Abstract
Purpose
The present study aimed to evaluate whether combining the magnetic-activated cell sorting (MACS) with density-gradient (DG) or swim-up (SU) sperm separation techniques can improve sperm selection to obtain higher quality spermatozoa.
Methods
Two commonly used sperm selection techniques, SU and DG, were compared to MACS combined with either SU or DG. Spermatozoa obtained from normozoospermic (n = 10) and oligozoospermic (n = 10) cases were grouped as SU, DG, SU+MACS, and DG+MACS followed by the analysis of sperm morphology, motility, DNA integrity, and the levels of Izumo-1 and PLCZ proteins.
Results
Although spermatozoa obtained by SU or DG when combined with MACS have improved aspects when compared to SU or DG alone, results did not reach a statistically significant level. Moreover, separation with MACS caused a significant loss in the numbers of total and rapid progressive spermatozoa.
Conclusions
Considering the cost/benefit ratio, MACS application together with traditional techniques may only be preferred in certain cases having higher concentrations of spermatozoa, but it does not seem to be an ideal and practical sperm selection technique for routine use.
Keywords: Sperm selection, Magnetic cell sorting, DNA integrity, Izumo-1, PLC-ζ
Introduction
Selecting the competent spermatozoa by sperm selection techniques has been employed in in vitro fertilization (IVF) techniques, particularly in intracytoplasmic sperm injection (ICSI) cycles. These techniques can be classified in a wide range from sperm washing to advanced sperm selection methods including the removal of apoptotic cells, sperm birefringence, the ability to bind to hyaluronic acid, and the assessment of sperm morphology under ultra-high magnification [1]. Magnetic-activated cell sorting (MACS) was introduced as a technique that separates apoptotic spermatozoa from non-apoptotic ones. One of the early signs of apoptosis is the externalization of the phosphatidylserine phospholipids in the cell membrane due to the loss of membrane integrity, and hence, annexin V labeling can be used as an apoptotic marker because of its high affinity to externalized phosphatidylserine molecules. Hence, in the MACS technique, spermatozoa are incubated with a buffer containing annexin V-conjugated microbeads and are then exposed to a magnetic field in an affinity column, which separates apoptotic sperm cells from the non-apoptotic ones [2]. Although many reports indicate that MACS is a beneficial technique to remove apoptotic spermatozoa and provides higher IVF outcomes compared to canonical sperm selection techniques, the possible beneficial effects of this technique in clinical application are still debatable. Recently, a meta-analysis and an original research article were published, where contradictory results were consecutively presented in the same journal. The meta-analysis, considering the potential beneficial effects of MACS on IVF outcomes, reported that MACS has positive effects on IVF outcomes by increasing the clinical pregnancy rate and decreasing the miscarriage rate [3]. On the other hand, in the original research article, the authors separated non-apoptotic spermatozoa from apoptotic ones and concluded that removing apoptotic cells from unselected males with MACS technology does not improve the reproductive outcome of ICSI in oocyte donation cycles [4]. In both studies, the authors indicated that the application of MACS still needs a more detailed study. By taking into consideration all these data, we determined to select higher quality spermatozoa after the removal of the apoptotic ones with MACS following conventional sperm selection methods. For this purpose, sperm morphology, DNA integrity, TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling) positivity, and the distribution and the quantity of two proteins essential for oocyte fertilization (Izumo-1, sperm-oocyte fusion protein) [5] and activation (phospholipase C zeta (PLC-ζ), oocyte-activating protein) [6] were investigated in the current study. Meanwhile, we also aimed to evaluate whether MACS has an additional benefit when combined with commonly used sperm separation techniques including swim-up (SU) and density gradient (DG). In order to test this, each semen sample was grouped as follows: (i) SU, (ii) DG, (iii) SU+MACS, and (iv) DG+MACS.
Briefly, overall data indicated that the MACS application resulted in selecting slightly higher quality spermatozoa, without any significance compared to the SU- or DG-alone groups.
