Abstract
Glycosylation can exert a profound impact on the structures and biological functions of antibodies. Glycosylation remodeling using the endoglycosidase-catalyzed deglycosylation and transglycosylation approach is emerging as a promising platform to produce homogeneous glycoforms of antibodies, but the broad application of this method will require the availability of highly efficient glycosynthase mutants. We describe in this paper a systematic site-directed mutagenesis of an endoglycosidase from Streptococcus pyogenes of serotype M49 (Endo-S2) and the evaluation of the resulting mutants for their hydrolysis and transglycosylation activities. We found that mutations at the Asp-184 residue gave mutants that demonstrated significantly different properties, some possessed potent transglycosylation activity with diminished hydrolysis activity but others did not, which would be otherwise difficult to predict without the comparative study. In contrast to the previously reported Endo-S mutants that are limited to action on complex type N-glycans, the Endo-S2 glycosynthases described here, including D184M and D184Q, were found to have remarkably relaxed substrate specificity and were capable of transferring three major types (complex, high-mannose, and hybrid type) of N-glycans for antibody glycosylation remodeling. In addition, the Endo-S2 glycosynthase mutants were found to be much more active in general than the Endo-S mutants for transglycosylation. The usefulness of these Endo-S2 glycosynthase mutants was exemplified by an efficient glycosylation remodeling of two therapeutic monoclonal antibodies, rituximab and trastuzumab (Herceptin).
Keywords: antibody engineering, glycoprotein, glycosidase, glycosylation, monoclonal antibody, Herceptin, chemoenzymatic synthesis, glycoengineering, glycosynthase, transglycosylation
Introduction
Monoclonal antibodies (mAbs) represent a major class of therapeutic proteins used for the treatment of cancers, inflammatory disorders, and infectious diseases (1–3). Compelling experimental data have shown that glycosylation can have profound impacts on the stability, biological functions, and overall therapeutic efficacy of antibodies (4–7). For example, core-fucosylation of Fc glycans could significantly reduce the antibody-dependent cellular cytotoxicity, and antibodies with low content of core-fucosylation have shown improved therapeutic efficacy in anti-cancer therapy (8, 9). On the other hand, it has been reported that the terminal α2,6-sialylated Fc glycoform, a minor component in the intravenous immunoglobulin, is responsible for the anti-inflammatory activity of intravenous immunoglobulin as demonstrated in animal models (10–13). However, natural and recombinant antibodies are usually produced as heterogeneous glycoforms that are difficult to separate for further probing the structure-activity relationships of antibodies. Moreover, for a majority of anti-cancer mAbs on the market that rely on antibody-dependent cellular cytotoxicity as a major mechanism of the therapeutic efficacy, the most active non-fucosylated glycoforms are usually present as minor fractions among the heterogeneous mixtures (8, 9). Thus, methods that can lead to the production of structurally well defined, homogeneous glycoforms of antibodies are highly desirable for both functional studies and for the development of better antibody-based therapeutics. In parallel to the attempt to control glycosylation through host glycosylation pathway engineering (14–20), a chemoenzymatic glycosylation remodeling method, which involves endoglycosidase-catalyzed deglycosylation and subsequent transglycosylation of intact antibodies, has been emerging as a promising approach to obtain homogeneous antibody glycoforms (21). We have initially shown that the Fc glycans of recombinant IgG-Fc domain could be remodeled through the enzymatic deglycosylation-transglycosylation steps under the catalysis of an appropriate endoglycosidase, including Endo-A2 and Endo-D, without the need of denaturing the proteins (22–24). In 2012, we reported the first example of glycosylation remodeling of an intact therapeutic monoclonal antibody and intravenous immunoglobulin, which was enabled by the discovery of glycosynthase mutants of Endo-S, an endoglycosidase from Streptococcus pyogenes (25). In this approach, the heterogeneous Fc glycans of a monoclonal antibody such as rituximab are removed by Endo-S-catalyzed deglycosylation to yield the antibody protein backbone carrying only the α1,6-fucosylated GlcNAc acceptor at the glycosylation site, then a desired N-glycan is transferred to the GlcNAc acceptor by an Endo-S glycosynthase mutant (EndoS-D233A or D233Q) in a site- and stereo-specific manner to reconstitute a homogeneous glycoform of the antibody. The Endo-S glycosynthase mutants have been used by several research groups for the synthesis of different homogeneous glycoforms of antibodies for structural and functional studies (26–31). Although the Endo-S glycosynthases were able to transfer biantennary complex type and modified Man3GlcNAc core, these mutants showed only marginal activity in transferring high-mannose type N-glycans. More recently, we have generated glycosynthase mutants from Endo-F3, another GH18 family endoglycosidase (32). It was found that the Endo-F3 glycosynthases, such as the D165A mutant, was able to transfer triantennary N-glycan to Fc domain of intact antibody, but they required α1,6-fucosylated GlcNAc moiety as the acceptor for transglycosylation and were unable to transfer to non-fucosylated GlcNAc acceptors. These studies have demonstrated that the glycosynthases so far available still have limitations due to substrate specificity and also vary in transglycosylation efficiency. As an effort to expand the scope of the glycosylation remodeling strategy, we turned our attention to Endo-S2, an endoglycosidase from S. pyogenes of serotype M49 (33, 34). Endo-S2 shows only 37% sequence identity to Endo-S from the same bacteria and demonstrates a broader glycan substrate specificity in Fc deglycosylation than Endo-S (34). However, it was not clear if Endo-S2 had potential transglycosylation activity. If yes, it was yet to evaluate whether efficient glycosynthases could be generated from Endo-S2 and whether they would potentially have broader substrate specificity in transglycosylation than those previously reported. In this paper, we describe a systematic mutagenesis at Asp-184 that was identified as a critical residue for hydrolysis by sequence alignment, and the evaluation of the 19 mutants for transglycosylation activities and for their feasibility in antibody glycosylation remodeling. We found that Endo-S2 did possess potent transglycosylation activity, and the systematic site-directed mutagenesis led to the discovery of several glycosynthase mutants, including D184M and D184Q, that showed remarkable transglycosylation activity without apparent product hydrolysis activity. Moreover, we found that the Endo-S2 glycosynthases demonstrated remarkably relaxed substrate specificity, being capable of transferring three major types (complex, high-mannose, and hybrid type) of N-glycans for antibody glycosylation remodeling. In a comparative study, we also found that the Endo-S2 glycosynthase mutants were much more active in general than the Endo-S mutants for transglycosylation. A highly efficient glycosylation remodeling of two therapeutic monoclonal antibodies, rituximab and trastuzumab (Herceptin), is described.
Results
Cloning, Expression, and Characterization of Endo-S2
cDNA sequence encoding Endo-S2 (38–819) was cloned into a pET22b-CPD vector, which adds to the C terminus of the expressed protein the cysteine protease domain (CPD) of the Vibrio cholerae MARTX toxin and a ×10 histidine tag (35). We have recently reported that a high-level, soluble expression of Endo-F3 and its mutants could be achieved using this vector (32). Following a similar method, the Endo-S2 was successfully expressed in Escherichia coli and was readily purified using immobilized metal ion affinity chromatography to obtain the soluble enzyme with a yield of more than 20 mg/liters. The recombinant Endo-S2 showed high hydrolysis activity as demonstrated by its rapid deglycosylation of commercial rituximab, which was monitored by LC-MS analysis.
