Abstract
Substrate ambiguity and relaxed reaction specificity underlie the diversity of reactions catalyzed by the transsulfuration pathway enzymes, cystathionine β-synthase (CBS) and γ-cystathionase (CSE). These enzymes either commit sulfur metabolism to cysteine synthesis from homocysteine or utilize cysteine and/or homocysteine for synthesis of H2S, a signaling molecule. We demonstrate that a kinetically controlled heme-dependent metabolite switch in CBS regulates these competing reactions where by cystathionine, the product of CBS, inhibits H2S synthesis by the second enzyme, CSE. Under endoplasmic reticulum stress conditions, induction of CSE and up-regulation of the CBS inhibitor, CO, a product of heme oxygenase-1, flip the operating preference of CSE from cystathionine to cysteine, transiently stimulating H2S production. In contrast, genetic deficiency of CBS leads to chronic stimulation of H2S production. This metabolite switch from cystathionine to cysteine and/or homocysteine renders H2S synthesis by CSE responsive to the known modulators of CBS: S-adenosylmethionine, NO, and CO. Used acutely, it regulates H2S synthesis; used chronically, it might contribute to disease pathology.
Keywords: endoplasmic reticulum stress (ER stress), enzyme kinetics, homocysteine, hydrogen sulfide, metabolic regulation
Introduction
Hydrogen sulfide (H2S) is a signaling molecule that regulates physiological processes ranging from neuromodulation (1) to cardioprotection (2) and inflammation (3). Two enzymes in the transsulfuration pathway, cystathionine β-synthase (CBS)3 and γ-cystathionase (CSE), produce H2S (4, 5). In humans, the canonical role of these enzymes is to commit sulfur, obtained from the diet as methionine, to cysteine (see Fig. 1a). CBS catalyzes the rate-limiting step, the condensation of serine and homocysteine, to generate cystathionine (6, 7). The latter is cleaved by CSE to yield cysteine. Unlike enzymes that synthesize the other gas signaling molecules, CO and NO, and exhibit high reaction specificity, CBS and CSE display significant substrate ambiguity and relaxed reaction specificity resulting in a multitude of possible reactions, many of which generate H2S (see Fig. 1a). Both CBS and CSE can use cysteine and homocysteine as substrates to produce H2S. The kinetic properties and preferences of CBS and CSE for their canonical substrates (4, 5) suggest that the transsulfuration pathway enzymes are poised to carry out their housekeeping function of cysteine synthesis at physiologically relevant substrate concentrations. How then is the substrate preference switched to catalyze H2S production to initiate signaling? The transsulfuration pathway is regulated in response to various conditions such as oxidative stress (8, 9) and insulin signaling (10, 11). Furthermore, growing evidence indicates cross-talk between the endoplasmic reticulum (ER) stress response and the transsulfuration pathway (12, 13). However, the mechanism by which the transsulfuration pathway switches to H2S production is not known.
FIGURE 1.
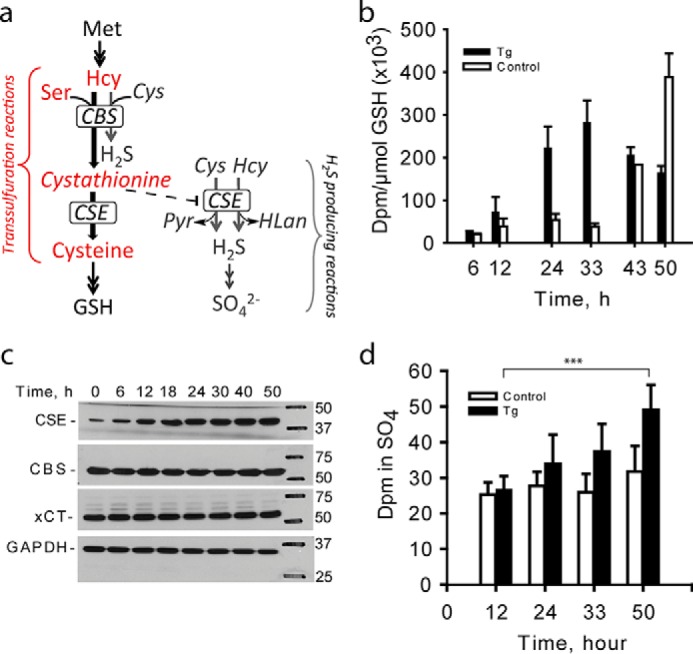
ER stress induces switching from the canonical to H2S-producing reactions in the transsulfuration pathway. a, schematic of the transsulfuration pathway in which the canonical (red) and H2S-generating (gray) reactions are shown. Hcy, Pyr, and HLan denote homocysteine, pyruvate, and homolanthionine, respectively. For clarity, only three of the eight H2S-generating reactions catalyzed by CBS and CSE are shown. A complete list of H2S-generating reactions catalyzed by CBS and CSE are described in Refs. 4 and 5. b, ER stress decreases flux through the transsulfuration pathway in HEK293 cells as monitored by the rate of radiolabel incorporation from [35S]methionine into GSH in untreated (open bars) versus thapsigargin (Tg)-treated (filled bars) cells. ER stress was induced with 0.5 μm thapsigargin, and [35S]methionine was added to medium 5 h prior to sample collection as described under “Experimental Procedures.” Error bars represent ± S.D., n = 3. c, representative Western blotting analysis of CSE, CBS, and xCT during the ER stress response. GAPDH was used as equal loading control. d, ER stress increases H2S production as measured by the rate of radiolabel incorporation from [35S]methionine into the sulfate pool in untreated (open bars) versus thapsigargin-treated (filled bars) cells. The values represent mean ± S.D. with n ranging from 4 to 8 (***, p < 0.001).