Material and methods
Case selection, study groups, and routine semen analysis
Ethical approval was obtained from the Local Ethical Review Board for clinical research. Informed consents were obtained from male donors who had applied to the andrology laboratory for routine sperm analysis. The analysis of sperm concentration and motility was performed both in raw and subgrouped semen samples. Briefly, following liquefaction for 30 min (for raw semen) or incubation at 37 °C for 5 min (for liquefied samples), a semen drop of 5 μL was loaded onto the application area of a Makler counting chamber (Sefi Medical Instruments Ltd., Haifa, Israel) after gentle pipetting of the sample. The coverslip ring containing the counting grids was applied, and a 0.1-mm2 smear was obtained to evaluate the sperm concentration and motility. The concentration of the sample was determined by counting sperm heads in successive 10 squares. A mean was detected after counting five different 10-square-in-rows. Motility was detected using the same chamber, counting at least 200 spermatozoa per sample and calculating the sum of rapid progressive sperm percentage. All of the evaluations were performed under a phase-contrast microscope (Nikon, Germany) with ×20 magnification. Following the routine sperm evaluation procedure, cases were grouped as follows: normozoospermic (NZS, n = 10) when sperm concentration was higher than 20 million/mL and oligozoospermic (OZS, n = 10) when sperm concentration was between 5 and 15 × 106/mL. Each semen sample from NZS or OZS cases was further subgrouped as follows: (i) SU (only); (ii) DG (only); (iii) SU+MACS; and (iv) DG+MACS.
Preparation and fixation of spermatozoa
Following the liquefaction of the semen sample, the total volume in each individual subject was equally divided into four subgroups.
(i) SU (only) group: Pipetted semen samples in a 15-mL conical centrifuge tube were gently overlayered with an equal volume of sperm washing medium (SpermRinse, Vitrolife, Sweden). The tube was inclined at an angle of about 45°, to increase the surface area of the semen-culture medium interface, and then incubated for 1 h at 37 °C. After gently returning the tube to the upright position, the uppermost half of the medium was transferred to another test tube and then resuspended with an equal volume of the medium. The pipetted mixture was centrifuged for 10 min at 400g. After discarding the supernatant, the pellet was resuspended with 0.5 mL of sperm washing medium.
(ii) DG (only) group: A density-gradient medium (SpermGrad, Vitrolife, Sweden) was layered as 1 mL of 40 % (v/v) density-gradient medium upper and 1 mL of 80 % (v/v) density-gradient medium lower in a test tube. Semen samples were placed above the density-gradient medium and centrifuged at 400g for 10 min. The supernatant was discarded; the pellet was resuspended with 5 mL of medium by gentle pipetting and then centrifuged at 200g for 10 min.
(iii) and (iv) SU+MACS and DG+MACS groups: Following the aforementioned sperm selection techniques executed in groups (i) and (ii), the MACS procedure was applied to the samples. For this purpose, spermatozoa were incubated with annexin V-conjugated microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) for 15 min at room temperature (RT). One hundred microliters of microbeads were used for each 1 × 107 spermatozoa. The sperm/microbead suspension was then loaded in a separation column containing iron globes, which was fitted in a magnet (MiniMACS; Miltenyi Biotec). The fraction composed of apoptotic spermatozoa was retained in the separation column, whereas the fraction with intact membranes was drained through the column and collected as non-apoptotic spermatozoa.
For the evaluation of four samples, final collections were divided into three groups. The first group was prepared for the sperm morphology assessments. The second group was fixed with 3.5 % paraformaldehyde (Sigma Chemical Co., St. Louis, MO, USA) for 30 min at RT for immunofluorescence assessment; the third group was prepared for flow cytometric analysis.