Generation of Glycosynthase Mutants from Endo-S2
We have previously generated glycosynthase mutants from endoglycosidases of both GH85 and GH18 families by site-directed mutation at a key residue that is responsible for promoting the formation of the oxazolinium ion intermediate during hydrolysis, which proceeds in a substrate-assisted mechanism. These include a key asparagine residue for endoglycosidases Endo-A (Asn-171) (36), Endo-M (Asn-175) (37, 38), and Endo-D (Asn-322) (24) of the GH85 family, or a key aspartic acid residue for the GH18 family endoglycosidases Endo-S (Asp-233) (25) and Endo-F3 (Asp-165) (32). Sequence alignment of Endo-S2 and Endo-S revealed that the Asp-184 of Endo-S2 was the residue equivalent to Asp-233 of Endo-S essential for promoting oxazolinium ion formation in hydrolysis (Fig. 1). To generate efficient glycosynthase mutants from Endo-S2, we systematically replaced the Asp-184 with other 19 natural amino acid residues using site-directed mutagenesis. The resulting 19 Asp-184 mutants were also expressed as soluble proteins in the pET22b-CPD vector and purified using immobilized metal ion affinity chromatography, in the same way as demonstrated for the wild type enzyme. The expression of the mutant enzymes gave comparable yields (15–20 mg/liters) as the expression of the wild type enzyme.
FIGURE 1.
Sequence alignment of Endo-S2 and Endo-S. The aspartic acid residue (Asp-233 of Endo-S and Asp-184 of Endo-S2) critical for promoting oxazolinium ion formation in hydrolysis and the catalytic general acid/base residue (Glu-235 of Endo-S and Glu-186 of Endo-S2) are marked.
Comparative Study on the Hydrolysis and Transglycosylation Activity of 19 Mutants and WT
The hydrolysis activities on Fc N-glycans in an intact antibody and the transglycosylation activities with glycan oxazoline were assessed following the scheme as shown in Fig. 2. The results indicate that most of the mutants at the Asp-184 residue led to significantly reduced or completely diminished hydrolysis activity on Fc N-glycan. Among them, D184F, D184H, D184K, D184R, and D184W mutants were completely devoid of hydrolytic activity, whereas several other mutants, including D184C, D184E, D184G, D184N, D184S, D184Y, were found to still retain significant hydrolysis activity (Table 1). On the other hand, the evaluation of transglycosylation indicated that almost all the mutants possessed transglycosylation activity when using the deglycosylated rituximab as the acceptor and a biantennary complex type glycan oxazoline as the donor substrate, but the activities varied significantly among different mutants (Fig. 2, Table 2). Among others, D184C, D184M, D184G, D184E, D184Y, D184S, and D184A were found to be the most active mutants. However, D184C, D184G, D184E, D184Y, D184S, and D184A also demonstrated significant residual hydrolytic activity. The most interesting mutant is D184M, which retained only marginal hydrolytic activity but showed extraordinarily high transglycosylation activity (only second to D184C), making it one of the best glycosynthase mutants to choose for glycosylation remodeling.
FIGURE 2.
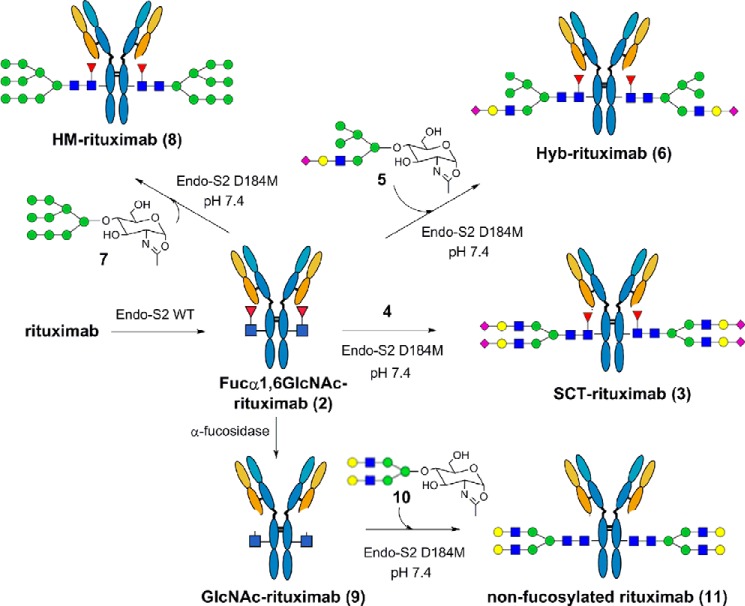
Schematic presentation of the hydrolysis and transglycosylation by Endo-S2 and its mutants using rituximab as the substrate. Fucα1,6GlcNAc-rituximab, the deglycosylated rituximab carrying the core-fucosylated GlcNAc moiety at the glycosylation site; SCT-rituximab, the sialyl complex type glycoform of rituximab.
TABLE 1.
Comparison of the specific hydrolysis activities of the Endo-S2 Asp-184 mutants using a synthetic complex glycoform of rituximab as the substrate
| Mutant | Specific hydrolysis activity (×100) | Percentage of specific hydrolysis activity |
|---|---|---|
| μmol/min/mg | % | |
| WT | 11.185 | 100.000 |
| D184A | 0.750 | 6.701 |
| D184C | 1.386 | 12.392 |
| D184E | 0.879 | 7.855 |
| D184F | 0.000 | 0.000 |
| D184G | 1.249 | 11.168 |
| D184H | 0.000 | 0.000 |
| D184K | 0.000 | 0.000 |
| D184R | 0.000 | 0.000 |
| D184I | 0.033 | 0.295 |
| D184L | 0.045 | 0.405 |
| D184M | 0.158 | 1.410 |
| D184N | 0.842 | 7.526 |
| D184P | 0.340 | 3.043 |
| D184Q | 0.049 | 0.441 |
| D184S | 1.387 | 12.398 |
| D184T | 0.212 | 1.899 |
| D184V | 0.409 | 3.659 |
| D184W | 0.000 | 0.000 |
| D184Y | 5.526 | 49.406 |
TABLE 2.