ER stress is induced when the protein folding capacity of cells is jeopardized by conditions such as infection, inflammation, increased synthesis of secreted proteins, or conditions triggering abnormal folding of proteins. Compromised ER function is a significant contributing factor in the development of cardiovascular, neurodegenerative, and metabolic diseases (14). Response to ER stress involves cellular reprograming including shutdown of general protein synthesis while expression of a select set of proteins is induced to reestablish cellular homeostasis (15). One such protein is CSE, whose expression is increased by a mechanism involving the activating transcription factor 4 (ATF4) (13), a chief transcriptional regulator of cellular responses to ER stress. Up-regulation of CSE, in turn, induces an H2S-dependent pro-survival signaling cascade (12).
In this study, we probed the canonical versus H2S-producing activities of the transsulfuration pathway enzymes to elucidate the mechanism of increased H2S synthesis during the ER stress response. We found that the activity of the transsulfuration pathway enzymes during the ER stress response switches from cysteine production to H2S synthesis via heme-dependent inhibition of CBS. When CBS is active, cystathionine is produced and kinetic control favors its subsequent conversion by CSE to cysteine. When CBS is inhibited acutely by binding of CO produced in response to stress, to its heme cofactor, or by disabling mutations associated with CBS-dependent homocystinuria, there is a paucity of cystathionine, which favors increased H2S synthesis by CSE.
Results
To investigate ER stress-induced regulation of the transsulfuration pathway, we monitored the incorporation of radiolabel from [35S]methionine into two downstream pathway products, GSH and sulfate in HEK293 cells during ER stress induced by thapsigargin (Fig. 1a). Flux through the canonical reactions, which leads to GSH production, initially increased in response to ER stress, but declined subsequently (Fig. 1b, supplemental Fig. S1a), begging the question as to why the flux to GSH decreased despite increased CSE expression (Fig. 1c). CSE levels remained elevated 50 h after thapsigargin treatment, whereas CBS levels were unchanged (Fig. 1c) as reported previously (12, 13). No change was seen in xCT, the cysteine/glutamate antiporter (Fig. 1c), which was reportedly induced in murine islets and MIN6 cells, albeit at a higher (1 μm) concentration of thapsigargin (12). Total GSH levels also declined in thapsigargin-treated cells for the duration of the experiment (supplemental Fig. S1b). In contrast, radiolabel incorporation into sulfate, an H2S oxidation product, continued to increase in thapsigargin-exposed cells (Fig. 1d, supplemental Fig. S1c), and total sulfate also increased (supplemental Fig. S1d). Increased synthesis of sulfate is consistent with enhanced production and oxidation of H2S. Similar results were obtained with HeLa cells for incorporation of radiolabel into GSH, although the GSH pool was more sensitive to ER stress in this cell line (supplemental Fig. S2, a and b).
We hypothesized that the change in the kinetics of radiolabeling reflected cellular switching from the canonical to H2S-producing reactions. This metabolite switching mechanism could operate under ER stress conditions due to the induction of heme oxygenase-1 (16), a source of CO, which binds to the heme cofactor in CBS and inhibits its activity (17–20). We hypothesized that low cystathionine and increased homocysteine resulting from CBS inhibition promote H2S synthesis by CSE (Fig. 1a). The catalytic efficiency of CSE is significantly greater for cysteine synthesis from cystathionine (kcat/Km = 82,000 m−1 s−1) than for H2S synthesis from cysteine (159 m−1 s−1) or from homocysteine (492 m−1 s−1) (5). Hence, under conditions where CBS is active, the canonical transsulfuration reactions for converting homocysteine to cysteine are expected to predominate. Conversely, inhibition of CBS and diminished cystathionine levels are expected to increase the efficiency of H2S generation from cysteine and homocysteine catalyzed by CSE.