Evaluation of sperm morphology
After liquefaction for 30 min (for raw semen) or incubation at 37 °C for 5 min (for liquefied samples), a semen drop of 10 μL was smeared on a glass slide. Air-thawed semen samples on glass slides were incubated in solution A (SpermBlue Histostain Kit, Microptic, Spain) for 20 s, washed in distilled water, and incubated in solution B for 20 min at RT. Cells were examined according to strict criteria [7, 8] under a light microscope (Nikon, Germany) equipped with a ×100 objective. At least 100 cells were counted in each group, and the percentage of normal spermatozoa was determined.
Analysis of DNA fragmentation
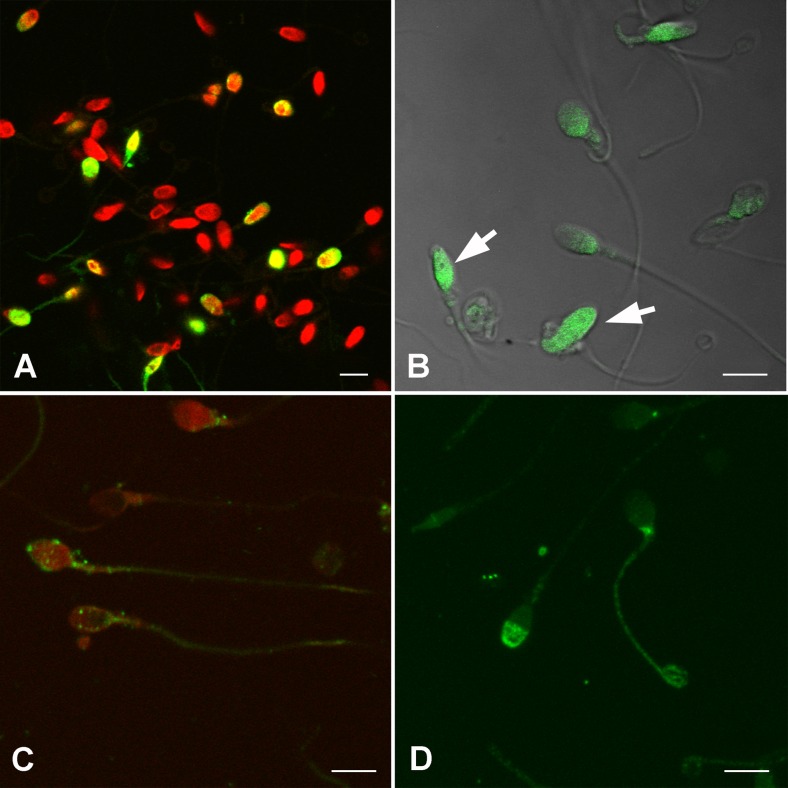
The evaluation of nuclear DNA fragmentation was performed by TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling) assay kit (Roche, Germany). Following the membrane permeabilization with 0.1 % Triton X-100 (Sigma), a 50-mL mixture (9:1) of labeling and enzyme solutions was applied to the slides followed by incubation for 60 min at 37 °C in a dark humidified chamber. As a negative control, the enzyme solution (terminal deoxynucleotidyl transferase) was omitted from the reaction mixture. The slides were then washed in a phosphate-buffered saline (PBS) solution (Sigma), and chromosomes were marked with 10 μM 7-aminoactinomycin D (7-AAD, Sigma) for 15 min at 37 °C. All microscopic observations were performed using Carl Zeiss LSM 510 Meta confocal laser scanning microscope equipped with 488-nm argon ion and 543-nm green helium neon laser lines, unless otherwise stated. The ratio of the total number of TUNEL-positive cells (Fig. 1a) to the total number of counted cells was defined as the TUNEL index.
Fig. 1.
