Abstract
Transmembrane adaptor proteins are molecules specialized in recruiting cytoplasmic proteins to the proximity of the cell membrane as part of the signal transduction process. A member of this family, SLP65/SLP76, Csk-interacting membrane protein (SCIMP), recruits a complex of SLP65/SLP76 and Grb2 adaptor proteins, known to be involved in the activation of PLCγ1/2, Ras, and other pathways. SCIMP expression is restricted to antigen-presenting cells. In a previous cell line-based study, it was shown that, in B cells, SCIMP contributes to the reverse signaling in the immunological synapse, downstream of MHCII glycoproteins. There it mainly facilitates the activation of ERK MAP kinases. However, its importance for MHCII glycoprotein-dependent ERK signaling in primary B cells has not been analyzed. Moreover, its role in macrophages and dendritic cells has remained largely unknown. Here we present the results of our analysis of SCIMP-deficient mice. In these mice, we did not observe any defects in B cell signaling and B cell-dependent responses. On the other hand, we found that, in dendritic cells and macrophages, SCIMP expression is up-regulated after exposure to GM-CSF or the Dectin-1 agonist zymosan. Moreover, we found that SCIMP is strongly phosphorylated after Dectin-1 stimulation and that it participates in signal transduction downstream of this important pattern recognition receptor. Our analysis of SCIMP-deficient dendritic cells revealed that SCIMP specifically contributes to sustaining long-term MAP kinase signaling and cytokine production downstream of Dectin-1 because of an increased expression and sustained phosphorylation lasting at least 24 h after signal initiation.
Keywords: dendritic cell, ERK, innate immunity, p38, pattern recognition receptor (PRR), phosphotyrosine signaling, signal transduction, Dectin-1, SCIMP
Introduction
Dectin-1 is a pattern recognition receptor from the C-type lectin receptor family (1). It is expressed in macrophages, dendritic cells, neutrophils, and a subset of T and B cells (2–4). Through its carbohydrate recognition domain, it specifically recognizes β-1,3-glucan (5), which is a typical component of fungal cell walls (6). Dectin-1 is considered a major receptor for β-glucans and plays an important role in the defense against various species of pathogenic fungi in mice, including Candida albicans, Aspergillus fumigatus, and Pneumocystis carinii (7–11). The importance of dectin-1 for antifungal defense has also been demonstrated by studies of human patients with disrupted dectin-1 function who display increased mucosal colonization with Candida species and suffer from recurrent mucocutaneous fungal infections (12, 13).
Dectin-1 signaling is initiated by phosphorylation of the hemITAM motif in its intracellular tail, leading to the recruitment and activation of the protein tyrosine kinase Syk. This is followed by sequential activation of PLCγ2 and PKCδ. Stimulation of this pathway as well as of additional Syk-independent pathways results in the activation of the transcription factors NF-κB, nuclear factor of activated T cells (NFAT), and IRF1/5 and initiation of signaling by the MAP kinases ERK, p38, and JNK, which then contribute to downstream cellular responses (14–16). Activation of Dectin-1 leads to phagocytosis of fungi or any other β-glucan-containing particles. In addition, it also triggers the production of reactive oxygen species and proinflammatory cytokines (7, 17, 18). Cytokines produced in response to Dectin-1 stimulation also promote Th1 and Th17 polarization of helper T cells necessary for defeating fungal infection (14–16). Interestingly, only β-glucan in the form of particles can elicit the full activity of Dectin-1, whereas soluble β-glucans, which also bind to the receptor, lack strong activating properties and can inhibit the responses to particulate β-glucan (19). The difference is thought to be caused by the ability of particulate β-glucan to induce the formation of a phagocytic synapse that excludes CD45 and CD148 phosphatases (19).
As any important receptor, Dectin-1 is tightly regulated. This regulation occurs not only at the level of signaling pathways but also at the level of expression. Dectin-1 is highly up-regulated after IL-4, IL-13, and GM-CSF treatment, whereas IL-10, LPS, and dexamethasone down-regulate its expression (20).
To elicit the full antifungal immune response, Dectin-1 cooperates with several TLRs4 (most importantly TLR2) (17). Its function is also complemented by other C-type lectin receptors, such as Dectin-2, which recognizes mannan structures in fungal cell walls (1). In addition, Dectin-1 interacts with tetraspanin molecules, which form the basis of tetraspanin-enriched microdomains and were suggested to be involved in Dectin-1 trafficking (21–23). However, the effects of tetraspanins on Dectin-1 signal transduction are at present unclear.
Tetraspanin-enriched microdomains in some Dectin-1-expressing cells also interact with MHCII glycoproteins (MHCIIgp)and a small palmitoylated transmembrane adaptor protein, SCIMP (23–25). Expression of SCIMP is highly specific for the tissues of the immune system, where it is confined to the professional antigen-presenting cells (dendritic cells, B cells, and macrophages). In B cells, SCIMP is phosphorylated after MHCIIgp cross-linking, and it is thought to be involved in the reverse signaling at the APC side of the immunological synapse. In the K46 B cell line, it was shown to be mainly responsible for supporting ERK signaling upon MHCIIgp stimulation (24).