As a test of our model, in HEK293 cells, we overexpressed the constitutively expressed heme oxygenase-2 (HO-2), which also releases CO. HO-2 overexpression inhibited incorporation of radiolabel from [35S]methionine into GSH in control and thapsigargin-treated cells (Fig. 2a). The decrease in GSH levels observed in untransfected cells exposed to thapsigargin was not seen in HO-2-overexpressing cells (Fig. 2b), suggesting a protective antioxidant effect as discussed below. Radiolabel incorporation into sulfate was decreased in HO-2-overexpressing cells and was unaffected by thapsigargin treatment (Fig. 2c), although total sulfate levels increased in treated cells (Fig. 2d). The decreased incorporation of radiolabel despite the increased production of sulfate in HO-2-overexpressing cells (± thapsigargin) can be explained by the increased expression of the cystine transporter xCT under these conditions (supplemental Fig. S3). The consequent increased import of unlabeled cystine from the extracellular medium by HO-2-overexpressing cells leads to radiolabel dilution in the cysteine pool. We speculate that the higher xCT levels reflect an adaptation to decreased cysteine synthesis via the transsulfuration pathway in HO-2-overexpressing cells. Up-regulation of xCT and consequent import of the GSH substrate, cysteine, would also explain why the GSH pool size is unaffected while radiolabel incorporation into GSH from methionine is inhibited in HO-2-overexpressing cells. The increased synthesis of sulfate versus GSH from methionine in HO-2-overexpressing versus control cells is consistent with CBS inhibition and increased H2S synthesis under these conditions (Fig. 1a).
FIGURE 2.
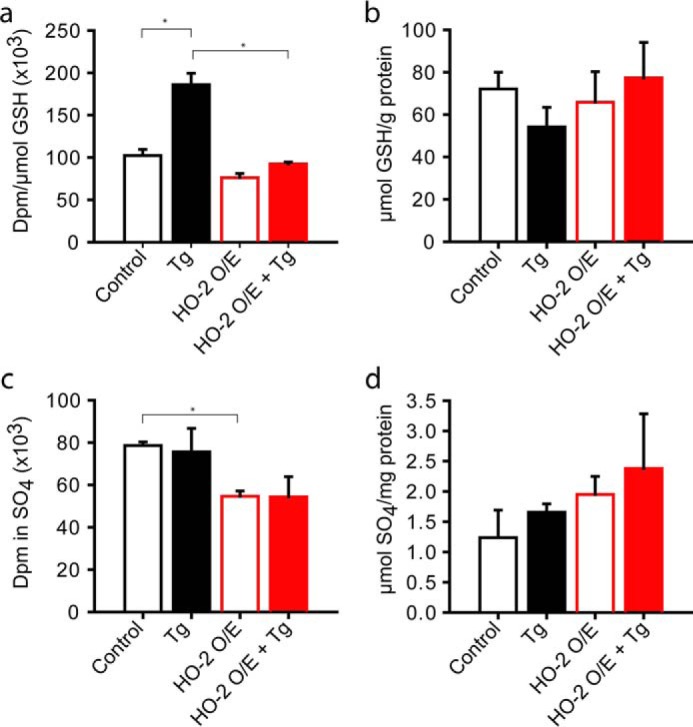
Inhibition of CBS by CO-producing HO-2 activates the metabolite switch in the absence of ER stress. a, HO-2 overexpression decreases incorporation of radiolabel from [35S]methionine into GSH in the presence or absence of thapsigargin (Tg) treatment. ER stress was induced in HEK293 cells with 0.5 μm thapsigargin, and samples were analyzed after 24 h in control versus HO-2-overexpressing (O/E) cells. b, effect of HO-2 overexpression on GSH levels. c and d, radiolabel incorporation from [35S]methionine into sulfate (c) and total sulfate levels in the absence (open bars) and presence (closed bars) of ER stress induced by 0.5 μm thapsigargin (d). Representative data from one of 3 independent experiments are shown with the values representing mean ± S.D. (*, p < 0.05).