Analysis of spermatozoa for DNA integrity and the capacity of fertilization. (a) Spermatozoa were stained with the TUNEL kit. Green signals show TUNEL-positive cells. (b) Chromomycin A3 staining was applied to evaluate sperm DNA integrity. Spermatozoa with low DNA integrity are observed relatively brighter green, as CMA3 binds to unpacked guanine bases. (c) Green signals indicating anti-PLC-ζ1 protein was observed in different localizations in the spermatozoa. (d) Izumo-1 protein (green signals) was detected particularly in peri-acrosomal or neck locations. Red signals: sperm DNA with 7-AAD. Scale bar: 5 μm
Chromomycin A3 (CMA3) staining
CMA3 was introduced as a tool for the assessment of sperm chromatin integrity, and it was noted that deficiency of protamine, which is a nuclear protein that plays a key role in sperm DNA integrity, is observed as bright after CMA3 staining [9]. Fixed spermatozoa were incubated in a 25 μg/mL (in PBS with 1 % MgCl2) of CMA3 solution (Sigma) for 20 min at RT. Following washing in PBS, the slides were mounted with a 1:1 mixture of glycerol/PBS solution. Spermatozoa with low DNA integrity were observed relatively brighter, as CMA3 binds to unpacked guanine bases. Approximately 1000 cells were examined in each slide (Fig. 1b). The ratio of the total number of bright cells to the total number of counted cells was determined as the CMA3 index.
Detection and quantification of Izumo-1 and PLC-ζ proteins
Prior to flow cytometric analysis, spermatozoa were gated based on the forward scattered cell (FSC) and side scattered cell (SSC) profiles. To confirm the spermatozoa gate in FSC vs. SSC plots, cells were stained with a DNA-binding dye thiazole orange (BD Biosciences, Ann Arbor, MI, USA), and the positive cells were backgated on FSC vs. SSC plots. The correlation between the serial dilutions of known concentration of spermatozoa and the number of cells in the spermatozoa gate was observed for further confirmation. Then, the FSC threshold was set to 300,000 to exclude debris and enrich the analysis of spermatozoa for the following experiments. For further flow cytometric analysis, fixed spermatozoa were permeabilized with 0.01 % Triton X-100 for 15 min and then washed in PBS. Each washing step was followed by a centrifugation at 800g for 10 min during the entire procedure. Cells were then blocked in a solution containing 3 % bovine serum albumin (BSA) for 30 min and then incubated in a 1:20 dilution of anti-PLC-ζ1 rabbit polyclonal antibody (Abcam, Cambridge, UK) for 30 min. Cells were then washed in PBS and incubated in a 1:250 dilution of an affinity-purified fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Sigma) for 30 min. The same protocol was performed for the anti-Izumo-1 antibody (Sigma, 1:100 in PBS). All procedures were executed at RT, and then, cells were analyzed on a BD Accuri C6 Cytometer (USA).
In order to verify the flow cytometer outcomes and to determine the localization of those proteins, immunofluorescence staining was applied. For this purpose, following the permeabilization of fixed spermatozoa with 0.1 % of Triton X-100, they were incubated in either 25 μg/mL of anti-PLC-ζ1 rabbit polyclonal antibody (Abcam, Cambridge, UK) (Fig. 1c) or anti-Izumo-1 rabbit polyclonal antibody (Sigma, 1:100 in PBS) (Fig. 1d) overnight at 4 °C. Following washing in PBS, the cells were incubated in a 1:100 dilution of an affinity-purified FITC-conjugated goat anti-rabbit IgG (Sigma). Chromosomes were marked with 7-AAD, and spermatozoa were mounted between glass slides (as described above).
Statistical analysis
All statistical analyses were performed by an SPSS software package (version 15.0; SPSS Inc., Chicago, IL). The Shapiro-Wilk test for normality was used to test whether the data were normally distributed or not. Logarithmic transformation was applied to not normally distributed data, when applicable. Normally distributed data were presented as mean ± standard deviation (SD) and analyzed by one-way analysis of variance (ANOVA) to investigate the differences between the groups, and then, a post hoc Bonferroni test was implemented. The significance level was set at p < 0.05.
Results
Assessments of sperm count, motility, and morphology
Compared to fresh sperm samples, sperm concentration and rapid progressive sperm concentration significantly decreased in both SU+MACS and DG+MACS groups in the NZS cases (Table 1). The decrease in the sperm concentration and rapid progressive sperm number was found to be comparable between SU and SU+MACS, DG and DG+MACS, and also among the OZS groups.