Comparison of the specific transglycosylation activity of Endo-S2 Asp-184 mutants using sialoglycan oxazoline as donor substrate and deglycosylated rituximab as the acceptor substrate
| Mutants | Specific transglycosylation activity (×10) | Percentage of specific transglycosylation activity |
|---|---|---|
| μmol/min/mg | % | |
| WT | 1.712 | 44.31 |
| D184A | 1.023 | 26.46 |
| D184C | 3.864 | 100.00 |
| D184E | 2.006 | 51.907 |
| D184F | 0.044 | 1.14 |
| D184G | 2.270 | 58.73 |
| D184H | 0.026 | 0.68 |
| D184K | 0.021 | 0.54 |
| D184R | 0.022 | 0.57 |
| D184I | 0.066 | 1.71 |
| D184L | 0.099 | 2.55 |
| D184M | 2.866 | 74.18 |
| D184N | 0.879 | 22.75 |
| D184P | 0.160 | 4.13 |
| D184Q | 0.383 | 9.92 |
| D184S | 1.164 | 30.13 |
| D184T | 0.828 | 21.42 |
| D184V | 0.147 | 3.79 |
| D184W | 0.046 | 1.20 |
| D184Y | 1.198 | 30.99 |
Endo-S2 Glycosynthases Show Remarkably Broad Substrate Specificity in Transglycosylation
Rituximab, a therapeutic monoclonal antibody, was used as a model to examine the transglycosylation activity of the Endo-S2 Asp-184 mutants. The major Fc glycans of commercial rituximab are core-fucosylated biantennary complex type oligosaccharides carrying 0–2 galactose moieties named G0F, G1F, and G2F glycoforms, respectively. The general glycosylation remodeling approach was presented in Fig. 3 and the reaction products were assessed by LC-MS analysis (Fig. 4). Treatment of rituximab (1) with wild type Endo-S2 resulted in complete deglycosylation of rituximab, as demonstrated by the conversion of the glycoform mixtures (G0F, G1F, and G2F) found in commercial rituximab (Fig. 4A) to the Fucα1,6GlcNAc-glycoform (2) of rituximab (Fig. 4B). The Fucα1,6GlcNAc-rituximab (2) was purified away from the WT endoglycosidase and released glycans by Protein A affinity chromatography and used as the acceptor in the transglycosylation reactions. We found that Endo-S2 D184M was able to efficiently transfer the sialylated biantennary complex type (SCT) N-glycan from the corresponding glycan oxazoline (4) to the Fucα1,6GlcNAc-rituximab acceptor (2) to form the S2G2F glycoform of rituximab (3). The reaction could be readily pushed to completion with 20 molar eq (i.e. 10 molar eq per monomeric Fc domain) of the glycan oxazoline. The reaction yield was estimated by LC-MS analysis to be over 95% as almost no starting material was detected, which confirmed the completion of the transglycosylation. LC-MS analysis of the transglycosylation products (3) carrying the SCT N-glycan revealed that the heavy chain of 3 appeared as a single species at 51,412 (deconvolution data), which is in good agreement with the calculated molecular mass (M = 51,421 Da) for the heavy chain carrying a SCT N-glycan (with core-fucose), respectively (Fig. 4C).
FIGURE 3.
Evaluation of substrate specificity of Endo-S2 mutants on various glycans (HM, CT, and Hyb type). GlcNAc-rituximab, the rituximab glycoform carry only the first GlcNAc moiety at the Fc glycosylation site; Fucα1,6GlcNAc-rituximab, the deglycosylated rituximab carrying the core-fucosylated GlcNAc moiety at the glycosylation site; SCT-rituximab, the sialyl complex type glycoform of rituximab; HM-rituximab, the high-mannose type glycoform of rituximab; Hyb-ituximab, the hybrid type glycoform of rituximab.
FIGURE 4.
ESI-MS analysis of the glycosylation remodeling of rituximab using Endo-S2 D184M. A, ESI-MS (after deconvolution) of the heavy chain of the commercial rituximab; B, ESI-MS of the heavy chain of the Fucα1,6GlcNAc-rituximab (2); C and D, ESI-MS of the heavy chain and light chain of transglycosylation product 3 (SCT-rituximab), respectively; E and F, ESI-MS of the heavy chain and light chain of transglycosylation product 8 (HM-rituximab); G and H, ESI-MS of the heavy chain and light chain of transglycosylation product 6 (Hyb-rituximab); I and J, ESI-MS of the heavy chain and light chain of transglycosylation product 11 (non-fucosylated rituximab).
In addition to the complex type N-glycan, the specificity of Endo-S2 was further tested with high-mannose type (HM) Man9GlcNAc oxazoline (7) and sialo hybrid type (Hyb) Neu5AcGalGlcNAcMan5GlcNAc oxazoline (5) as donor substrate in the transglycosylation reactions. The reactions led to the formation of the corresponding homogeneous glycoforms, (8) and (6), respectively (Fig. 4, E and G). The deconvoluted ESI-MS of the heavy chain of the transglycosylation product (8) showed a single species at 51,074, as shown in Fig. 4E, which matched well with the calculated molecular mass (M = 51,081 Da) of the rituximab heavy chain carrying a Man9GlcNAc2 glycan. Similarly, the deconvoluted ESI-MS of the heavy chain of transglycosylation product (6) showed a single species at 51,082, as shown in Fig. 4G, which was in good agreement with the calculated molecular mass (M = 51,090 Da) of the rituximab heavy chain carrying a Neu5AcGalGlcNAcMan5GlcNAc2 glycan. Under the same conditions, we found that the previously reported Endo-S mutants, including D233A and D233Q mutants of Endo-S (25) showed only marginal transglycosylation activity with high-mannose and hybrid type N-glycans, although they could efficiently transfer biantennary complex type N-glycan. In addition, the recently reported D165A mutant of Endo-F3 (32) was unable to transfer the high-mannose or hybrid type N-glycans but could work on bi- and triantennary complex type sugars. Thus, these Endo-S2-derived mutants represent the first glycosynthases that can efficiently transfer high-mannose and hybrid type N-glycans to core-fucosylated GlcNAc acceptor in an intact antibody. It should be mentioned that the Endo-A mutant (N171A and N171Q) could transfer high-mannose type N-glycan to the GlcNAc-Fc domain, but they were unable to use core-fucosylated GlcNAc-Fc as acceptor (22, 23, 36). These studies also show that the combined use of wild type Endo-S2 and Endo-S2 glycosynthase mutants provides a particularly efficient glycosylation remodeling approach to various homogeneous glycoforms of antibodies starting from a single precursor.
Endo-S2-based Glycosylation Remodeling for Making Nonfucosylated Glycoforms
For anticancer therapy, nonfucosylated IgG glycoforms are desirable as it has been previously demonstrated that mAbs with low-fucose contents of Fc glycosylation showed enhanced antibody-dependent cellular cytotoxicity activity in vitro and enhanced anticancer efficacy in vivo, particularly for those patients carrying the low affinity Phe-158 allele of the FcγIIIa receptor (8, 9, 39, 40). To test whether Endo-S2 can glycosylate non-fucosylated IgG, Fucα1,6GlcNAc-rituximab (2) was incubated with a recombinant α1,6-fucosidase from Lactobacillus casei (41) to give GlcNAc-rituximab (9) lacking the core-fucose. Endo-S2 D184M catalyzed transglycosylation of GlcNAc-rituximab (9) was carried out with asialo biantennary complex type (CT) N-glycan oxazoline (10). It was found that the D184M mutant could efficiently transfer the N-glycan to the GlcNAc acceptor in the antibody to give the fully galactosylated and nonfucosylated glycoform (11) in an essentially quantitative conversion. The deconvoluted ESI-MS of the heavy chain of transglycosylation product (11) showed a single species at 50,684, as shown in Fig. 4I, which was in good agreement with the calculated molecular mass (M = 50,693 Da) of rituximab heavy chain carrying a fully galactosylated biantennary complex type N-glycan without the core-fucose. In addition to the confirmation of site-specific glycosylation of the heavy chain by LC-MS analysis combined with enzymatic transformation, the light chain of the transglycosylation products (3, 6, 8, 11) also appeared as a single species at 23,034, which matches the calculated molecular mass of the light chain of rituximab (M = 23,039 Da) (Fig. 4, D, F, H, and J) without any modifications. These results indicated that there were no non-enzymatic modifications occurring on the heavy and light chains except the attachment of the transferring N-glycan at the GlcNAc acceptor at the Fc domain during the Endo-S2 catalyzed glycosylation remodeling processes. It should be pointed out that the fully galactosylated and non-fucosylated glycoform of rituximab (11) was previously shown to have at least 20-fold enhanced affinity for the FcγIIIA receptor in comparison with the commercial rituximab, which is an indication of a significantly enhanced antibody-dependent cellular cytotoxicity (25).