Consistent with our model, we found that CSE-dependent H2S synthesis by murine liver lysate is inhibited at increasing concentrations of cystathionine (between 50 and 1,000 μm) in the presence of 1 mm cysteine (Fig. 3a). CSE is the dominant source of H2S under these conditions (4). H2S synthesis diminished up to 250 μm cystathionine; however, the inhibition was reversed at higher concentrations. The CSE-catalyzed cleavage of cystathionine to cysteine likely contributes to the U-shaped dependence because the concentration of cysteine, an H2S-producing substrate, rises with increasing concentration of cystathionine. This result is consistent with the model that the supply of cystathionine by CBS steers CSE activity away from H2S synthesis within a certain concentration window. Despite the similar catalytic efficiencies (kcat/Km = 2,650 m−1 s−1 for serine versus 2,882 m−1 s−1 for cysteine), serine is expected to inhibit H2S production by CBS due to its lower Kd than cysteine (4). Indeed, the addition of serine, the canonical substrate for CBS, inhibited H2S production by CBS in liver lysate in the presence of high concentrations of cysteine and homocysteine, which supported H2S synthesis by CSE (supplemental Fig. S4). These results, combined with the higher cellular concentration of serine versus cysteine, indicate that CBS is poised to catalyze the canonical transsulfuration reaction in vivo.
FIGURE 3.
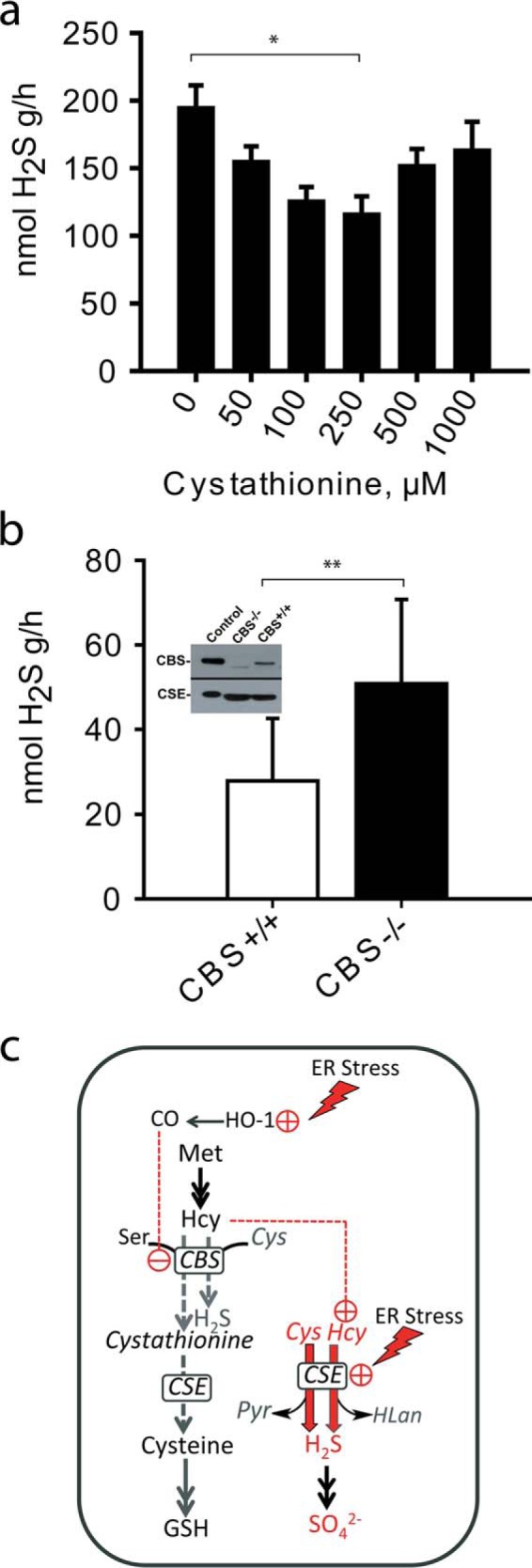
Cystathionine inhibits H2S production and operates metabolite switching. a, H2S production by CSE in liver lysates from wild-type mice was measured in the presence of increasing concentrations of cystathionine. Reactions (20 ml with 0.4-ml aqueous phase and 19.6-ml gas phase), containing 1 mm l-cysteine in 100 mm HEPES buffer, pH 7.4, were started by the addition of liver homogenate and incubated at 37 °C with shaking for 10 min. H2S produced was quantified in the gas phase as described under “Experimental Procedures.” The data represent the average of 3–7 independent experiments ± S.D. (**, p < 0.01). b, liver H2S production is increased in Cbs−/− mice. H2S production was measured in reaction mixtures containing 100 μm cysteine and 10 μm homocysteine in 100 mm HEPES buffer, pH 7.4, and liver homogenate from wild-type or Cbs−/− mice in 20-ml reaction volume as described under “Experimental Procedures.” The data represent the mean ± S.D. from n = 18 independent experiments using liver tissue from three mice (*, p < 0.05). Inset: a representative Western blot of CBS and CSE protein levels in liver homogenates from wild-type and Cbs−/− mice. c, schematic showing how switching occurs between the canonical (gray) and CSE-catalyzed H2S-producing (red) reactions under ER stress conditions. We postulate that induction of HO-1 in response to ER stress stimulates CO synthesis, which inhibits CBS. This leads to a build-up of homocysteine and a decrease in cystathionine, which combine to stimulate H2S synthesis by CSE. ER stress also leads to induction of CSE expression. Hcy, Pyr, and HLan denote homocysteine, pyruvate, and homolanthionine, respectively.