Table 1.
Comparison of sperm concentration, rapid progressive sperm concentration, and morphology
| (mean ± SD ×106/mL) | Fresh | SU | SU+MACS | DG | DG+MACS | |
|---|---|---|---|---|---|---|
| NZS | Sperm concentration | 43.0 ± 21.3 | 21.1 ± 11.9 | 8.3 ± 6.1* | 20.0 ± 11.2 | 12.1 ± 7.6** |
| Rapid progressive sperm concentration | 19.6 ± 14.3 | 9.3 ± 6.9 | 4.1 ± 2.7*** | 6.0 ± 4.5 | 2.5 ± 1.8**** | |
| Normal sperm morphology (%, mean ± SD) | 4.6 ± 3.9 | 3.3 ± 2.8 | 1.8 ± 3.0 | 2.0 ± 2.2 | 2.1 ± 2.2 | |
| OZS | Sperm concentration | 9.6 ± 3.8 | 3.8 ± 2.9 | 2.5 ± 2.1 | 6.2 ± 2.8 | 3.5 ± 3.4 |
| Rapid progressive sperm concentration | 2.4 ± 0.8 | 1.4 ± 1.0 | 0.02 ± 0.04 | 0.9 ± 0.8 | 1.5 ± 2.5 | |
| Normal sperm morphology (%, mean ± SD) | 3.7 ± 3.1 | 6.4 ± 1.1***** | 1.8 ± 1.9 | 4.0 ± 2.5 | 1.2 ± 1.9 |
*p < 0.001 SU+MACS vs. fresh; **p = 0.001 DG+MACS vs. fresh; ***p = 0.049 SU+MACS vs. fresh; ****p = 0.001 DG+MACS vs. fresh; *****p = 0.01 SU+MACS vs. SU
Morphology assessment was performed after each sperm separation technique (Table 1), and no significant difference was noted among the NZS groups. A significant reduction (p = 0.008) was observed in morphologically normal spermatozoa in SU+MACS when compared to SU alone.
Evaluation of DNA integrity and DNA fragmentation
The assessment of the sperm chromatin integrity was assessed by CMA3 staining. No significant difference was observed among groups either in the NZS or OZS cases (Table 2 (A)). DNA strand breaks were analyzed by TUNEL assay, in a similar manner. A decrease in the TUNEL positivity was observed after MACS application, which did not reach statistically significant levels (Table 2 (B)).
Table 2.
Analysis of sperm DNA integrity
| SU | SU+MACS | DG | DG+MACS | |
|---|---|---|---|---|
| A. CMA3 index % (mean ± SD) | ||||
| NZS | 15.2 ± 6.8 | 15.0 ± 4.9 | 18.6 ± 5.8 | 21.0 ± 6.4 |
| OZS | 29.8 ± 10.7 | 21.2 ± 11.5 | 18.5 ± 12.2 | 22.8 ± 23.6 |
| B. TUNEL index % (mean ± SD) | ||||
| NZS | 21.4 ± 16.6 | 10.3 ± 9.6 | 18.7 ± 10.4 | 17.7 ± 13.6 |
| OZS | 12.0 ± 11.4 | 9.4 ± 9.4 | 10.6 ± 8.4 | 8.4 ± 6.3 |
Analysis of fertilization capacity of the spermatozoa
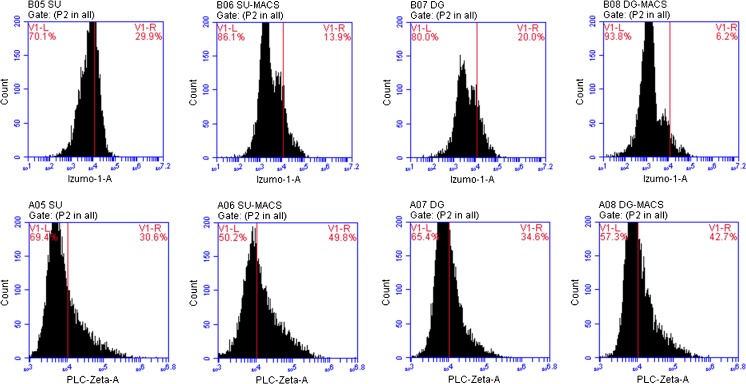
The ability of a sperm to adhere to an oocyte was tested by flow cytometric analysis. The expression levels of the Izumo-1 protein, which is located on the sperm cell surface, was assessed in the NZS cases. The ratio of the total number of Izumo-1-positive cells to the total number of analyzed cells and the signal intensity obtained from each group were compared, and no significant difference was noted between groups (Table 3, Fig. 2). Similarly, the levels of PLC-ζ, generally considered as an oocyte-activating protein, were analyzed by flow cytometry, and no significant difference was observed among the groups either (Table 3, Fig. 2).