The SCIMP molecule has four potential tyrosine phosphorylation sites. When phosphorylated, it binds Grb2, SLP-65, or SLP-76 and Csk via their Src homology 2 (SH2) domains. Through a proline-rich sequence, SCIMP is constitutively associated with the Src family kinase Lyn. Despite the interaction with a negative regulator of the Src family kinases Csk, SCIMP plays an overall positive regulatory function mediated by the recruitment of the Grb2·SLP-65 complex, whereas Csk binding seems to be only responsible for negative feedback regulation of this process (24, 25).
Here we have investigated SCIMP function in vivo using a SCIMP-deficient mouse model. Although we did not observe any effects of SCIMP deficiency on MHCIIgp signaling, we found that it is involved in the signaling by Dectin-1 in dendritic cells and macrophages, where it is important for sustaining prolonged MAP kinase activity and pro-inflammatory cytokine production.
Results
Normal Leukocyte Development and Distribution in SCIMP-deficient Mice
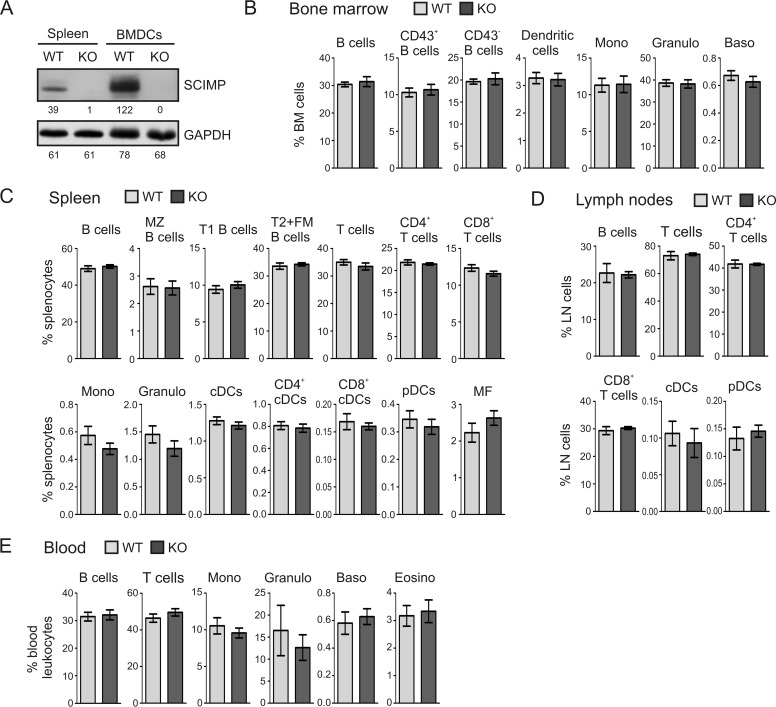
To determine the physiological function of SCIMP, we obtained the SCIMP-deficient mouse strain Scimptm1a(KOMP)Wtsi (hereafter termed Scimp−/−) from the International Knockout Mouse Consortium (for details, see “Experimental Procedures”). First we verified SCIMP deficiency at the protein level. As expected, there was no detectable SCIMP protein present in the lysates of Scimp−/− splenocytes and dendritic cells (Fig. 1A).
FIGURE 1.
Verification of SCIMP protein deficiency and leukocyte subset analysis in Scimp−/− mice. A, lysates of splenocytes and BMDCs prepared from WT and Scimp−/− (KO) mice were probed for the presence of SCIMP and GAPDH (loading control) by Western blotting. B–E, percentages of major leukocyte subsets in the bone marrows (B), spleens (C), lymph nodes (D), and peripheral blood (E) of WT and Scimp−/− mice determined by flow cytometry. The individual subpopulations were gated as follows. Bone marrow: B cells (B220+), CD43+ B cells (B220+, CD43+), CD43− B cells (B220+, CD43−), dendritic cells (F4/80−, Ly6C−, CD11blow, CD11chi), monocytes (Mono, F4/80−, CD11b+, Gr1low, Ly6Chi), granulocytes (Granulo, F4/80−, Ly6C+, Gr1hi), and basophils (Baso, ckit−, CD49b+, FcϵR+). Spleen and lymph nodes: B cells (CD19+), MZ B cells (B220+, IgMhi, IgD−, CD1d+), T1 B cells (B220+, IgM+, IgD−), T2+FM B cells (B220+, IgD+), T cells (CD3+), CD4 T cells (CD3+, CD4+), CD8 T cells (CD3+, CD8+), monocytes (F4/80−, CD11b+, Gr1low, Ly6Chi), granulocytes (CD3−, CD19−, CD11b+, Gr1hi), classical dendritic cells (CD11c+, Ly6C−), CD4+ classical dendritic cells (CD11c+, Ly6C−,CD4+), CD8+ classical dendritic cells (CD11c+, Ly6C−,CD8+), plasmacytoid dendritic cells (CD11clow, Ly6C+, B220+), and macrophages (CD11blow, F4/80+). Peripheral blood: B cells (CD19+), T cells (CD3+), monocytes (SSAlow, CD11b+), granulocytes (CD11b+, LyGhi, Ly6Clow), basophils (ckit−, CD49b+, FcϵRIα+), and eosinophils (Eosino, CD11b+, Ly6Clow, Ly6G−, SSAhi).
