Abstract
AIM
To determine the risk of second primary malignancy (SPM) and survival of patients with essential thrombocythemia (ET).
METHODS
We identified all patients with ET diagnosed during 2001 to 2011 from the Surveillance, Epidemiology and End Results (SEER) 18 database. Actuarial and relative survival methods were used to calculate the survival statistics. We utilized the SEER 13 database to calculate SPM. We used multiple primary standardized incidence ratio (SIR) session of the SEER*Stat software (version 8.1.5) to calculate SIR and excess risk of SPM for ET patients.
RESULTS
Age standardized five-year cause-specific survival was greater for patients < 50 years vs those ≥ 50 years (99.4% vs 93.5%, P < 0.01). Five-year cause-specific survival was lower for men vs women (70.2% vs 79.7%). A total of 201 patients (2.46%) developed SPM at a median age of 75 years. SPMs occurred at an observed/expected (O/E) ratio of 1.26 (95%CI: 1.09-1.45, P = 0.002) with an absolute excess risk (AER) of 37.44 per 10000 population. A significantly higher risk was noted for leukemia (O/E 3.78; 95%CI: 2.20-6.05, P < 0.001; AER 11.28/10000).
CONCLUSION
ET patients have an excellent cause-specific five-year survival but are at an increased risk of SPM, particularly leukemia, which may contribute to excess deaths.
Keywords: Essential thrombocythemia, Second primary malignancy, Survival
Core tip: Second primary malignancy (SPM) contributes to worse survival in essential thrombocythemia (ET). We utilized the Surveillance, Epidemiology and End Results database to analyze the risk of SPM in ET patients diagnosed during 2001-2011. Two hundred and one patients (2.46%) developed SPM at a median age of 75 years. SPMs occurred at an observed/expected (O/E) ratio of 1.26 (95%CI: 1.09-1.45, P = 0.002) with an absolute excess risk of 37.44 per 10000 population. A significantly higher risk was noted for leukemia (O/E 3.78; 95%CI: 2.20-6.05, P < 0.001). An increased risk of SPM, particularly leukemia, may contribute to excess deaths in ET.
INTRODUCTION
Essential thrombocythemia (ET) is a subtype of myeloproliferative disorder, which lacks BCR-ABL fusion transcript[1,2]. In United States, the estimated annual incidence is approximately 2.5 cases per 100000 population, whereas the prevalence is estimated to be approximately 24 cases per 100000 population[3,4]. Patients with ET generally have an excellent survival. A large study has shown a median survival of 20 years for the entire cohort of patients with ET and a median survival of 33 years for those < 60 years. Nonetheless, the life expectancy of patients with ET is inferior to sex- and age-matched population[5]. Another study has also demonstrated a median survival of approximately 19 years but the survival was worse than the general population, particularly after the first decade[6]. Risk factors associated with inferior survival include advanced age at diagnosis, leukocytosis and thrombosis; the mutational status of janus kinase 2 (JAK2) and calreticulin (CALR) genes may not definitely influence survival[7-9]. The thrombohemorrhagic events and second primary malignancy (SPM) are among the frequent causes of death in ET[10-12]. ET may progress to other myeloid malignancies such as myelofibrosis, myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML)[2,12]. The probability of transformation to leukemia is 1%-5% during the first decade but increases significantly in the subsequent decades[2,6]. Additionally, patients with ET may also develop lymphoid malignancies such as non-Hodgkin’s lymphoma (NHL) and solid organ malignancies[13,14]. The treatment with cytotoxic chemotherapy decreases the incidence of thrombohemorrhagic events but may increase the risk of hematological SPM[2]. The risk of SPM may be high in patients exposed to alkylating agents and radioactive phosphorus, more so when used in high doses[2,12,15,16]. A study from Italy has demonstrated that the use of alkylating agents such as melphalan may be associated with a higher risk of developing second hematologic malignancies but not non-hematological malignancies[17]. Prior studies assessing the risk of SPM in ET utilized data mainly from outside the Unites States. Although the risk of MDS/AML in ET has been studied, the risk of other malignancies is not well determined in United States population-based studies. This United States population-based database analysis aimed to determine the probability of SPM and survival in patients with ET[18]. The influence of age at diagnosis and disease duration on the probability of SPM was also analyzed.