Comparison of Transglycosylation Efficiency on Different N-Glycan Substrates by Endo-S2 D184M Mutant
To further characterize the N-glycan substrate preference of Endo-S2 D184M, three parallel transglycosylation reactions were carried out with the N-glycan oxazolines of the complex (SCT) type, high-mannose (HM) type, and hybrid (Hyb) type, respectively. The reaction progresses were monitored by LC-MS analysis of reaction aliquots taken at multiple time points and the results were summarized in Fig. 5. Under the same reaction conditions, the transglycosylation reaction with SCT-oxazoline (4) was completed within 20 min to give S2G2F-rituximab (3), whereas the transglycosylation with HM-oxazoline (7) and Hyb-oxazoline (5) were much slower. These results suggest that Endo-S2 D184M prefers complex type over the high-mannose type and hybrid type N-glycan despite of having a remarkably relaxed N-glycan specificity.
FIGURE 5.

Comparison of transglycosylation efficiency of different types of glycans by Endo-S2 D184M mutant. The transglycosylation reaction was carried out using deglycosylated rituximab 2 as the acceptor and different types of glycan oxazolines as the donor substrate under the catalysis of Endo-S2 D184M (0.05 mg/ml). The molar ratio of donor to acceptor was 20:1. The data sets presented here are representative of two independent experiments.
Comparison of the Transglycosylation Efficiency of Typical Endo-S2 and Endo-S Mutants
We have previously generated the first Endo-S glycosynthase mutants D233Q and D233A that transglycosylate rituximab efficiently with complex type N-glycan oxazoline (25). The Endo-S Asp-233 mutants have been recently used by us and several other groups to generate homogeneous monoclonal antibodies for structural and functional studies (26–31). To compare the transglycosylation efficiency of the glycosynthase mutants from Endo-S2 and Endo-S, we selected to use the Endo-S D233Q mutant, the equivalent Endo-S2 D184Q mutant, and the Endo-S2 D184M mutant to catalyze three parallel transglycosylation reactions. The time course of the transglycosylation reactions was monitored by LC-MS analysis and summarized in Fig. 6. Under the reaction conditions, the D184M mutant of Endo-S2 showed remarkably potent transglycosylation activity, and reached completion of the glycan transfer within 10 min. The other Endo-S2 mutant could also transfer the glycan smoothly and reached the completion within 1 h. However, the corresponding Endo-S mutant (D233Q) was much less efficient, reaching about 10% of transglycosylation at 1 h under the same conditions (Fig. 6). As demonstrated in a separate experiment, much more (10-fold) Endo-S D233Q mutant and a larger excess of the glycan oxazoline were required to achieve the same level of the transglycosylation catalyzed by the Endo-S2 D184Q mutant, and the D184M mutant of Endo-S2 was even much more efficient than the D184Q mutant. These studies suggest that the newly discovered Endo-S2 Asp-184 mutants are superior to the previously reported Endo-S mutants for antibody glycosylation remodeling in both efficiency of reactions and the breadth of substrate diversity.
FIGURE 6.

Comparison of the transglycosylation with SCT by Endo-S2 D184Q, Endo-S2 D184M, and Endo-S D233Q. The transglycosylation was performed using deglycosylated rituximab 2 as the acceptor and SCT glycan oxazoline 4 as the donor substrate under the catalysis of different endoglycosidase mutants at a fixed concentration of 0.05 mg/ml. The molar ratio of donor to acceptor was 20:1. The data sets presented here are representative of two independent experiments.
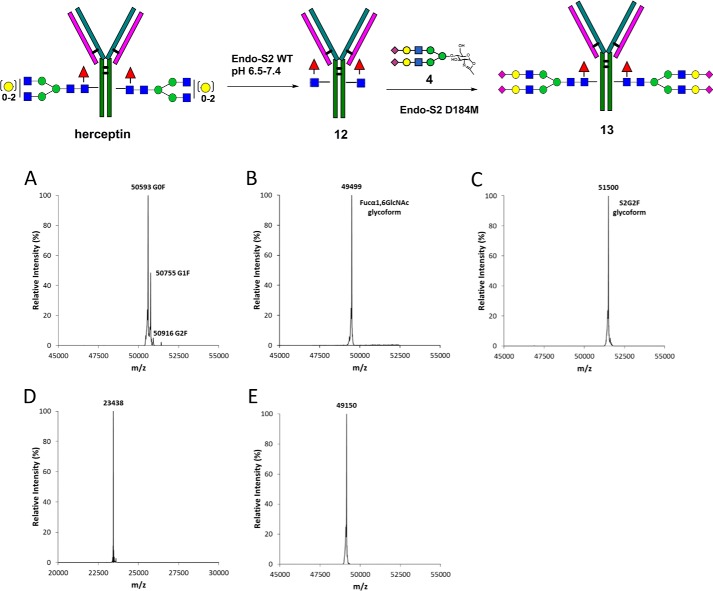
Glycosylation Remodeling of Trastuzumab (Herceptin) Using a Pair of Endo-S2 Enzymes
To demonstrate that the observed enzymatic properties of Endo-S2 and its mutants are generally applicable to antibody glycosylation remodeling, we also performed glycosylation remodeling of another monoclonal antibody, trastuzumab (Herceptin), which is widely used for treatment of breast cancer. The glycosylation remodeling was assessed by the synthesis of a sialylated glycoform of trastuzumab (Fig. 7). Treatment of trastuzumab with wild type Endo-S2 resulted in complete deglycosylation, as demonstrated by the conversion of the glycoform mixtures (G0F, G1F, and G2F) found in commercial trastuzumab (Fig. 7A) to the Fucα1,6GlcNAc-glycoform (12) of trastuzumab (Fig. 7B). The deglycosylated trastuzumab was purified away from the endoglycosidase and released glycans by Protein A affinity chromatography and used as the acceptor in the transglycosylation reactions. Incubation of the Fucα1,6GlcNAc-trastuzumab (12) with the donor substrate SCT-oxazoline (4) in the presence of Endo-S2 D184M mutant resulted in rapid conversion of the deglycosylated trastuzumab (12) to the fully glycosylated trastuzumab (13). The reaction was essentially quantitative to give a single transglycosylation product (13). LC-MS analysis of the transglycosylation product (13) revealed that the heavy chain of 13 appeared as a single species at 51,500 Da (deconvolution data) (Fig. 7C), indicating the attachment of a single sialylated N-glycan on the heavy chain. On the other hand, the light chain of the transglycosylation product (13) appeared as a single species at 23,438, which is in good agreement with the light chain of trastuzumab without any additional modifications (Fig. 7D).
FIGURE 7.