Next, we assessed leukocyte development and leukocyte subset representation in lymphoid organs obtained from SCIMP-deficient mice. Bone marrows, spleens, lymph nodes, and peripheral blood were isolated from 6- to 8-week-old wild-type and Scimp−/− animals. After preparation of single cell suspensions, a multiparametric flow cytometry analysis was carried out. This analysis determined the percentages of T cells, B cells, and their subpopulations as well as the representation of major myeloid cell subsets. However, Scimp−/− mice did not show any statistically significant differences from wild-type animals under steady-state conditions (Fig. 1, B–E).
Normal Function of SCIMP-deficient B Cells
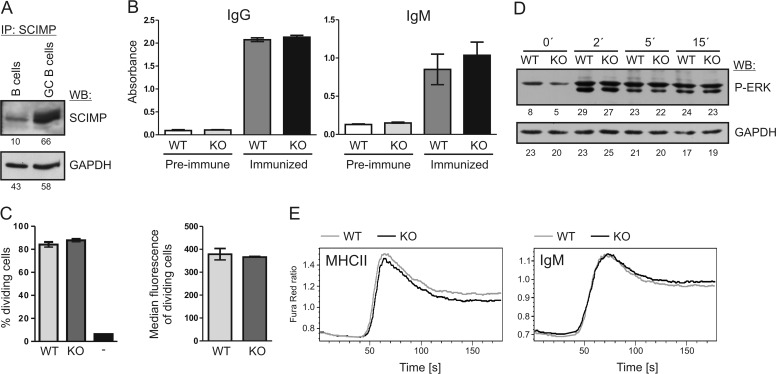
In B cells, SCIMP was shown to be involved in signal transduction downstream of MHCIIgp (24). Moreover, we found that GL7+ germinal center B cells express higher levels of SCIMP than naïve B cells (Fig. 2A). This suggests that SCIMP may participate in signaling in B cells during the process of obtaining antigen-specific T cell help in the germinal centers of lymphoid follicles. This eventually results in B cell differentiation into plasma cells and secretion of specific antibodies. To test the effect of SCIMP deficiency on this process, we immunized wild-type and Scimp−/− mice intradermally with ovalbumin. After the immunization, we collected the serum from immunized animals and measured antigen-specific IgM and IgG by ELISA. Although there was a significant increase in immunoglobulin production after immunization, there were no significant differences between wild-type and Scimp−/− animals in the detected levels of antigen-specific IgM or IgG (Fig. 2B). Similar results were also obtained when we used different antigen (a small recombinant fragment from ARHGEF4 protein, data not shown).
FIGURE 2.
Normal responses of Scimp−/− B cells. A, sorted naïve mouse B cells from control mouse or GL7+ germinal center (GC) B cells from sheep red blood cell-immunized mice were lysed in SDS-PAGE sample buffer and probed for SCIMP protein by immunoblotting. GAPDH staining served as a loading control. IP, immunoprecipitation; WB, Western blotting. B, sera from WT (n = 5) or Scimp−/− (KO, n = 6) mice immunized intradermally with ovalbumin in incomplete Freund adjuvant were collected, and immunoglobulins specific to ovalbumin were detected by ELISA. As a control, preimmune sera from the same mice were used. C, proliferation of OTII transgenic T cells labeled with CFSE was measured after 48 h co-culture with NP-ovalbumin-fed B1–8i transgenic B cells (WT or Scimp−/−) by flow cytometry. T cells cultured alone served as a negative control (−). Percentages of dividing cells and median CFSE fluorescence of cells that underwent at least one division are shown. D, ERK1/2 phosphorylation after MHCIIgp cross-linking in WT and Scimp−/− primary splenic B cells was analyzed by immunoblotting. E, splenocytes isolated from WT and Scimp−/− mice were stained with APC-conjugated anti-CD3 and anti-CD11b antibodies. The increase in calcium flux after MHCIIgp or IgM cross-linking was evaluated in B cells (gated as CD3− CD11b−) using a Fura Red calcium-sensitive probe.
Another option was that SCIMP may support the function of B cells as antigen-presenting cells. To test this, we performed a B cell antigen presentation assay. Scimp−/− and wild-type mice were crossed with the transgenic mouse strain B1–8i expressing B cell antigen receptor (BCR) specific for 4-hydroxy-3-nitrophenylacetyl (NP) hapten. Then we isolated B cells from these mice and fed them with NP-ovalbumin to allow its processing and presentation on MHCII molecules. Next we tested the ability of these B cells to stimulate OTII T cells expressing transgenic T cell antigen receptor (TCR) specific for ovalbumin peptide. The proliferation and activation of antigen-specific T cells were measured by flow cytometry (Fig. 2C). Similar to the previous experiment, we did not observe any differences between WT and Scimp−/− B cells in their ability to activate antigen-specific T cells.