MATERIALS AND METHODS
We utilized Surveillance, Epidemiology and End Results (SEER) database to extract data on all ET patients diagnosed and treated between 1973 and 2011. The SEER is a program of the National Cancer Institute (NCI) that provides cancer data from population-based cancer registries and covers 28% of the total United States population. The database covers data from 25% of all White population, 26% of African Americans, 38% of Hispanics, 50% of Asians, 44% of American Indians and Alaska Natives and 67% of Hawaiian/Pacific Islanders[19]. Patients with ET were identified using International Classification of Diseases for oncology, 3rd edition (ICD)-O-3 code 9962/3 from the SEER 18 registry. Prior studies have used a similar approach in identifying patients with ET from the SEER database[20,21]. Cases with unknown age or survival time and those diagnosed at autopsy and by death certificate only were excluded. SEER started reporting data on chronic myeloproliferative disorders including ET from 2001; as a result our analysis was restricted to cases diagnosed after 2001 only[21]. Actuarial and relative survival methods were used to calculate the survival statistics. We computed age-standardized cause-specific survival using the cause-specific death classification available from the SEER registry. Cause-specific survival is a net survival measure that computes survival from cancer related causes of death in the absence of other causes of death. A prior study from SEER registry has shown that cause-specific survival rates may be a reliable alternative to relative survival methods when suitable life tables are not available[22]. Relative survival rate, which measures net cancer survival controlling for differences in mortality for causes other than cancer, was defined as the ratio of observed survival of a group of cancer patients to the expected survival of a comparable cohort of cancer free patients. Expected survival was computed using the Ederer II method. All calculations were age standardized to the International Cancer Survival Standard for age 15+ years[23].
For the calculation of SPM, we utilized the SEER 13 registry using patients with ET diagnosed between 2001-2010. Using Warren and Gates criteria as modified by NCI[24], SPM was defined as a metachronous malignancy developing at least six months after the diagnosis of ET. A similar approach for the definition of SPM has been used in prior SEER based studies[25,26]. We used multiple primary standardized incidence ratio (SIR) session of the SEER*Stat software (version 8.1.5) to calculate SIR and absolute excess risk (AER) of SPM for ET patients. SIR is obtained by dividing the observed number of second malignancies by the expected number of cases that would occur in a reference population without the index malignancy. Confidence intervals (at 95%) and P-values were calculated using Poisson exact methods for the ratio of observed to expected events. AER was defined as the excess (observed-expected) number of second cancers in patients with index ET, per 1000 person years at risk. For patients who developed more than one malignancies after the primary disease, all of the subsequent malignancies were counted in the numerator for the calculation of SIR. The strata for SPMs were defined as a priori, and we included at least ten observed occurrences in each stratum.
Statistical analysis was done using SEERstat version 8.1.5 (National Cancer Institute, Bethesda, Maryland) and STATA (Stata-Corp, College-Station, Texas). Differences between survival rates of two groups were analyzed using the Z test for comparison of population proportions. All P-values were two sided and the level of significance was chose at 0.05. Institutional review board waiver was obtained from the University of Nebraska Medical Center Institutional Review Board prior to conducting this study.
RESULTS
A total of 8152 cases were identified from the SEER 18 registry, out of which 36 cases met the exclusion criteria (23 had unknown survival and 13 cases diagnosed prior to 2001). The remaining 8116 cases included 39.4% males (n = 3195) and 60.6% (n = 4921) females, and had a median age at diagnosis of 68 years (range < 1-107) (Table 1). Only 17 patients were less than 18 years old (0.7% of total). Ethnicity included 78% Whites, 12% African Americans, 7% others (American Indian/Alaska Native or Asian/Pacific Islander) and 3% unknown. The median year at diagnosis was 2007 (range 2001-2011).
Table 1.
Characterstics of patients with essential thrombocythemia
| Characteristics | n (%) |
| Age | |
| < 50 yr | 1600 (19.7) |
| 50-70 yr | 2958 (36.5) |
| > 70 yr | 3558 (43.8) |
| Sex | |
| Male | 3195 (39.4) |
| Female | 4921 (60.6) |
| Race | |
| White | 6332 (78) |
| African American | 971 (12) |
| Other1 | 576 (7.1) |
| Unknown | 237 (2.9) |
| Marital status | |
| Single/unmarried | 1152 (14.2) |
| Married | 3815 (47) |
| Divorced/separated/widowed | 2125 (26.2) |
| Unknown | 1024 (12.6) |
| Year at diagnosis | |
| 2001-2005 | 3035 (37.3) |
| 2006-2010 | 4251 (52.4) |
| 2011-present | 840 (10.3) |
Other race indicates Asian, Hispanic, Native North American and other racial groups.