Table 3.
Flow cytometry outcomes of Izumo-1 and PLC-ζ proteins
| Flow cytometer results | Groups | |||
|---|---|---|---|---|
| SU | SU+MACS | DG | DG+MACS | |
| Izumo-1 % (mean ± SD) | 17.6 ± 18.9 | 17.5 ± 19.6 | 7.7 ± 8.7 | 12.1 ± 15.1 |
| Izumo-1 intensity (mean ± SD) | 211,144 ± 380,965 | 221,066 ± 393,916 | 383,435 ± 840,201 | 293,764 ± 569,052 |
| PLC-ζ % (mean ± SD) | 39.2 ± 26.7 | 46.4 ± 29.2 | 31.5 ± 25.4 | 39.6 ± 29.2 |
| PLC-ζ intensity (mean ± SD) | 443,388 ± 501,436 | 647,879 ± 781,284 | 462,729 ± 449,742 | 884,534 ± 989,250 |
Fig 2.
Flow cytometry outcomes of Izumo-1 and PLC-ζ1 (lower row) proteins. The upper row indicates the outcomes of the Izumo-1 protein, and the lower row depicts the PLC-ζ1 protein in sperm samples from each group
Discussion
Selecting competent spermatozoa is still one of the core issues of IVF and assisted reproductive technologies. In this study, we analyzed certain characteristics of spermatozoa, like motility, morphology, DNA integrity, and the existence of specific proteins required for oocyte attachment and fertilization which enable the spermatozoa to reach and fertilize the oocyte after applying single and/or double sperm selection techniques.
The finding that MACS significantly reduces total and rapid progressive sperm number compared to fresh samples may not be considered as a problem for ICSI cycles, but it should be taken into account in the classical IVF and IUI applications. Recently, Curti et al. [10] applied SU+MACS and SU techniques to semen samples obtained from five infertile human subjects and analyzed spermatozoa under a transmission electron microscope. They found no significant ultrastructural differences between groups. Here, in addition to PLC-ζ or Izumo-1 distribution patterns at a high-resolution 3-D confocal microscopic level, we also analyzed the sperm morphology according to strict criteria and found no significant difference between groups in the NZS cases.
Our finding that SU+MACS or DG+MACS resulted in lower DNA fragmentation and higher DNA integrity compared to SU or DG alone at the statistically insignificant level indicated that SU or DG alone are also beneficial techniques to select spermatozoa with high DNA quality as it has been indicated by previous studies [11, 12]. Very recently, Bucar et al. [13] demonstrated that the application of MACS following DG and SU gave a significantly lower number of total or rapid progressive spermatozoa compared to others. However, they did not find a significant difference between DG+SU, DG+MACS+SU, MACS+DG+SU, and MACS+SU regarding progressive motility, morphology, and DNA fragmentation. Although our experiment design was not identical, the finding that there was no difference among the SU, DG, SU+MACS, and DG+MACS groups regarding TUNEL positivity was considered parallel to their findings.