Finally, we also tested signaling downstream of MHCIIgp in SCIMP-deficient B cells. Specifically, we analyzed ERK MAP kinase activation triggered by MHCIIgp cross-linking, which was shown previously to be reduced in the K46 B cell line after SCIMP down-regulation (24). However, in contrast to the K46 B cell line, murine purified B cells isolated from Scimp−/− spleen did not show any defects in ERK phosphorylation when compared with wild-type B cells (Fig. 2D). Moreover, calcium responses after MHCIIgp and IgM cross-linking were also not altered (Fig. 2E). This lack of differences at the single cell level in vitro may explain the lack of differences during the antigen presentation assay and antigen-specific antibody response in vivo.
SCIMP Expression in Myeloid Cells
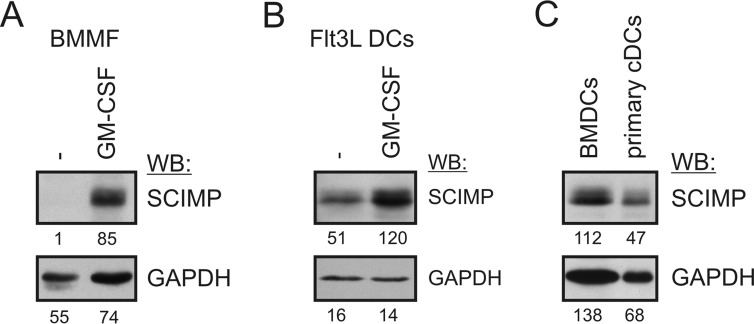
SCIMP is expressed not only in the B cell lineage but also in myeloid cells, such as dendritic cells and macrophages. Strong expression of SCIMP was observed in dendritic cells differentiated from human monocytes using GM-CSF- and IL-4-supplemented medium (24). Interestingly, in bone marrow-derived macrophages (BMMFs), SCIMP expression is relatively low. However, it can be strongly up-regulated after GM-CSF treatment (Fig. 3A). Therefore, we wanted to find out whether SCIMP expression in BMDCs prepared by culturing bone marrow cells in GM-CSF-containing medium is caused by the presence of GM-CSF or whether it is their intrinsic quality. To test this, we used another method of BMDC generation from bone marrow cells that employs Flt3L instead of GM-CSF. As Fig. 3B shows, SCIMP is expressed even in BMDCs differentiated by Flt3L, although it is still further up-regulated after GM-CSF exposure (Fig. 3B).
FIGURE 3.
Expression of SCIMP and its regulation in dendritic cells and macrophages. A, BMMFs were differentiated from bone marrow cells for 7 days in M-CSF-containing medium. On day 7, the medium was replaced with GM-CSF-containing medium, and after an additional 24 h, cells were lysed in SDS-PAGE sample buffer. SCIMP was detected by immunoblotting. WB, Western blotting. B, dendritic cells were generated using Flt3L- and stem cell factor-containing medium. On day 8, plasmacytoid dendritic cells were removed using anti-mPDCA-1 magnetic beads, and the rest of the dendritic cells were cultured for 24 h in GM-CSF-containing medium followed by analysis of SCIMP expression by immunoblotting. C, splenic cDCs (CD11c+, B220−, Ly6C−) were sorted from the spleens of wild-type mice, lysed, and subjected to immunoblotting with SCIMP and GAPDH antibodies. BMDCs served as a positive control.
Finally, to assess SCIMP expression in dendritic cells in vivo, we sorted classical dendritic cells from murine spleens and analyzed SCIMP expression in these cells by Western blotting. These results clearly showed the presence of SCIMP in primary DCs (Fig. 3C). From these results we can conclude that SCIMP is expressed in dendritic cells in vitro and in vivo and that its expression is enhanced by the presence of GM-CSF.
SCIMP Is Phosphorylated After Stimulation of Dendritic Cells and Macrophages with Zymosan
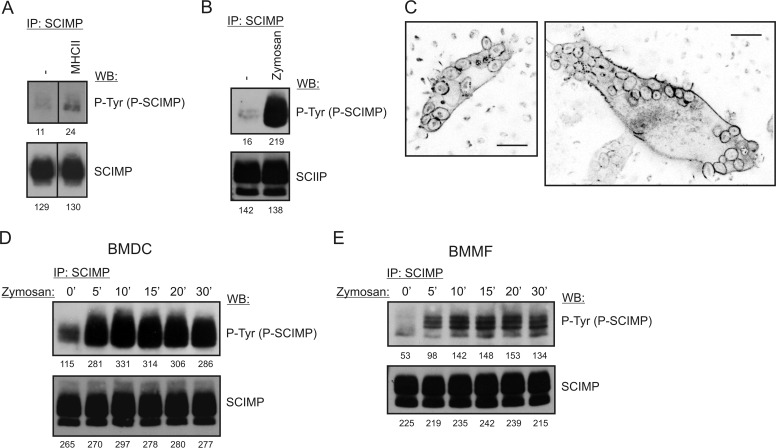
Because of the strong SCIMP expression in BMDCs, we decided to search for its function in this cell type. Surprisingly, we observed only a marginal increase in SCIMP phosphorylation after MHCIIgp cross-linking on the surface of these cells (Fig. 4A). Thus we hypothesized that, in BMDCs, SCIMP acts downstream of a different receptor. Dectin-1 was a very good candidate because, similar to SCIMP, it is present in tetraspanin-enriched microdomains (24), and it is up-regulated in the presence of GM-CSF. We used zymosan as a well established Dectin-1 activator. Zymosan is a preparation of small particles rich in the Dectin-1 ligand β-glucan, prepared from the yeast Saccharomyces cerevisiae (26). Strikingly, treatment of BMDCs with zymosan resulted in a strong increase of SCIMP phosphorylation (Fig. 4B). Moreover, SCIMP localized into zymosan-containing phagosomes and remained there for at least 30 min (Fig. 4C and supplemental Movie 1). SCIMP phosphorylation was very stable, with only a minor reduction during the 30-min experiment (Fig. 4D). A similar observation was also made with BMMFs (Fig. 4E).