Next, we tested metabolite switching in an animal model of CBS deficiency (Fig. 3b, inset) in which plasma homocysteine levels are ∼40-fold higher than in wild-type controls (21). Our kinetic studies have predicted a graded increase in CSE-derived H2S with increasing homocysteine concentrations, and linked the resulting H2S synthesized to homolanthionine (5), a metabolite found in urine of homocystinuric individuals but not in normal individuals (22). We measured H2S production in liver from CBS knock-out mice at physiologically relevant concentrations of cysteine and homocysteine. The rate of H2S synthesis was ∼2-fold higher in liver lysates from Cbs−/− mice as compared with Cbs+/+ controls (Fig. 3b). Under these conditions, the concentration of cystathionine in tissue homogenates is negligible due to dilution, whereas inhibitory concentrations of cystathionine are produced from the exogenously supplied substrates of CBS, which is present only in wild-type tissue.
Discussion
This is the first report of heme-dependent metabolite switching that can transiently regulate H2S production via the transsulfuration pathway (Fig. 3c). Under basal conditions, when CBS is active, cystathionine is synthesized and kinetic control favors its conversion by CSE to cysteine. Diminished cystathionine and increased homocysteine via inhibition of CBS, e.g. by CO produced in response to ER stress, favor increased H2S synthesis by CSE. Similarly, we predict that conditions that increase NO levels, decrease S-adenosylmethionine, an allosteric activator and stabilizer of CBS (23), or disable CBS via mutations, as in CBS-dependent homocystinuria, will enhance H2S production by CSE. CSE is a major source of H2S in liver at physiologically relevant substrate concentrations (24) and in the cardiovascular system (25). Our model suggests that the activity of CBS dampens H2S production by CSE. H2S plays an important role in the cardiovascular system, and we speculate that the low CBS in endothelial cells is a mechanism for promoting H2S synthesis by CSE.
Although the metabolite switch from cystathionine to cysteine and/or homocysteine could be protective in an acute response, its chronic operation, as in homocystinuria in a background of high homocysteine, is likely to have pathological consequences. Interestingly, cystathionine administration to homocystinuric mice attenuated liver injury and steatosis without protecting against the pathological effects of ER stress due to high homocysteine (26), which can now be explained by the metabolite switching model. We speculate that other conditions in which regulation of H2S synthesis is perturbed include type 1 diabetes, Down syndrome, and caloric restriction. Patients with insulin-dependent diabetes without nephropathy have decreased plasma homocysteine (27), which is recapitulated in the streptozotocin-induced rat diabetic model that exhibits increased hepatic CBS and CSE activities (10, 11). In Down syndrome, trisomy of chromosome 21 results in an extra copy of CBS, decreased plasma homocysteine, and increased plasma cystathionine (28). We predict that CSE-dependent H2S production is down-regulated in both type I diabetes and Down syndrome patients. In contrast, caloric restriction leads to significantly lower hepatic methionine and cysteine as compared with ad libitum fed mice, and leads to a paradoxical increase in H2S production (29). We posit that decreased liver cystathionine and increased CSE levels accompanying dietary restriction (29) activate the metabolite switch, promoting H2S production by CSE, with consequent attenuation of injury during ischemia/reperfusion. Furthermore, the reported increase in endothelial NO (30) associated with caloric restriction suggests that increased NO bioavailability could inhibit CBS, activate the metabolite switch, and promote H2S biogenesis by CSE. Finally, heme-dependent metabolite switching could explain the reported enhancement of H2S production from cysteine in response to an NO donor (31) and provide a molecular mechanism for the cross-talk between NO and H2S signaling pathways.