The overall 1-year and 5-year age-standardized cause-specific survival of the study cohort was 99.1% and 94.9% respectively. The 5-year cause-specific survival was significantly different for patients below 50 years vs those 50 years and above (99.4% vs 93.5%; P < 0.01; Figure 1A) as well as for men vs women (93.1% vs 96.0%; P < 0.01; Figure 1B) (Table 2). Similarly, the age-standardized 1-year and 5-year relative survival rates were 96.6% and 88.4% respectively.
Figure 1.
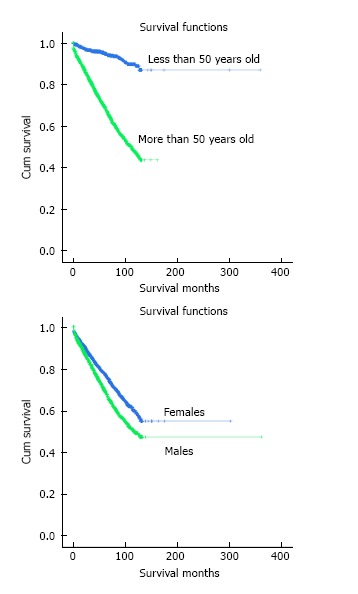
Cumulative survival (Kaplan-Meier estimates) of patients with essential thrombocythemia based on age (A) and gender (B). The log rank test was statistically significant with a P-value of < 0.01.
Table 2.
Age standardized cause-specific survival rates of the study population
| Survival at defined time period | Entire cohort | Male | Female | Patients < 50 yr | Patients ≥ 50 yr |
| 12 mo | 99.10% | 98.80% | 99.30% | 99.90% | 98.90% |
| 24 mo | 98.30% | 97.80% | 98.70% | 99.80% | 97.90% |
| 36 mo | 97.20% | 96.20% | 97.90% | 99.70% | 96.50% |
| 48 mo | 96.10% | 94.80% | 96.90% | 99.70% | 95.00% |
| 60 mo | 94.90% | 93.10% | 96.00% | 99.40% | 93.50% |
The percentage indicates cause-specific survival of specified population at defined time period.
At a median follow-up of 3 years (range, 6-129 mo), 201 patients (2.46%) out of 2913 patients with ET developed SPM. The median age at diagnosis was 75 years (range, 39-94 years). None of the patients less than 18 years old developed SPM. SPMs occurred at an SIR of 1.26 (95%CI: 1.09-1.45; P = 0.002) with an AER of 37.44 per 10000 population. The risk for developing leukemia (SIR 3.78; 95%CI: 2.20-6.05, P < 0.001; AER 11.28/10000), particularly AML (SIR 7.74; 95%CI: 3.71-14.24, P < 0.001; AER 7.86/10000), and kidney cancer (SIR 2.40; 95%CI: 1.15-4.42, P = 0.01; AER 5.27/10000) was high (Table 3).
Table 3.
Risk of second primary malignancies among the study population
| SPM | Observed | SIR | 95%CI | AER | P-value |
| All sites | 201 | 1.26 | 1.09-1.45 | 37.44 | 0.002 |
| Solid | 162 | 1.15 | 0.98-1.35 | 19.43 | 0.074 |
| Colorectal | 12 | 0.66 | 0.34-1.15 | -5.62 | 0.916 |
| Lung/bronchus | 31 | 1.31 | 0.89-1.86 | 6.67 | 0.133 |
| Breast | 24 | 1.06 | 0.68-1.58 | 1.29 | 0.743 |
| Prostate | 24 | 1.11 | 0.71-1.65 | 2.14 | 0.594 |
| Kidney | 10 | 2.40 | 1.15-4.42 | 5.27 | 0.014 |
| Lymphoma | 12 | 1.59 | 0.82-2.78 | 4.02 | 0.127 |
| Leukemia | 17 | 3.78 | 2.20-6.05 | 11.28 | < 0.001 |
| AML | 10 | 7.74 | 3.71-14.24 | 7.86 | < 0.001 |
AER: Absolute excess risk; SIR: Standardized incidence ratio; SPM: Second primary malignancies.