FIGURE 4.
SCIMP is phosphorylated in response to zymosan stimulation. A, SCIMP phosphorylation in SCIMP immunoprecipitates (IP) prepared from BMDCs stimulated for 5 min by MHCIIgp cross-linking. WB, Western blotting. B, SCIMP phosphorylation in SCIMP immunoprecipitates prepared from BMDCs stimulated for 5 min with 300 μg/ml zymosan. C, localization of SCIMP-YFP into zymosan-containing phagosomes in BMDCs retrovirally transduced with a SCIMP-YFP coding construct. Zymosan is visible because of its strong autofluorescence. Scale bars = 10 μm. D, sustained SCIMP phosphorylation in SCIMP immunoprecipitates prepared from BMDCs stimulated for the indicated times with 300 μg/ml zymosan. E, sustained SCIMP phosphorylation in SCIMP immunoprecipitates prepared from BMMFs stimulated for the indicated times with 300 μg/ml zymosan.
SCIMP Is Involved in Dectin-1 Signaling
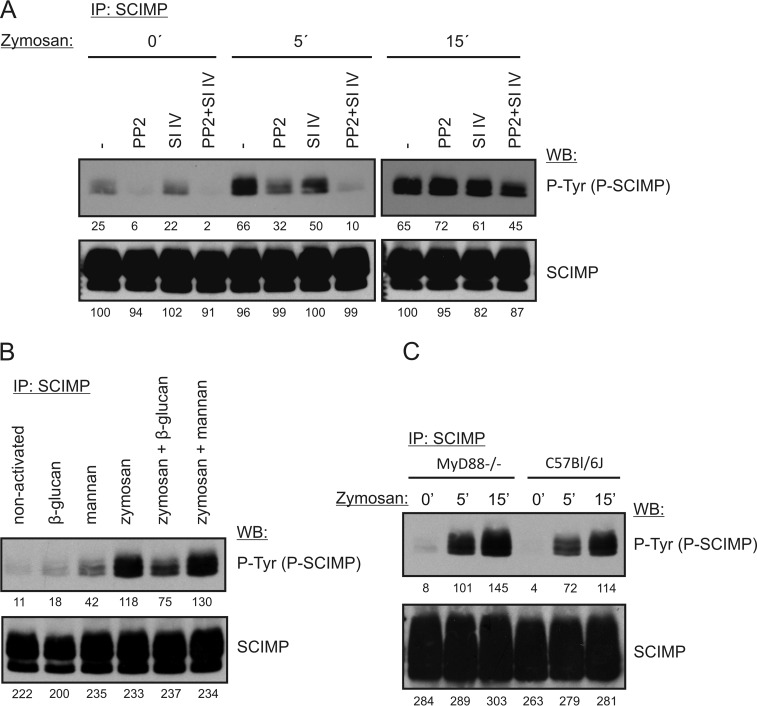
Dectin-1 signal transduction is initiated by Src family kinases and Syk (15). To test which kinases are responsible for SCIMP phosphorylation after zymosan treatment, we stimulated BMDCs with zymosan in the presence of specific inhibitors of Src family kinases and Syk. The results suggested that both Src family and Syk kinases are required for SCIMP phosphorylation after zymosan treatment because SCIMP phosphorylation was most profoundly reduced when both Src family kinase (PP2) and Syk (Syk inhibitor IV) inhibitors were combined (Fig. 5A).
FIGURE 5.
SCIMP is part of the Dectin-1 signaling pathway. A, BMDCs were incubated with 10 μm PP2 or 2 μm Syk inhibitor IV (SI IV) or a combination of them for 5 min. Next, 300 μg/ml zymosan was added, and cells were incubated in its presence for the indicated times, lysed, and subjected to SCIMP immunoprecipitation (IP) followed by immunoblotting with phosphotyrosine (P-Tyr) and SCIMP antibodies. WB, Western blotting. B, BMDCs were incubated at 37 °C with 600 μg/ml mannan or 600 μg/ml soluble β-glucan alone or in combination with 300 μg/ml zymosan. The level of SCIMP phosphorylation was detected as in A. C, MyD88-deficient or wild-type BMDCs were activated with 300 μg/ml zymosan for 5 or 15 min, and SCIMP phosphorylation was detected as in A.