The metabolite switching model helps explain why large randomized controlled trials for lowering homocysteine had limited success in reducing cardiovascular disease outcomes in patients (32–34) and suggests instead that a strategy targeting H2S might be effective. In light of the metabolite switching mechanism for regulating H2S, the current approaches for treating the orphan disease, homocystinuria, and chronic diseases such as type I diabetes, where H2S dysregulation and ER stress are implicated (12, 35), should be reevaluated.
Experimental Procedures
Animal Tissues
Livers from Cbs−/− (Tg-I278T) and wild-type mice were a generous gift from Dr. Warren Kruger (Fox Chase Cancer Center, Philadelphia, PA). Briefly, the mice express a human Cbs transgene carrying the pathogenic I278T mutation under the control of a zinc-inducible promoter (36) to overcome the neonatal lethality associated with the Cbs−/− genotype (21). The livers were harvested from female mice maintained on zinc-free water for 4 months, frozen, and shipped to our laboratory.
Cell Culture and Metabolic Labeling
HEK293 cells were grown in 10-cm dishes in minimum essential medium (Lonza) supplemented with 10% FBS and 2 mm l-glutamine until they reached 60–80% confluency. ER stress was induced by adding thapsigargin to the culture medium to a final concentration of 0.5 μm, and cells were grown until the indicated times before sample collection. For metabolic labeling studies, 5–10 μCi of [35S]methionine (PerkinElmer) was added per 5 ml of medium 4–5 h prior to sample collection.
GSH Determination
For analysis of GSH and GSSG, an aliquot of cell suspension was mixed with an equal volume of metaphosphoric acid solution (135 mm metaphosphoric acid, 5 mm EDTA, and 150 mm NaCl). After a freeze-thaw cycle, precipitated proteins were removed by centrifugation (10,000 × g for 5 min). To the resulting protein-free supernatant, iodoacetic acid was added to a final concentration of 10 mm, to block free thiol groups. The pH was adjusted to 8–9 with potassium carbonate, and the reaction mixture was incubated for 1 h at room temperature in the dark. An equal volume of 2,3-dinitrofluorobenzene (1.5% v/v in absolute ethanol) was added to derivatize amino groups and incubated at room temperature for 4 h in the dark before HPLC analysis. The second aliquot of the cell suspension was used to measure the protein concentration using the Bradford reagent (Bio-Rad) and for Western blotting analysis.
HPLC Analysis
Derivatized samples were analyzed by HPLC using a Bondapak-NH2 300 × 3.9-mm column (Waters) with a methanol/acetate gradient as described previously (37). Radiolabel incorporation into GSH and GSSG was determined by scintillation counting of the corresponding HPLC fractions. The results were normalized to protein concentration to determine GSH and GSSG concentrations as described previously (38).
Sulfate Analysis
Sulfate concentration in the culture medium was measured using a turbidity assay as described previously (39). To measure radioactivity associated with sulfate, BaCl2 and HCl were added to a final concentration of 50 mm and 0.5 n, respectively, to 3 ml of culture medium to precipitate sulfate. After 20 min of incubation at room temperature, the barium sulfate precipitate was collected by centrifugation and washed with the same precipitating solution in water. The pellet was dissolved in 1 m NaOH, and the radioactivity was measured in a scintillation counter.
H2S Production Assay
Reactions for H2S production were prepared in polypropylene syringes as described previously (20) with minor modifications. Reactions containing tissue homogenate and the substrates (cysteine and homocysteine or cysteine alone as indicated in the figure legends in a total liquid volume of 1 ml) were mixed in 20-ml syringe barrels. Syringes were sealed with plungers and immediately made anaerobic by flushing the headspace with nitrogen using a three-way stopcock, and then left under nitrogen in a final volume (liquid + gas) of 20 ml. Syringes were incubated at 37 °C with gentle shaking (80 rpm) for the times indicated in the figure legends. Control reactions containing only tissue homogenate or only substrates were prepared in parallel. Aliquots (200 μl) from the gas were collected through a septum attached to the stopcock, and then injected into an HP 6890 gas chromatograph. A standard curve was prepared using pure H2S gas from Cryogenic Gases with a stock concentration of 40 ppm. The amount of H2S in the injected volume was calculated from the peak areas using the calibration coefficient obtained from the standard curve.