Glycosylation remodeling of herceptin (trastuzumab) using a pair of Endo-S2 enzymes (Endo-S2 WT and Endo-S2 D184M mutant). A, ESI-MS of the heavy chain of commercial trastuzumab (Herceptin); B, ESI-MS of the heavy chain of deglycosylated herceptin 12; C, ESI-MS of the heavy chain of transglycosylation product 13 (S2G2F-trastuzumab); D, ESI-MS of the light chain of transglycosylation product 13; E, ESI-MS of the heavy chain of transglycosylation product 13 after PNGase F catalyzed deglycosylation.
To confirm that the N-glycan was attached specifically to the Asn-297 N-glycosylation site of the Fc domain, instead of other sites of the polypeptide backbone that might occur by non-enzymatic reactions as implicated in a recent publication (30), we treated the transglycosylation product (13) with PNGase F and examined the protein portion by mass spectrometry analysis. PNGase F was highly specific and could release the N-glycans only when they were attached to the Asn side chain in an N-glycosylamide linkage at the conserved glycosylation site in N-glycoproteins. LC-MS analysis of the heavy chain of the PNGase F-treated transglycosylation product (13) gave a single species of 49,150 (deconvolution data), which corresponds to the polypeptide backbone of the heavy chain without any additional modifications (Fig. 7E). Taken together, these results clearly indicated that a single sialylated biantennary N-glycan was conjugated to the antibody heavy chain, and the intact N-glycan was attached at the conserved N-glycosylation site without any non-enzymatic glycations of the antibody. The highly efficient transformation catalyzed by the Endo-S2 glycosynthase mutants allowed much less reaction time and the use of much less excess of glycan oxazoline to achieve a quantitative conversion.
Discussion
We describe in this paper the discovery of a new class of glycosynthases derived from an endoglycosidase (Endo-S2) of the S. pyogenes M49 serotype, which showed broader substrate specificity and much more potent transglycosylation activity for antibody glycosylation remodeling than the previously reported glycosynthases, such as those derived from Endo-A, Endo-M, Endo-S, and Endo-F3. These findings were enabled by a systematic mutagenesis at the critical residue Asp-184, coupled with comparative analysis of the hydrolysis and transglycosylation activities of the resulting 19 mutants. Our experimental data also revealed a remarkable difference in both the hydrolysis and transglycosylation activities among the 19 mutants (Tables 1 and 2), which would be difficult to predict without this comparative study. Several notable mutants, including the D184M and D184Q mutants, were identified that showed high transglycosylation activity for glycosylation remodeling but retained only marginal hydrolysis activity.
Comparison of the hydrolysis and transglycosylation activities of the mutants reveals several interesting features. First, most of the mutants that showed high transglycosylation activity, including the D184C, D184G, D184E, D184Y, D184S, and D184A mutants, also possessed relatively high residual hydrolysis activity. An exception is the D184M mutant, which demonstrated remarkable transglycosylation activity but retained only residual hydrolysis activity, making it the most efficient glycosynthase for glycosylation remodeling. Second, most the mutants with the Asp-184 residue being replaced by amino acids with positively charged side chains (Lys, Arg, and His) or bulky hydrophobic side chains (Ile, Leu, Phe, and Trp) showed very low activities in both transglycosylation and hydrolysis. But, interestingly, the D184Y mutant retained the highest hydrolytic activity among all the mutants and also possessed a relatively high transglycosylation activity. A detailed understanding of the molecular mechanism behind these observations may rely on structural studies of the Endo-S2 mutants and their complex with respective substrates.
Another important discovery is the findings of the much broader substrate specificity of the Endo-S2-derived glycosynthase mutants than that of the previously reported glycosynthases. We found that the D184M and D184Q mutants, two notable Endo-S2 glycosynthases identified, were able to efficiently transfer all three major types of N-glycans including high-mannose type, complex type, and hybrid type, in antibody glycosylation remodeling. In addition, the Endo-S2 glycosynthases could recognize both core-fucosylated and non-fucosylated GlcNAc moiety at the Fc domain as an acceptor for transglycosylation. These findings significantly expand the scope of the glycosylation remodeling strategy. For example, the previously reported Endo-S and Endo-F3 are specific for complex type N-glycans and are unable to efficiently transfer high-mannose and hybrid type N-glycans, and the Endo-F3 is efficient only for core-fucosylated GlcNAc acceptor (32).
A direct comparison of the transglycosylation activity of the typical Endo-S2 and Endo-S glycosynthase mutants reveals that the Endo-S2 mutants are generally much more active than the corresponding Endo-S mutant. By an estimate of the initial rate, the D184Q mutant of Endo-S2 was at least 10-fold more active than the corresponding D233Q mutant of Endo-S, and the best Endo-S2 mutant, D184M, was estimated to be 100-fold better than the Endo-S D233Q mutant in glycosylating the deglycosylated rituximab (Fig. 6). Finally, in addition to the remodeling of rituximab, the highly efficient glycosylation remodeling of trastuzumab (Herceptin) by using a pair of Endo-S2 (the wild type and the D184M mutant) enzymes to give a single homogeneous glycoform without side reactions (Fig. 7) showcases the power of the newly discovered glycosynthases. It is expected that these highly efficient glycosynthases, which show also remarkably relaxed substrate specificity, will find a wide range of applications for producing various homogeneous glycoforms of antibodies for structural and functional studies, and for developing more effective antibody-based therapeutics as well.
Experimental Procedures
Materials
Monoclonal antibodies rituximab and trastuzumab (Herceptin) were products from Genentech Inc., (South San Francisco, CA). Asialo and sialoglycan complex type oxazoline was synthesized following the previously reported procedure (42). The high-mannose type (HM) glycan (Man9GlcNAc) was prepared from soybean flour by the previously described procedure (43). The synthesis of hybrid type (Hyb) glycan (Neu5AcGalGlcNAcMan5GlcNAc) was achieved by a sequential enzymatic glycosylation of the Man5GlcNAc (43) under the catalysis of a β1,2-GlcNAc transferase (GnT1) (44), a β1,4-galactosyltransferase (45), and an α2,6-sialyltransferase (46). The details of the enzymatic synthesis will be reported elsewhere.3 The HM and hybrid glycan oxazolines were synthesized following the previously described one-pot transformation procedure (42). Endo-S D233Q from S. pyogenes was overexpressed and purified following our previous procedure (25).