The composition of zymosan is relatively complex. In addition to β-glucan, it also contains mannans (ligands of Dectin-2) and TLR2 ligands (26, 27). To confirm Dectin-1 involvement in the induction of SCIMP phosphorylation, we exploited the much stronger responsiveness of Dectin-1 to particulate β-glucan than to soluble β-glucan as a unique feature of this receptor. As a result, many aspects of Dectin-1 signaling after stimulation with particulate β-glucan can be inhibited by soluble forms of β-glucan (19). Thus, in the next experiment, we stimulated BMDCs with zymosan particles in the presence or absence of soluble β-glucan. Indeed, treatment with soluble β-glucan substantially inhibited SCIMP phosphorylation, whereas soluble mannan had no effect (Fig. 5B), suggesting that SCIMP is indeed a part of the Dectin-1 signaling pathway. To further support this conclusion, we investigated SCIMP phosphorylation in MyD88-deficient BMDCs, which have impaired function of multiple TLRs, including TLR2, the main TLR activated by zymosan (17, 28, 29). SCIMP phosphorylation was not affected by the disruption of TLR signaling (Fig. 5C). Based on these experiments, we concluded that Dectin-1 is responsible for inducing SCIMP phosphorylation after zymosan treatment.
SCIMP Is Important for Sustained MAP Kinase Signaling and Cytokine Production After Stimulation with Zymosan
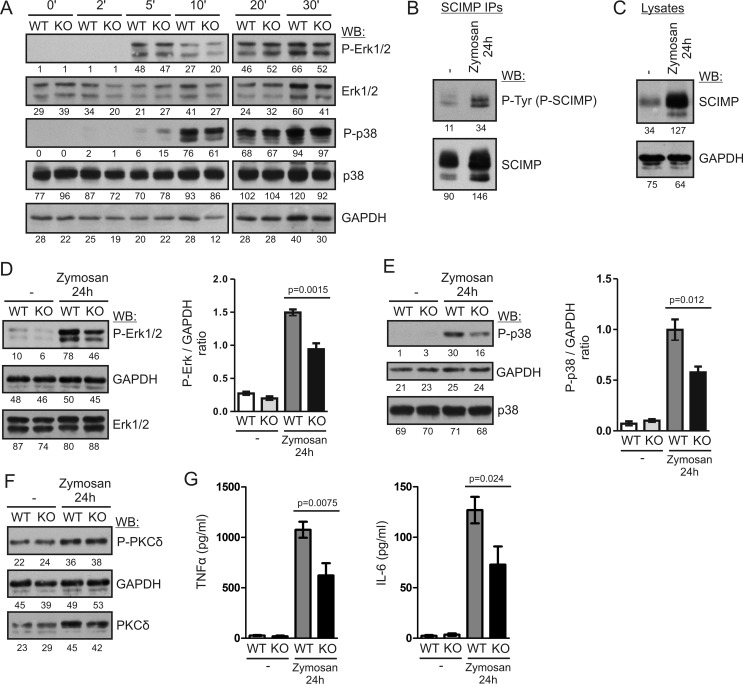
To test the functional significance of SCIMP in Dectin-1 signaling, we first measured ERK activation in zymosan-stimulated BMDCs because the ERK pathway was affected in the previous study of the SCIMP-deficient K46 B cell line (24). We stimulated BMDCs with zymosan for 30 min and followed ERK phosphorylation in the lysates prepared from these cells at various time points. Surprisingly, we could not detect any reproducible differences in ERK phosphorylation between wild-type and Scimp−/− BMDCs (Fig. 6A). Similar results were also obtained for p38 and JNK MAP kinases (Fig. 6A and data not shown). We also crossed Scimp−/− mice with a MyD88-deficient strain to avoid interference from the TLR pathways. However, even in MyD88-deficient BMDCs, no differences in signaling caused by SCIMP deficiency could be detected (data not shown).
FIGURE 6.
SCIMP enhances long-term MAP kinase activation and pro-inflammatory cytokine production after stimulation with zymosan. A, BMDCs from WT and Scimp−/− (KO) mice were stimulated with 300 μg/ml zymosan for the indicated times, and ERK1/2 and p38 phosphorylation was analyzed by immunoblotting. WB, Western blotting. B, BMDCs from C57Bl/6J mice were stimulated with 300 μg/ml zymosan for 24 h or left unstimulated, lysed, and subjected to SCIMP immunoprecipitation followed by immunoblotting with phosphotyrosine (P-Tyr) and SCIMP antibodies. C, levels of SCIMP protein in the lysates of BMDCs stimulated with 300 μg/ml zymosan for 24 h or left unstimulated were analyzed by immunoblotting with SCIMP antibodies. D, phosphorylation of ERK1/2 in the lysates of WT and Scimp−/− BMDCs stimulated with zymosan for 24 h or left unstimulated (left panel). The right panel shows the results of densitometric quantification of ERK phosphorylation in samples prepared from 4 mice/genotype. E, a similar analysis of p38 phosphorylation in the samples from D. F, a similar analysis of PKCδ phosphorylation. G, late production of TNFα and IL-6 by WT and Scimp−/− BMDCs. BMDCs were stimulated with 300 μg/ml zymosan for 48 h, washed, and cultured for an additional 24 h in the absence of stimulus. After this period, culture supernatants were collected and analyzed by ELISA for the presence of TNFα and IL-6. In the experiments shown in B–G, mice with a MyD88-deficient genetic background were used.