The risk of SPM was higher after 24 mo of diagnosis of ET (Table 4). Before 24 mo, the risk of development of leukemia (O/E 3.88, CI: 1.42-8.44, P < 0.05), particularly AML (O/E 9.02, CI: 2.46-23.09, P < 0.05) was noted to be higher than expected. After a follow-up of 24 mo, the risk of SPM in general (O/E 1.31, 95%CI: 1.1-1.55, P = 0.002), kidney cancer (O/E 3.27, 95%CI: 1.5-6.21, P = 0.003), and leukemia (O/E 3.72, 95%CI: 1.86-6.66, P < 0.001), particularly AML (O/E 7.08, 95%CI: 2.6-15.4, P < 0.001) was determined to be higher.
Table 4.
Risk of second primary malignancies based on the follow-up duration
| SPM | Overall SIR (95%CI) | SIR at 6-23 mo follow-up (95%CI) | SIR at > 24 mo follow-up (95%CI) |
| All sites | 1.26 (1.09-1.45)1 | 1.16 (0.9-1.49) | 1.31 (1.1-1.55)1 |
| Solid malignancies | 1.15 (0.98-1.35) | 1.09 (0.82-1.43) | 1.18 (0.97-1.43) |
| Colorectal | 0.66 (0.34-1.15) | 0.47 (0.10-1.38) | 0.76 (0.35-1.44) |
| Lung/bronchus | 1.31 (0.89-1.86) | 1.48 (0.76-2.58) | 1.23 (0.74-1.92) |
| Breast | 1.06 (0.68-1.58) | 0.91 (0.37-1.88) | 1.14 (0.66-1.82) |
| Prostate | 1.11 (0.71-1.65) | 1.03 (0.45-2.03) | 1.15 (0.66-1.87) |
| Kidney | 2.40 (1.15-4.42)1 | 0.71 (0.02-3.95) | 3.27 (1.5-6.21)1 |
| Hematologic malignancies | 2.36 (1.63-3.29)1 | 2.03 (0.97-3.73) | 2.53 (1.62-3.76)1 |
| Lymphoma | 1.59 (0.82-2.78) | 1.56 (0.42-3.99) | 1.61 (0.69-3.17) |
| Leukemia | 3.78 (2.2-6.05)1 | 3.88 (1.42-8.44)1 | 3.72 (1.86-6.66)1 |
| AML | 7.74 (3.71-14.24)1 | 9.02 (2.46-23.09)1 | 7.08 (2.6-15.4)1 |
Indicates a statistically significant value. AML: Acute myeloid leukemia; SIR: Standardized incidence ratio; SPM: Second primary malignancies.
The risk of AML was noted to be higher among all age groups (Table 5). The risk of SPM in general (O/E 1.78), solid tumors (O/E 1.75), and AML (O/E 12.7) were noted to be higher among patients 18-60 years old. Among patients ≥ 60 years old, the risk of kidney cancer (O/E 2.42) and leukemia (O/E 3.78), particularly AML (O/E 7.06) was higher.
Table 5.
Risk of secondary primary malignancies based on age at the time of diagnosis of essential thrombocythemia
| SPM | Overall SIR (95%CI) | SIR for age group, 18-60 yr (95%CI) | SIR for age group, > 60 yr (95%CI) |
| All sites | 1.26 (1.09-1.45)1 | 1.78 (1.31-2.36)1 | 1.15 (0.98-1.35) |
| Solid tumors | 1.15 (0.98-1.35) | 1.75 (1.27-2.35)1 | 1.03 (0.85-1.23) |
| Colorectal | 0.66 (0.34-1.15) | 1.33 (0.27-3.88) | 0.56 (0.26-1.07) |
| Lungs/bronchus | 1.31 (0.89-1.86) | 2.36 (0.86-5.13) | 1.19 (0.77-1.75) |
| Breast | 1.06 (0.68-1.58) | 1.08 (0.40-2.35) | 1.06 (0.63-1.67) |
| Prostate | 1.11 (0.71-1.65) | 1.91 (0.82-3.76) | 0.92 (0.52-1.49) |
| Kidney | 2.40 (1.15-4.42)1 | 2.32 (0.28-8.37) | 2.42 (1.05-4.78)1 |
| Lymphoma | 1.59 (0.82-2.78) | 1.72 (0.21-6.23) | 1.57 (0.75-2.88) |
| Leukemia | 3.78 (2.2-6.05)1 | 3.74 (0.45-13.51) | 3.78 (2.12-6.24)1 |
| AML | 7.74 (3.71-14.24)1 | 12.73 (1.54-45.98)1 | 7.06 (3.05-13.90)1 |
Indicates a statistically significant value. AML: Acute myeloid leukemia; SIR: Standardized incidence ratio; SPM: Second primary malignancies.