Site-directed Mutagenesis and Expression and Purification of Recombinant Endo-S2
cDNA encoding amino acids 38–819 of Endo-S2 from S. pyogenes NZ131 (serotype M49) was amplified by PCR and cloned into the pCPD-Lasso vector (a pET22b-CPD derivative) (35). For saturation mutagenesis of the Asp-184 residue, a forward primer, 5′-CGTAAATTCGTGCTCAATNNNAATATCTAGTCCATCGACACCACGATCAGTT-3′, and a reverse primer, 5′-AACTGATCGTGGTGTCGATGGACTAGATATTNNNATTGAGCACGAATTTACG-3′, were used. Mutations were confirmed by DNA sequencing. The plasmids containing mutated Endo-S2 genes were transformed into E. coli BL21(DE3). For simultaneous production of 20 Endo-S2 Asp-184 variants, the transformants were cultured in 20 ml of 2×YT broth media supplemented with 100 μg/ml of carbenicillin. Cultures were grown at 37 °C until the cells reached an A600 of 0.8–1.0. Then 0.5 mm isopropyl β-d-1-thiogalactopyranoside was added to the culture to induce protein overproduction at 20 °C. After 24 h the cells were harvested by centrifugation. The cell pellets were lysed by Bacterial Cell Lysis Buffer (Gold Biotechnology, Inc.) following the manufacturer's instructions. His10-tagged Endo-S2/CPD fusion proteins were purified with nickel-nitrilotriacetic acid spin columns (Qiagen). The purified Endo-S2 proteins were desalted into PBS (pH 7.4) using centrifugal diafiltration with Amicon Ultrafiltration (10 kDa, Millipore). The protein purity was confirmed by SDS-PAGE and the concentration was measured on a NanoDrop 2000c using absorbance at 280 nm. For large-scale purification of selected Endo-S2 variants, 1 liter of culture was used. The cell lysate was applied to a HisTrap HP column (GE Healthcare) and washed with PBS with 0.5 m NaCl and 20 mm imidazole (pH 7.4). Bound His-tagged protein was eluted with a gradient of 0–250 mm imidazole in PBS buffer. Eluted fractions containing Endo-S2 protein were pooled, concentrated, and further purified by size exclusion chromatography through a HiPrep 16/60 Sephacryl S-200 HR column (GE Healthcare).
Liquid Chromatography Mass Spectrometry (LC-ESI-MS) of IgG
The LC-MS analysis was performed on an Exactive Plus Orbitrap (Thermo Scientific). For intact antibody, the analysis was performed with a Waters XBridgeTM BEH300 C4 column (3.5 μm, 2.1 × 50 mm) with a linear gradient of 5–90% MeCN containing 0.1% formic acid within 9 min at a flow rate of 0.4 ml/min. For analysis of antibody light chain and heavy chain, the IgG antibody were treated with 50 mm tris(2-carboxyethyl)phosphine and heated at 37 °C for 20 min, then subjected to LC-MS analysis with an Agilent Poroshell 300SB-C8 column (5 μm, 75 × 1 mm). The analysis was performed at 60 °C eluting with a linear gradient of 25–35% MeCN containing 0.1% formic acid within 6 min at a flow rate of 0.40 ml/min. The LC-MS analysis of PNGase F-treated antibody glycoforms was performed in the same manner but included a 3-h incubation with PNGaese F prior to tris(2-carboxyethyl)phosphine treatment. Raw data were deconvoluted using MagTran (Amgen).
Deglycosylation of Rituximab by Wild Type Endo-S2 To Give (Fucα1,6)GlcNAc-Rituximab
Commercial rituximab in its original buffer was incubated with wild type Endo-S2 for 1 h at 37 °C with an antibody-to-enzyme ratio of 500:1 (by weight). LC-MS analyses indicated the complete cleavage of the N-glycans on the heavy chain. The deglycosylated rituximab was purified by protein A chromatography. LC-MS: calculated for the heavy chain of (Fucα1,6)GlcNAc-rituximab (2), M = 49,420 Da; found (m/z), 49,412 (deconvolution data).
Defucosylation of (Fucα1,6)GlcNAc-Rituximab by Bacterial α-Fucosidase
A solution of (Fucα1,6)GlcNAc-rituximab (2) in a Tris-HCl buffer (50 mm, pH 7.4) was incubated with the α-fucosidase AlfC from L. casei at 37 °C, with an antibody-to-enzyme ratio of 50:1. After 16 h incubation, LC-MS monitoring indicated the complete defucosylation of (Fucα1,6)GlcNAc-rituximab (2) to give the product, GlcNAc-rituximab (9). The defucosylated rituximab was purified by protein A chromatography. LC-MS: calculated for the heavy chain of GlcNAc-rituximab (9) carrying a GlcNAc moiety, M = 49,274 Da; found (m/z), 49,265 (deconvolution data).
Enzyme Assay
Assay for the hydrolysis activity of each Endo-S2 variant (0.1 μg) was performed at 30 °C with pure sialo-complex type (S2G2F) rituximab 3 (10 μg, 7.0 μm) as substrate in PBS buffer (pH 7.4, 10 μl). An aliquot of each reaction mixture was diluted in 0.1% formic acid to stop the reaction and analyzed by LC-MS. The relative amount of the substrate and the hydrolysis products were quantified after deconvolution of the raw data and integration of the corresponding MS peaks using MagTran. The transglycosylation reaction with synthetic sugar oxazoline was assayed as follows: (Fucα1,6)GlcNAc-rituximab (100 μg, 69 μm), and SCT-ox (1.38 mm, 20 eq) was incubated with 0.1 μg of each Endo-S2 variants at 30 °C in PBS (pH 7.4, 10 μl). The reactions were stopped and analyzed as in the hydrolysis assay stated above. Experiments of each mutant were repeated at least twice to ensure the consistency of the results.
Transglycosylation of Fuc1,6GlcNAc-Rituximab with SCT-ox, Hyb-ox, and HM-ox by Endo-S2 D184M: Synthesis of 3, 6, 8
A solution of Fuc1,6GlcNAc-Rituximab (1 mg, 69 μm) (2) and SCT-ox (1.38 mm, 20 eq) (4) was incubated with Endo-S2 D184M (5 μg) at 30 °C in 100 μl of 100 mm Tris buffer (pH 7.4) for 15 min. LC-MS analysis indicated the completion of the transglycosylation reaction. The product 3 was purified using protein A chromatography. LC-MS: calculated for the heavy chain of 3 carrying the fully sialylated biantennary N-glycan, M = 51,421 Da; found (m/z), 51,412 (deconvolution data).
A solution of Fuc1,6GlcNAc-Rituximab (1 mg, 69 μm) (2) and Hyb-ox (1.38 mm, 20 eq) (5) was incubated with Endo-S2 D184M (5 μg) at 30 °C in 100 μl of 100 mm Tris buffer (pH 7.4) for 30 min. Then another 10 eq of Hyb-ox (5) was added and the reaction was monitored by LC-MS of aliquots. When LC-MS analysis indicated the near completion of the transglycosylation reaction, the product 6 was purified using protein A chromatography. LC-MS: calculated for the heavy chain of 5 carrying the sialylated hybrid type N-glycan, M = 51,090 Da; found (m/z), 51,082 (deconvolution data).
A solution of Fuc1,6GlcNAc-Rituximab (1 mg, 69 μm) (2) and HM-ox (1.38 mm, 20 eq) (7) was incubated with Endo-S2 D184M (5 μg) at 30 °C in 100 μl of 100 mm Tris buffer (pH 7.4) for 30 min. Then another 10 eq of HM-ox (7) was added and the reaction was monitored by LC-MS of aliquots. When LC-MS analysis indicated the completion of the transglycosylation reaction, the product 8 was purified using protein A chromatography. LC-MS: calculated for the heavy chain of 12 carrying the high-mannose type (Man9GlcNAc2) N-glycan, M = 51,081 Da; found (m/z), 51,074 (deconvolution data).
Transglycosylation of GlcNAc-Rituximab with Complex Type-ox by Endo-S2 D184M: Synthesis of 11
A solution of GlcNAc-Rituximab (1 mg, 69 μm) (9) and CT-ox (1.38 mm, 20 eq) (10) was incubated with Endo-S2 D184M (5 μg) at 30 °C in 100 μl of 100 mm Tris buffer (pH 7.4) for 30 min. LC-MS analysis indicated the completion of the transglycosylation reaction. The product 11 was purified using protein A chromatography. LC-MS: calculated for the heavy chain of 11 carrying the biantennary N-glycan, M = 50,693 Da; found (m/z), 50,684 (deconvolution data).