To the best of our knowledge, this is the first study that has analyzed the potential beneficial effects of MACS application on the existence and density of two vital proteins to adhere (Izumo-1) or activate (PLC-ζ) the oocyte. Although we did not observe any significant difference between the groups, the slight increase in Izumo-1 and PLC-ζ protein intensity in the MACS-applied groups may indicate that selecting sperms with intact cytoplasmic membrane may result in a higher fertilization capacity.
We believe that it would be interesting to apply a telomere length analysis in our study groups; however, it is controversial whether telomere length affects fertilization rates and embryo development, even though some authors [14, 15] suggest a positive correlation between them. Additionally, it could also be informative to analyze spermatozoa obtained from repeated ejaculations from the same individuals since sperm numbers and quality alters among ejaculates obtained from the same individuals in different ejaculation periods. Moreover, examining the effects of sperms selected with these techniques on IVF outcomes, like fertilization rates and embryo development rates, would further help to determine the optimal sperm selection procedure.
Conclusions
The beneficial effect of MACS to select higher quality spermatozoa is still debatable due to various reports of the related studies. In the current study, our scope was to analyze the effects of MACS together with routinely used techniques to select competent spermatozoa. Our results showed that the application of MACS technique cause a depletion in the numbers of total and rapid progressive spermatozoa and do not significantly increase the concentration of spermatozoa having higher DNA integrity, Izumo-1, or PLC-ζ proteins. Therefore, we think that further studies should be performed to verify the efficiency of MACS compared to other selection techniques.
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK, 213S019) and Ankara University Scientific Research Project Coordination (AU-BAP 13O3330001). Preliminary outcomes of this study were presented in the ESHRE 2015 in Lisbon, Portugal.
Footnotes
Capsule
MACS application together with traditional techniques may be preferred in certain cases having higher concentrations of spermatozoa, although it seems that more powerful and practical sperm selection techniques are still needed for routine use.
References
- 1.McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2014;10:CD010461. doi: 10.1002/14651858.CD010461.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Said TM, Grunewald S, Paasch U, Glander HJ, Baumann T, Kriegel C, et al. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. 2005;10(6):740–6. doi: 10.1016/S1472-6483(10)61118-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102(4):998–1005 e8. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Romany L, Garrido N, Motato Y, Aparicio B, Remohi J, Meseguer M. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertil Steril. 2014;102(6):1567–75 e1. doi: 10.1016/j.fertnstert.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434(7030):234–8. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 6.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization DoRHaR. WHO laboratory manual for the examination and processing of human semen. Fifth ed. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland World Health Organization; 2010.
- 8.Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5(5):586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 9.Simoes R, Feitosa WB, Mendes CM, Marques MG, Nicacio AC, de Barros FR, et al. Use of chromomycin A3 staining in bovine sperm cells for detection of protamine deficiency. Biotech Histochem. 2009;84(3):79–83. doi: 10.1080/10520290902843595. [DOI] [PubMed] [Google Scholar]
- 10.Curti G, Skowronek F, Vernochi R, Rodriguez-Buzzi AL, Rodriguez-Buzzi JC, Casanova G, et al. Morphological evaluation of sperm from infertile men selected by magnetic activated cell sorting (MACS) Reprod Biol. 2014;14(4):289–92. doi: 10.1016/j.repbio.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Santiso R, Tamayo M, Gosalvez J, Meseguer M, Garrido N, Fernandez JL. Swim-up procedure selects spermatozoa with longer telomere length. Mutat Res. 2010;688(1–2):88–90. doi: 10.1016/j.mrfmmm.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online. 2015;31(1):44–50. doi: 10.1016/j.rbmo.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Bucar S, Goncalves A, Rocha E, Barros A, Sousa M, Sa R. DNA fragmentation in human sperm after magnetic-activated cell sorting. J Assist Reprod Genet. 2015;32(1):147–54. doi: 10.1007/s10815-014-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287(4):803–7. doi: 10.1007/s00404-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 15.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]