Ercan CM, Yilmaz A, Babacan A, Korkmaz C, Duru NK, et al. Random-start controlled ovarian hyperstimulation with letrozole for fertility preservation in cancer patients: case series and review of literature. J Pak Med Assoc. 2014;64(7):830–2. [PubMed] [Google Scholar]
- 38.Schuffner A, Skroch R, Garbelini M, Neto J, Peixoto A, da Rosa V. Ovarian stimulation in the follicular, late follicular and luteal phases: an ideal protocol for the preservation of fertility? J Bras Reprod Assist. 2012;16:91–3. [Google Scholar]
- 39.Reynolds KA, Grindler NM, Rhee JS, Cooper AR, Ratts VS, Carson KR, et al. Variability in the practice of fertility preservation for patients with cancer. PLoS One. 2015;10(5):e0127335. doi: 10.1371/journal.pone.0127335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]