Comparison of Transglycosylation Activity of Endo-S2 D184M Mutant Using SCT-, HM- and Hyb-oxazoline as Donor Substrates
In three separate reactions, Fuc1,6GlcNAc-Rituximab (0.2 mg, 69 μm) (2) together with SCT-ox (4), Hyb-ox (5), or HM-ox (7) (1.38 mm, 20 eq) was incubated with Endo-S2 D184M (1 μg) at 30 °C in 20 μl of 100 mm Tris buffer (pH 7.4). An aliquot of reaction (0.5 μl) was taken out at several time points and diluted in 0.1% formic acid to stop the reaction. All aliquots were analyzed by LC-MS and % transglycosylation was calculated from the deconvoluted data.
Comparison of Transglycosylation Activity of Endo-S2 D184M and D184Q Mutants, and Endo-S D233Q Mutant Using SCT-oxazoline as the Donor Substrate
In three separate reactions, Fuc1,6GlcNAc-Rituximab (0.2 mg, 69 μm) (2) and SCT-ox (4) (1.38 mm, 20 eq) were incubated with Endo-S2 D184M, Endo-S2 D184Q, or Endo-S D233Q (1 μg) at 30 °C in 20 μl of 100 mm Tris buffer (pH 7.4). An aliquot of reaction (0.5 μl) was taken out at several time points and diluted in 0.1% formic acid to stop the reaction. All aliquots were analyzed by LC-MS and % transglycosylation was calculated from the deconvoluted data.
Transglycosylation of Fuc1,6GlcNAc-trastuzumab with SCT-ox by Endo-S2 D184M
Commercial trastuzumab (lyophilized powder) was dissolved in water and deglycosylated with wild type Endo-S2 following the same method for rituximab. For transglycosylation, a solution of Fuc1,6GlcNAc-trastuzumab (12) (1 mg, 69 μm) and SCT-ox (4) (1.38 mm, 20 eq) was incubated with Endo-S2 D184M (5 μg) at 30 °C in 100 μl of 100 mm Tris buffer (pH 7.4) for 15 min. LC-MS analysis indicated the completion of the transglycosylation reaction. The product 13 was purified using protein A chromatography.
Author Contributions
L. X. W. conceived the idea and supervised the study; T. L., X. T., Q. Y., and J. P. G. performed the experiments; T. L., X. T., Q. Y., J. P. G., and L. X. W. analyzed the experimental data; T. L., X. T., and L. X. W. wrote and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank members of the Wang lab for technical assistance and helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 GM080374 and R01 GM096973 (to L. X. W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
T. Li and L-X. Wang, unpublished data.
- Endo-A
- endo-β-N-acetylglucosaminidase from Arthrobacter
- CPD
- cysteine protease domain
- CT
- complex type
- Endo-D
- endo-β-N-acetylglucosaminidase D from S. pneumoniae
- Endo-F3
- endo-β-N-acetylglucosaminidase F3 from Elizabethkingia miricola
- Endo-M
- endo-β-N-acetylglucosaminidase from Mucor hiemalis
- Endo-S
- the endo-β-N-acetylglucosaminidase from S. pyogenes
- Endo-S2
- the endo-β-N-acetylglucosaminidase from S. pyogenes of serotype M49
- ESI-MS
- electron spray ionization-mass spectrometry
- GlcNAc
- N-acetylglucosamine
- HM
- high-mannose type
- Hyb
- hybrid type
- Neu5Ac
- N-acetyl neuraminic acid
- PNGase F
- peptide-N-glycosidase F
- SCT
- sialylated biantennary complex type
- ox
- oxazoline.
References
- 1. Adams G. P., and Weiner L. M. (2005) Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23, 1147–1157 [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal S. R. (2012) What's fueling the biotech engine-2011 to 2012. Nat. Biotechnol. 30, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal S. R. (2014) A survey of breakthrough therapy designations. Nat. Biotechnol. 32, 323–330 [DOI] [PubMed] [Google Scholar]
- 4. Jefferis R. (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 [DOI] [PubMed] [Google Scholar]
- 5. Dalziel M., Crispin M., Scanlan C. N., Zitzmann N., and Dwek R. A. (2014) Emerging principles for the therapeutic exploitation of glycosylation. Science 343, 1235681. [DOI] [PubMed] [Google Scholar]
- 6. van de Bovenkamp F. S., Hafkenscheid L., Rispens T., and Rombouts Y. (2016) The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 196, 1435–1441 [DOI] [PubMed] [Google Scholar]
- 7. Le N. P., Bowden T. A., Struwe W. B., and Crispin M. (2016) Immune recruitment or suppression by glycan engineering of endogenous and therapeutic antibodies. Biochim. Biophys. Acta 1860, 1655–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niwa R., Shoji-Hosaka E., Sakurada M., Shinkawa T., Uchida K., Nakamura K., Matsushima K., Ueda R., Hanai N., and Shitara K. (2004) Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 64, 2127–2133 [DOI] [PubMed] [Google Scholar]
- 9. Illidge T., Cheadle E. J., Donaghy C., and Honeychurch J. (2014) Update on obinutuzumab in the treatment of B-cell malignancies. Expert Opin. Biol. Ther. 14, 1507–1517 [DOI] [PubMed] [Google Scholar]
- 10. Kaneko Y., Nimmerjahn F., and Ravetch J. V. (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 [DOI] [PubMed] [Google Scholar]
- 11. Anthony R. M., Nimmerjahn F., Ashline D. J., Reinhold V. N., Paulson J. C., and Ravetch J. V. (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwab I., Mihai S., Seeling M., Kasperkiewicz M., Ludwig R. J., and Nimmerjahn F. (2014) Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur. J. Immunol. 44, 1444–1453 [DOI] [PubMed] [Google Scholar]
- 13. Washburn N., Schwab I., Ortiz D., Bhatnagar N., Lansing J. C., Medeiros A., Tyler S., Mekala D., Cochran E., Sarvaiya H., Garofalo K., Meccariello R., Meador J. W. 3rd, Rutitzky L., Schultes B. C., et al. (2015) Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc. Natl. Acad. Sci. U.S.A. 112, E1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Umaña P., Jean-Mairet J., Moudry R., Amstutz H., and Bailey J. E. (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 [DOI] [PubMed] [Google Scholar]
- 15. Yamane-Ohnuki N., Kinoshita S., Inoue-Urakubo M., Kusunoki M., Iida S., Nakano R., Wakitani M., Niwa R., Sakurada M., Uchida K., Shitara K., and Satoh M. (2004) Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 87, 614–622 [DOI] [PubMed] [Google Scholar]
- 16. Stanley P., Sundaram S., Tang J., and Shi S. (2005) Molecular analysis of three gain-of-function CHO mutants that add the bisecting GlcNAc to N-glycans. Glycobiology 15, 43–53 [DOI] [PubMed] [Google Scholar]
- 17. Cox K. M., Sterling J. D., Regan J. T., Gasdaska J. R., Frantz K. K., Peele C. G., Black A., Passmore D., Moldovan-Loomis C., Srinivasan M., Cuison S., Cardarelli P. M., and Dickey L. F. (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat. Biotechnol. 24, 1591–1597 [DOI] [PubMed] [Google Scholar]
- 18. Strasser R., Castilho A., Stadlmann J., Kunert R., Quendler H., Gattinger P., Jez J., Rademacher T., Altmann F., Mach L., and Steinkellner H. (2009) Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous β1,4-galactosylated N-glycan profile. J. Biol. Chem. 284, 20479–20485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H., Sethuraman N., Stadheim T. A., Zha D., Prinz B., Ballew N., Bobrowicz P., Choi B. K., Cook W. J., Cukan M., Houston-Cummings N. R., Davidson R., Gong B., Hamilton S. R., Hoopes J. P., Jiang Y., et al. (2006) Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24, 210–215 [DOI] [PubMed] [Google Scholar]