Western Blotting Analysis
Anti-HO-2 antibody (LSBio Inc.) was used at a 1:1000 dilution. Anti-xCT antibody (Santa Cruz Biotechnology) was used at a 1:1000 dilution, and anti-CBS and anti-CSE antibodies were raised in chicken against human proteins and affinity-purified in our laboratory using the respective recombinant human proteins. Frozen tissue was homogenized in 100 mm HEPES, pH 7.4, in an ice bath using a glass homogenizer.
Author Contributions
O. K. and V. Y. designed, performed, and analyzed the experiments. O. K. and R. B. helped conceive the experiments and wrote the manuscript. All authors edited and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Warren Kruger and Sapna Gupta (Fox Chase Cancer Center) for tissues from wild-type and Cbs−/− mice.
This work was supported in part by National Institutes of Health Grant HL58984 (to R. B.) and American Heart Association Grant 13SDG17070096 (to O. K.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was selected as a Paper of the Week.

This article contains supplemental Figs. S1–S4.
- CBS
- cystathionine β-synthase
- CSE
- γ-cystathionase
- ER
- endoplasmic reticulum
- ATF4
- activating transcription factor 4
- HO-2
- heme oxygenase-2.
References
- 1. Abe K., and Kimura H. (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elrod J. W., Calvert J. W., Morrison J., Doeller J. E., Kraus D. W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., Kimura H., Chow C. W., and Lefer D. J. (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 104, 15560–15565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiorucci S., Antonelli E., Distrutti E., Rizzo G., Mencarelli A., Orlandi S., Zanardo R., Renga B., Di Sante M., Morelli A., Cirino G., and Wallace J. L. (2005) Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129, 1210–1224 [DOI] [PubMed] [Google Scholar]
- 4. Singh S., Padovani D., Leslie R. A., Chiku T., and Banerjee R. (2009) Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., and Banerjee R. (2009) H2S biogenesis by cystathionine γ-lyase leads to the novel sulfur metabolites, lanthionine and homolanthionine, and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee R., and Zou C.-G. (2005) Redox Regulation and reaction mechanism of human cystathionine-β-synthase, a PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 433, 144–156 [DOI] [PubMed] [Google Scholar]
- 7. Miles E. W., and Kraus J. P. (2004) Cystathionine β-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 279, 29871–29874 [DOI] [PubMed] [Google Scholar]
- 8. Niu W. N., Yadav P. K., Adamec J., and Banerjee R. (2015) S-Glutathionylation enhances human cystathionine β-synthase activity under oxidative stress conditions. Antioxid. Redox. Signal. 22, 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mosharov E., Cranford M. R., and Banerjee R. (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39, 13005–13011 [DOI] [PubMed] [Google Scholar]
- 10. Jacobs R. L., House J. D., Brosnan M. E., and Brosnan J. T. (1998) Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes 47, 1967–1970 [DOI] [PubMed] [Google Scholar]
- 11. Ratnam S., Maclean K. N., Jacobs R. L., Brosnan M. E., Kraus J. P., and Brosnan J. T. (2002) Hormonal regulation of cystathionine β-synthase expression in liver. J. Biol. Chem. 277, 42912–42918 [DOI] [PubMed] [Google Scholar]
- 12. Gao X. H., Krokowski D., Guan B. J., Bederman I., Majumder M., Parisien M., Diatchenko L., Kabil O., Willard B., Banerjee R., Wang B., Bebek G., Evans C. R., Fox P. L., Gerson S. L., et al. (2015) Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the Integrated Stress Response. Elife 4, e10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickhout J. G., Carlisle R. E., Jerome D. E., Mohammed-Ali Z., Jiang H., Yang G., Mani S., Garg S. K., Banerjee R., Kaufman R. J., Maclean K. N., Wang R., and Austin R. C. (2012) Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: cross-talk between integrated stress response and thiol metabolism. J. Biol. Chem. 287, 7603–7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao S. S., and Kaufman R. J. (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox. Signal. 21, 396–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baird T. D., and Wek R. C. (2012) Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 3, 307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu X. M., Peyton K. J., Ensenat D., Wang H., Schafer A. I., Alam J., and Durante W. (2005) Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J. Biol. Chem. 280, 872–877 [DOI] [PubMed] [Google Scholar]
- 17. Taoka S., West M., and Banerjee R. (1999) Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine β-synthase reveals nonequivalent active sites. Biochemistry 38, 2738–2744 [DOI] [PubMed] [Google Scholar]