As shown in Fig. 4D, SCIMP phosphorylation after zymosan activation was stable for at least 30 min. When we analyzed this further, we found that increased SCIMP phosphorylation could be observed even as late as 24 h after addition of zymosan (Fig. 6B). However, it also seemed that the amount of SCIMP immunoprecipitated from zymosan-treated cells was higher. Indeed, when we tested cell lysates from untreated and zymosan-activated BMDCs, we observed that SCIMP expression was up-regulated after zymosan-mediated activation (Fig. 6C). Therefore, we decided to investigate the signaling downstream of Dectin-1 in Scimp−/− BMDCs at 24 h after initiation of signaling. To avoid interference from TLR pathways, we used mice with a MyD88-deficient genetic background for this experiment. Consistent with the long-lasting SCIMP phosphorylation, we observed a significant reduction of ERK and p38 phosphorylation in SCIMP-deficient BMDCs at this late time point (Fig. 6, D and E). This effect seemed selective for MAP kinase signaling because we did not detect any differences in the phosphorylation of PKCδ in the same samples (Fig. 6F). We also tested the activation status of JNK and the members of the NF-κB pathway (p65 and IKKα/β), but we could not detect any phosphorylation of these proteins, which at this late stage of signaling could have already been down-regulated.
Finally, we tested whether the defects in MAP kinase signaling influence downstream functional responses in dendritic cells. In a recent study, activation of the ERK pathway was shown to be critical for inflammatory cytokine production in BMMFs (30). Because we were unable to detect any significant defects in cytokine production in Scimp−/− BMDCs during the typical experimental setup, where the readout was measured within the first 24 h of stimulation (data not shown), we focused our analysis on late phases of cytokine production beyond the 24-h time frame. At these late phases, the alterations to sustained MAP kinase activity observed in Scimp−/− BMDCs were more likely to have an effect. To measure the prolonged cytokine production, we washed away cytokines produced during the initial 48 h of stimulation and then cultured the cells for an additional 24 h before collecting the supernatants. This way we obtained samples containing only the cytokines produced between 48–72 h after zymosan stimulation. Under these conditions, we observed a significant reduction in the production of TNFα and IL-6 by Scimp/MyD88−/− BMDCs compared with MyD88−/− cells (Fig. 6G). These data are in agreement with our observations that SCIMP deficiency affects mainly the late phases of Dectin-1 signaling.
Discussion
In this article, we describe an initial analysis of SCIMP-deficient mice. Analysis of lymphocyte subsets in these mice at steady state showed that SCIMP is dispensable for leukocyte development and homeostasis. On the other hand, our results also showed that SCIMP is involved in Dectin-1 signaling, where it appears to be selectively involved in sustaining long-term ERK and p38 MAP kinase activation and pro-inflammatory cytokine production.
An earlier cell line-based study from our laboratory (24) suggested that, in B cells, SCIMP is involved in the reverse signaling at the B cell side of the immunological synapse downstream of MHCII glycoproteins. Specifically, it showed that SCIMP accumulates at the APC side of the immunological synapse and that shRNA-mediated SCIMP down-regulation results in a defect in ERK signaling downstream of MHCIIgp in the K46 murine B cell line. However, our analysis of primary murine B cells from SCIMP-deficient mice showed that, in contrast to the K46 B cell line, SCIMP deficiency has no significant impact on ERK signaling elicited by MHCIIgp cross-linking in primary mouse B cells. One possible explanation was that the K46 cell line may be more related to activated B cells, such as those present in the germinal center (31). Indeed, germinal center B cells express higher levels of SCIMP than naïve B cells and thus seem more likely to be affected by the loss of SCIMP. Nevertheless, we have not observed any effects of SCIMP deficiency on germinal center B cell numbers in Scimp−/− mice, and our experiments with isolated germinal center B cells did not reveal any differences in MHCIIgp signaling between WT and Scimp−/− GC B cells (data not shown). Moreover, intact antibody production by Scimp−/− mice (Fig. 2B) also suggested that germinal center reaction is not affected by SCIMP deficiency.