- 20. Zhou Q., Shankara S., Roy A., Qiu H., Estes S., McVie-Wylie A., Culm-Merdek K., Park A., Pan C., and Edmunds T. (2008) Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol. Bioeng. 99, 652–665 [DOI] [PubMed] [Google Scholar]
- 21. Wang L. X., and Amin M. N. (2014) Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions. Chem. Biol. 21, 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei Y., Li C., Huang W., Li B., Strome S., and Wang L. X. (2008) Glycoengineering of human IgG1-Fc through combined yeast expression and in vitro chemoenzymatic glycosylation. Biochemistry 47, 10294–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou G., Ochiai H., Huang W., Yang Q., Li C., and Wang L. X. (2011) Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain: presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J. Am. Chem. Soc. 133, 18975–18991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan S. Q., Huang W., and Wang L. X. (2012) Remarkable transglycosylation activity of glycosynthase mutants of Endo-D, an endo-β-N-acetylglucosaminidase from Streptococcus pneumoniae. J. Biol. Chem. 287, 11272–11281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang W., Giddens J., Fan S. Q., Toonstra C., and Wang L. X. (2012) Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 134, 12308–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quast I., Keller C. W., Maurer M. A., Giddens J. P., Tackenberg B., Wang L. X., Münz C., Nimmerjahn F., Dalakas M. C., and Lünemann J. D. (2015) Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 125, 4160–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giddens J. P., and Wang L. X. (2015) Chemoenzymatic glyco-engineering of monoclonal antibodies. Methods Mol. Biol. 1321, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin C. W., Tsai M. H., Li S. T., Tsai T. I., Chu K. C., Liu Y. C., Lai M. Y., Wu C. Y., Tseng Y. C., Shivatare S. S., Wang C. H., Chao P., Wang S. Y., Shih H. W., Zeng Y. F., et al. (2015) A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl. Acad. Sci. U.S.A. 112, 10611–10616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurogochi M., Mori M., Osumi K., Tojino M., Sugawara S., Takashima S., Hirose Y., Tsukimura W., Mizuno M., Amano J., Matsuda A., Tomita M., Takayanagi A., Shoda S., and Shirai T. (2015) Glycoengineered monoclonal antibodies with homogeneous glycan (M3, G0, G2, and A2) using a chemoenzymatic approach have different affinities for FcγRIIIa and variable antibody-dependent cellular cytotoxicity activities. PLoS ONE 10, e0132848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parsons T. B., Struwe W. B., Gault J., Yamamoto K., Taylor T. A., Raj R., Wals K., Mohammed S., Robinson C. V., Benesch J. L., and Davis B. G. (2016) Optimal synthetic glycosylation of a therapeutic antibody. Angew. Chem. Int. Ed. Engl. 55, 2361–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu R., Giddens J., McClung C. M., Magnelli P. E., Wang L. X., and Guthrie E. P. (2016) Evaluation of a glycoengineered monoclonal antibody via LC-MS analysis in combination with multiple enzymatic digestion. MAbs 8, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giddens J. P., Lomino J. V., Amin M. N., and Wang L. X. (2016) Endo-F3 glycosynthase mutants enable chemoenzymatic synthesis of core-fucosylated triantennary complex type glycopeptides and glycoproteins. J. Biol. Chem. 291, 9356–9370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sjögren J., Struwe W. B., Cosgrave E. F., Rudd P. M., Stervander M., Allhorn M., Hollands A., Nizet V., and Collin M. (2013) EndoS2 is a unique and conserved enzyme of serotype M49 group A Streptococcus that hydrolyses N-linked glycans on IgG and α1-acid glycoprotein. Biochem. J. 455, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sjögren J., Cosgrave E. F., Allhorn M., Nordgren M., Björk S., Olsson F., Fredriksson S., and Collin M. (2015) EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology 25, 1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen A., Lupardus P. J., Morell M., Ponder E. L., Sadaghiani A. M., Garcia K. C., and Bogyo M. (2009) Simplified, enhanced protein purification using an inducible, autoprocessing enzyme tag. PloS One 4, e8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang W., Li C., Li B., Umekawa M., Yamamoto K., Zhang X., and Wang L. X. (2009) Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc. 131, 2214–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Umekawa M., Huang W., Li B., Fujita K., Ashida H., Wang L. X., and Yamamoto K. (2008) Mutants of Mucor hiemalis endo-β-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J. Biol. Chem. 283, 4469–4479 [DOI] [PubMed] [Google Scholar]
- 38. Umekawa M., Li C., Higashiyama T., Huang W., Ashida H., Yamamoto K., and Wang L. X. (2010) Efficient glycosynthase mutant derived from Mucor hiemalis endo-β-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem. 285, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., and Watier H. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 [DOI] [PubMed] [Google Scholar]
- 40. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., and Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 41. Rodríguez-Díaz J., Monedero V., and Yebra M. J. (2011) Utilization of natural fucosylated oligosaccharides by three novel α-l-fucosidases from a probiotic Lactobacillus casei strain. Appl. Environ. Microbiol. 77, 703–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang W., Yang Q., Umekawa M., Yamamoto K., and Wang L. X. (2010) Arthrobacter endo-β-N-acetylglucosaminidase shows transglycosylation activity on complex-type N-glycan oxazolines: one-pot conversion of ribonuclease B to sialylated ribonuclease C. ChemBioChem 11, 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang L. X., Ni J., Singh S., and Li H. (2004) Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: implications for HIV-1 vaccine design. Chem. Biol. 11, 127–134 [DOI] [PubMed] [Google Scholar]
- 44. Saribas A. S., Johnson K., Liu L., Bezila D., and Hakes D. (2007) Refolding of human β-1–2 GlcNAc transferase (GnT1) and the role of its unpaired Cys 121. Biochem. Biophys. Res. Commun. 362, 381–386 [DOI] [PubMed] [Google Scholar]
- 45. Park J. E., Lee K. Y., Do S. I., and Lee S. S. (2002) Expression and characterization of β-1,4-galactosyltransferase from Neisseria meningitidis and Neisseria gonorrhoeae. J. Biochem. Mol. Biol. 35, 330–336 [DOI] [PubMed] [Google Scholar]
- 46. Yu H., Huang S., Chokhawala H., Sun M., Zheng H., and Chen X. (2006) Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural α-2,6-linked sialosides: a P. damsela α-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. Engl. 45, 3938–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]