DISCUSSION
Our study demonstrates excellent age-standardized cause-specific five-year survival with 99.4% of patients below 50 years of age and 93.5% of patients above 50 years of age alive at 5 years. The fiver-year overall survival was significantly lower in patients over 50 years of age as well as in males as compared to females.
A Mayo clinic study demonstrated significantly higher OS in patients younger than 60 years vs those 60 years or older (32 years vs 19 years, P < 0.001). While OS was similar to general population in the first decade of disease [risk ratio (RR), 0.72; 95%CI: 0.50-0.99], OS was significantly worse after the first decade (RR, 2.21; 95%CI: 1.74-2.76, at 20 years and RR, 3.37; 95%CI: 1.84-5.65, at 30 years). This is consistent with fact that the incidence of SPM such as leukemia and myelofibrosis is higher after the first decade[6,10,27]. A number of factors may determine the OS of these patients. The prognostic factors associated with the poor outcomes are age at diagnosis of ≥ 60 years, leukocyte count more than 15 × 109/L, a history of thrombosis, diabetes, hypertension and tobacco smoking[6].
In our study, a significantly elevated risk was noted for the development of AML and kidney cancer and especially after 24 mo of diagnosis of ET. Such increased risk has been noted in other studies also. In a retrospective population-based Danish cohort study, patients with ET, compared to those without, had a higher risk for developing hematological and solid malignancies with the SIR of 5 (95%CI: 3.6-6.9) and 1.2 (95%CI: 1.0-1.4) respectively[13]. Another retrospective study from Italy (n = 331) showed cumulative incidence of SPM of 13% from the time of diagnosis of ET[17]. The study determined a particularly higher incidence of AML[17]. The risk of developing AML in ET depends on multiple factors such as old age, decreased hemoglobin and increased platelet count ≥ 1000 × 109/L[28]. Additionally, the risk increases with longer follow-up, with one study demonstrating a relatively low probability of developing leukemia in the first decade (1.4%) but significantly increased risk in the second (8.1%) and third decades (24.0%)[6]. The probability of occurrence of AML may also increase with the use of leukemogenic therapy such as radioactive phosphorus (P32) and alkylators such as chlorambucil[2,7,29,30]. Although the presence of JAK2V617F mutation and cytogenetic abnormalities may not increase the probability of developing leukemia[2], the probability is increased in the presence of 2 or more somatic mutations[31].
In our study, the risk of SPM varied based on the age at diagnosis of ET and the follow-up duration. The risk of developing leukemia especially AML was increased after 6 mo of diagnosis of ET and persisted beyond 24 mo of follow-up, consistent with prior studies[6]. Although patients < 60 years had a higher risk of overall SPM, the increased risk of kidney cancer and leukemia was noted in those above 60 years. Prior studies have shown that the risk of SPM differed based on the age of patients. In Passamonti study, age > 65 years was determined to be a risk factor for developing SPM including leukemia[32]. A Danish study demonstrated that the probability of non-hematologic and hematologic malignancies was higher in younger patients (20-49 years vs 50-69 and > 70 years)[13]. Such conflicting results may be accounted by the differences in the characteristics of patients and other risk factors for SPM in different studies.
Potential limitations of this research are its retrospective study design, possibility of coding errors in the SEER database and inability to obtain data on potential impact of other factors such as therapy or other risk factors for SPM such as smoking. Detection bias is another potential shortcoming of the study, since many asymptomatic malignancies may be over diagnosed because of closer follow-up of patients following the diagnosis of ET. The strength of this study includes real world data from a relatively large number of ET patients. Our study demonstrated that patients with ET have an excellent five-year survival. Besides bleeding and thrombotic events, patients diagnosed with ET are also at risk of developing SPM. The risk of leukemia, particularly AML, can affect patients of all age groups and persists over time. Clinical trials on early detection of SPM may be considered in patients with ET. Additionally, patients with ET should be encouraged to undergo age and sex-appropriate cancer screening tests.