- 18. Taoka S., and Banerjee R. (2001) Characterization of NO binding to human cystathionine β-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J. Inorg. Biochem. 87, 245–251 [DOI] [PubMed] [Google Scholar]
- 19. Puranik M., Weeks C. L., Lahaye D., Kabil O., Taoka S., Nielsen S. B., Groves J. T., Banerjee R., and Spiro T. G. (2006) Dynamics of carbon monoxide binding to cystathionine β-synthase. J. Biol. Chem. 281, 13433–13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kabil O., Weeks C. L., Carballal S., Gherasim C., Alvarez B., Spiro T. G., and Banerjee R. (2011) Reversible heme-dependent regulation of human cystathionine β-synthase by a flavoprotein oxidoreductase. Biochemistry 50, 8261–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., and Maeda N. (1995) Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. U.S.A. 92, 1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry T. L., Hansen S., Bar H. P., and Macdougall L. (1966) Homocystinuria: excretion of a new sulfur-containing amino acid in urine. Science 152, 776–778 [DOI] [PubMed] [Google Scholar]
- 23. Prudova A., Bauman Z., Braun A., Vitvitsky V., Lu S. C., and Banerjee R. (2006) S-Adenosylmethionine stabilizes cystathionine β-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. U.S.A. 103, 6489–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabil O., Vitvitsky V., Xie P., and Banerjee R. (2011) The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox. Signal. 15, 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A. K., Mu W., Zhang S., Snyder S. H., and Wang R. (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maclean K. N., Greiner L. S., Evans J. R., Sood S. K., Lhotak S., Markham N. E., Stabler S. P., Allen R. H., Austin R. C., Balasubramaniam V., and Jiang H. (2012) Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J. Biol. Chem. 287, 31994–32005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robillon J. F., Canivet B., Candito M., Sadoul J. L., Jullien D., Morand P., Chambon P., and Freychet P. (1994) Type 1 diabetes mellitus and homocyst(e)ine. Diabete Metab. 20, 494–496 [PubMed] [Google Scholar]
- 28. Pogribna M., Melnyk S., Pogribny I., Chango A., Yi P., and James S. J. (2001) Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am. J. Hum. Genet. 69, 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B. C., Brace L., Longchamp A., Treviño-Villarreal J. H., Mejia P., Ozaki C. K., Wang R., Gladyshev V. N., Madeo F., Mair W. B., and Mitchell J. R. (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mattagajasingh I., Kim C. S., Naqvi A., Yamamori T., Hoffman T. A., Jung S. B., DeRicco J., Kasuno K., and Irani K. (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 104, 14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao W., and Wang R. (2002) H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 283, H474–480 [DOI] [PubMed] [Google Scholar]
- 32. Bønaa K. H., Njølstad I., Ueland P. M., Schirmer H., Tverdal A., Steigen T., Wang H., Nordrehaug J. E., Arnesen E., Rasmussen K., and NORVIT Trial Investigators (2006) Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 354, 1578–1588 [DOI] [PubMed] [Google Scholar]
- 33. Albert C. M., Cook N. R., Gaziano J. M., Zaharris E., MacFadyen J., Danielson E., Buring J. E., and Manson J. E. (2008) Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 299, 2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J. M., Bowman L., Clarke R. J., Wallendszus K., Bulbulia R., Rahimi K., Haynes R., Parish S., Sleight P., Peto R., and Collins R. (2010) Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 303, 2486–2494 [DOI] [PubMed] [Google Scholar]
- 35. Outinen P. A., Sood S. K., Pfeifer S. I., Pamidi S., Podor T. J., Li J., Weitz J. I., and Austin R. C. (1999) Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression human vascular endothelial cells. Blood 94, 959–967 [PubMed] [Google Scholar]
- 36. Gupta S., Kühnisch J., Mustafa A., Lhotak S., Schlachterman A., Slifker M. J., Klein-Szanto A., High K. A., Austin R. C., and Kruger W. D. (2009) Mouse models of cystathionine β-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 23, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garg S., Vitvitsky V., Gendelman H. E., and Banerjee R. (2006) Monocyte differentiation, activation, and mycobacterial killing are linked to transsulfuration-dependent redox metabolism. J. Biol. Chem. 281, 38712–38720 [DOI] [PubMed] [Google Scholar]
- 38. Yan Z., Garg S. K., Kipnis J., and Banerjee R. (2009) Extracellular redox modulation by regulatory T cells. Nat. Chem. Biol. 5, 721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundquist P., Mårtensson J., Sörbo B., and Ohman S. (1980) Turbidimetry of inorganic sulfate, ester sulfate, and total sulfur in urine. Clin. Chem. 26, 1178–1181 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