ACKNOWLEDGMENTS
This study utilized the SEER database. The interpretation of this data is the sole responsibility of the authors. The authors acknowledge the efforts of the New York State Cancer Registry, New York State Department of Health, and the SEER program tumor registries in the creation of the SEER database. The abstract of this paper was published as a proceeding of 2015 American Society of Clinical Oncology annual meeting held in Chicago from May 29 to June 2, 2015.
COMMENTS
Background
Patients with essential thrombocythemia (ET) have an excellent cause-specific five-year survival but are at an increased risk of second primary malignancy (SPM), particularly leukemia, which may contribute to excess deaths.
Research frontiers
A few United States studies have evaluated the risk of myelodysplastic syndrome/acute myeloid leukemia (AML) in ET but not the risk of other hematological or solid-organ malignancies.
Innovations and breakthroughs
This large Surveillance, Epidemiology and End Results database-based study confirmed an increased risk of SPM in patients with ET. A significantly higher risk was noted for leukemia. An increased risk of SPM, particularly leukemia, may contribute to excess deaths in ET. The risk of developing leukemia especially AML was increased after 6 mo of diagnosis of ET and persisted beyond 24 mo of follow-up, consistent with prior studies. Prior studies have shown conflicting results on the impact of age on the risk of SPM. In this study, the risk of overall SPM was higher in patients younger than the age of 60 years, however, the increased risk of kidney cancer and leukemia was noted in those above 60 years. The risk of leukemia, particularly AML, can affect patients of all age groups and persists over time.
Applications
The knowledge of an increased risk of SPM in patients with ET has implications in patient education, planning of preventive cancer screening strategies and may serve as a foundation for future research targeted at understanding the tumorigenesis in these patients.
Terminology
SPM: A metachronous malignancy developing at least six months after the diagnosis of ET; Relative survival rate: Ratio of observed survival of a group of cancer patients to the expected survival of a comparable cohort of cancer free patients. It measures net cancer survival controlling for differences in mortality for causes other than cancer; Cause-specific survival: A net survival measure that computes survival from cancer-related causes of death in the absence of other causes of death; Standardized incidence ratio: Ratio obtained by dividing the observed number of second malignancies by the expected number of cases in a reference population without the index malignancy; Absolute excess risk: Excess (observed-expected) number of second cancers in patients with ET, per 1000 person years at risk.
Peer-review
The paper is about an interesting topic. It is well written and the methods are sound. The conclusions are consistent with the results.
Footnotes
Supported by The University of Nebraska Medical Center, College of Medicine, Physician-Scientist Training Program Grant 2015-2016 (to Bhatt VR).
Institutional review board statement: Institutional review board waiver was obtained from the University of Nebraska Medical Center’s Institutional Review Board prior to conducting this study.
Informed consent statement: Patients were not required to give informed consent to the study because this database study utilized deidentified publicly available data.
Conflict-of-interest statement: None.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript Source: Invited manuscript
Specialty Type: Oncology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 14, 2016
First decision: March 21, 2016
Article in press: May 27, 2016
P- Reviewer: Korpanty GJ, Mocellin S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:507–516. doi: 10.1002/ajh.23417. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt VR. Leukemic transformation in essential thrombocythemia. Future Oncol. 2014;10:2593–2602. doi: 10.2217/fon.14.239. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976-1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Vanasse G, Cartmel B, Wang Y, Selinger HA. Prevalence of polycythemia vera and essential thrombocythemia. Am J Hematol. 2008;83:359–362. doi: 10.1002/ajh.21129. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, Gangat N, Fjerza R, Belachew AA, Lasho TL, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–2513; quiz 2615. doi: 10.1182/blood-2014-05-579136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–166. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, Randi ML, Vaidya R, Cazzola M, Rambaldi A, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passamonti F, Thiele J, Girodon F, Rumi E, Carobbio A, Gisslinger H, Kvasnicka HM, Ruggeri M, Randi ML, Gangat N, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;120:1197–1201. doi: 10.1182/blood-2012-01-403279. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Wassie EA, Lasho TL, Finke C, Belachew AA, Ketterling RP, Hanson CA, Pardanani A, Gangat N, Wolanskyj AP. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia. 2014;28:2300–2303. doi: 10.1038/leu.2014.148. [DOI] [PubMed] [Google Scholar]
- 10.Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, Orlandi E, Arcaini L, Brusamolino E, Pascutto C, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Murphy S. Current opinion in essential thrombocythemia: pathogenesis, diagnosis, and management. Blood Rev. 2001;15:121–131. doi: 10.1054/blre.2001.0158. [DOI] [PubMed] [Google Scholar]
- 12.Harrison CN. Current trends in essential thrombocythaemia. Br J Haematol. 2002;117:796–808. doi: 10.1046/j.1365-2141.2002.03474.x. [DOI] [PubMed] [Google Scholar]
- 13.Frederiksen H, Farkas DK, Christiansen CF, Hasselbalch HC, Sørensen HT. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish population-based cohort study. Blood. 2011;118:6515–6520. doi: 10.1182/blood-2011-04-348755. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt VR, Bociek RG, Yuan J, Fu K, Greiner TC, Dave BJ, Rajan SK, Armitage JO. Leukemic diffuse large B-cell lymphoma in a patient with myeloproliferative disorder. J Natl Compr Canc Netw. 2015;13:281–287. doi: 10.6004/jnccn.2015.0039. [DOI] [PubMed] [Google Scholar]
- 15.Murphy S. Therapeutic dilemmas: balancing the risks of bleeding, thrombosis, and leukemic transformation in myeloproliferative disorders (MPD) Thromb Haemost. 1997;78:622–626. [PubMed] [Google Scholar]
- 16.Finazzi G, Barbui T. Efficacy and safety of hydroxyurea in patients with essential thrombocythemia. Pathol Biol (Paris) 2001;49:167–169. doi: 10.1016/s0369-8114(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 17.Radaelli F, Onida F, Rossi FG, Zilioli VR, Colombi M, Usardi P, Calori R, Zanella A. Second malignancies in essential thrombocythemia (ET): a retrospective analysis of 331 patients with long-term follow-up from a single institution. Hematology. 2008;13:195–202. doi: 10.1179/102453308X316022. [DOI] [PubMed] [Google Scholar]
- 18.Uhlenhopp MB. An overview of the relationship between alkylating agents and therapy-related acute nonlymphocytic leukemia. Cancer Nurs. 1992;15:9–17. [PubMed] [Google Scholar]
- 19.Overview of the SEER Program. Surveillance, Epidemiology, and End Results Program. [accessed 2014 Dec 11] Available from: http://seer.cancer.gov/about/overview.html. [Google Scholar]
- 20.Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) medicare enrollees with non-CML myeloproliferative neoplasms (MPN) PLoS One. 2014;9:e90299. doi: 10.1371/journal.pone.0090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 22.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 24.Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Published by the National Cancer Institute, Bethesda, MD, USA, 2006: 492. J Epidemiol Community Health. 2008;62:375–376. [Google Scholar]
- 25.Khanal N, Giri S, Upadhyay S, Shostrom VK, Pathak R, Bhatt VR. Risk of second primary malignancies and survival of adult patients with polycythemia vera: A United States population-based retrospective study. Leuk Lymphoma. 2016;57:129–133. doi: 10.3109/10428194.2015.1071492. [DOI] [PubMed] [Google Scholar]
- 26.Giri S, Pathak R, Martin MG, Bhatt VR. Survival of de novo and secondary acute promyelocytic leukemia: a propensity-matched analysis of the SEER database. Leuk Lymphoma. 2015:1–7. doi: 10.3109/10428194.2015.1063142. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes F, Alvarez-Larrán A, Talarn C, Gómez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–790. doi: 10.1046/j.1365-2141.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- 28.Gangat N, Wolanskyj AP, McClure RF, Li CY, Schwager S, Wu W, Tefferi A. Risk stratification for survival and leukemic transformation in essential thrombocythemia: a single institutional study of 605 patients. Leukemia. 2007;21:270–276. doi: 10.1038/sj.leu.2404500. [DOI] [PubMed] [Google Scholar]
- 29.Björkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, Andreasson B, Birgegård G, Linder O, Malm C, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol. 2011;29:2410–2415. doi: 10.1200/JCO.2011.34.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, Gugliotta L, Landolfi R, Kutti J, Gisslinger H, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–2670. doi: 10.1182/blood-2004-09-3426. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, Girsberger S, Lehmann T, Passweg J, Stern M, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- 32.Passamonti F, Brusamolino E, Lazzarino M, Baraté C, Klersy C, Orlandi E, Canevari A, Castelli G, Merante S, Bernasconi C. Efficacy of pipobroman in the treatment of polycythemia vera: long-term results in 163 patients. Haematologica. 2000;85:1011–1018. [PubMed] [Google Scholar]