We also tested a possible involvement of SCIMP in MHCIIgp signaling in dendritic cells. However, despite the high levels of SCIMP in these cells, MHCIIgp cross-linking resulted only in a marginal increase in SCIMP phosphorylation, suggesting that, in dendritic cells, SCIMP may function differently from B cells and act downstream of a different receptor. Our candidate-based approach led to the identification of the β-glucan receptor Dectin-1 as a receptor employing SCIMP in its signal transduction. Because both SCIMP and Dectin-1 are associated with tetraspanins (21, 22), we hypothesized that, via tetraspanin-enriched microdomains, SCIMP and Dectin-1 may interact and, as a result, that SCIMP may be involved in coupling of Dectin-1 to downstream signaling pathways. Indeed, in both dendritic cells and GM-CSF-activated macrophages, SCIMP is strongly phosphorylated after Dectin-1 stimulation with zymosan. However, zymosan also contains ligands for additional pattern recognition receptors, and so we performed a series of experiments indicating that Dectin-1 is indeed the receptor triggering SCIMP phosphorylation. These experiments showed that SCIMP phosphorylation induced by zymosan treatment is not affected in Myd88−/− dendritic cells with dysfunctional TLR signaling. In addition, zymosan-triggered SCIMP phosphorylation was inhibited by soluble β-glucan, which can specifically bind to the receptor but does not fully activate it (19). In contrast, a soluble form of mannan, another major zymosan component, did not inhibit SCIMP phosphorylation after zymosan treatment. Interestingly, soluble mannan alone elicited moderate SCIMP phosphorylation, suggesting that there may be a minor contribution from a mannan receptor, such as Dectin-2 (32), to zymosan-induced SCIMP phosphorylation.
SCIMP phosphorylation lasts for many hours after the encounter with zymosan. Moreover, zymosan stimulation also enhances SCIMP expression. Because SCIMP typically displays a certain level of constitutive phosphorylation even in quiescent cells, SCIMP protein up-regulation can contribute to the increased phosphorylation observed at the very late time points. However, the relative contribution of increased SCIMP expression and Dectin-1-induced phosphorylation to global levels of phosphorylated SCIMP remains unclear. Phosphorylated SCIMP appears to be important for sustaining ERK and p38 activation for prolonged periods of time. The precise mechanism of how SCIMP mediates ERK and p38 signaling is not entirely clear. Dectin-1 activates, by an unclear mechanism, PLCγ2, which is indispensable for ERK activation (33). PLCγ2 products also activate PKCδ, which then phosphorylates the adaptor protein CARD9, resulting in activation of the NF-κB pathway (15). A recent study showed that CARD9 also plays a critical role in ERK activation by bringing together Ras and RasGRF1, resulting in the activation of Ras and ERK further downstream (30). However, the existence of CARD9-independent pathways cannot be ruled out. Previously published data showed that SCIMP binds the SLP65/SLP76 adaptor proteins, which are known to be involved in PLCγ1/2 activation (24, 34). In addition, SCIMP also binds Grb2, which can then recruit Sos proteins, well characterized exchange factors for Ras and activators of the Ras/ERK pathway. Products of PLCγ2 also activate another family of Ras exchange factors, RasGRP proteins, which activate Ras downstream of TCR, BCR, and other immunoreceptors (35). Clearly, there are multiple options of how SCIMP can contribute to Dectin-1-dependent ERK activation. It certainly is not the only player connecting Dectin-1 to the ERK pathway, and especially at the early stages of signaling, other pathways dominate the response. However, at the very late stages of Dectin-1 stimulation, SCIMP activity becomes more apparent.
In addition to ERK, SCIMP also contributes to the activation of p38 MAP kinase. The mechanism of p38 activation downstream of Dectin-1 is less well understood. In contrast to ERK, it was shown to be independent of CARD9 (30). Our observations of similar defects in ERK and p38 activation in SCIMP-deficient animals together with unperturbed PKCδ activity suggest that SCIMP affects a CARD9-independent pathway that may be involved in the activation of both of these MAP kinases.
Dectin-1 is an important innate immune receptor recognizing a number of pathogenic fungi. It supports the immune response by mediating phagocytosis of these pathogens by triggering an oxidative burst and by inducing the production of cytokines (7, 17, 18). We have analyzed all of these downstream responses in BMDCs from SCIMP-deficient mice, but we did not observe any differences between WT and SCIMP-deficient cells when stimulated by zymosan (data not shown). The only exceptions were the late phases of TNFα and IL-6 production (Fig. 6G). Our data are consistent with previous observations that ERK signaling is required for the production of TNFα and IL-6 downstream of Dectin-1 (30, 36). p38 signaling in macrophages and dendritic cells has also been shown to contribute to pro-inflammatory cytokine production (37, 38). Although the precise function of the late sustained production of these cytokines still remains to be clarified, the role of these cytokines in antifungal defense has already been well established. Mice deficient in TNFα or IL-6 were shown to be highly susceptible to fungal infections (39–42). Moreover, the use of agents blocking the function of TNFα or IL-6 in human patients is also associated with an increased incidence of infection with opportunistic fungi (43).
Taken together, our study describes a novel branch of Dectin-1 signaling that is driven by the small transmembrane adaptor SCIMP. Our data suggest that the early and late phases of Dectin-1 signaling are differentially regulated. The late sustained phase of Dectin-1 signaling is partly dependent on SCIMP, which in dendritic cells promotes MAP kinase signaling and production of TNFα and IL-6, important mediators of the inflammatory response during fungal infections.
Experimental Procedures
Antibodies
Antibodies to the following antigens were used for Western blotting detection: GAPDH (Sigma-Aldrich); phospho-ERK (Thr-202/Tyr-204), phospho-PKCδ/θ (Ser-643/676), PKCδ, phospho-p38 (Thr-180/182), and p38 MAPK, (Cell Signaling Technology); ERK1/2 (Promega); and phospho-tyrosine (4G10, Upstate Biotechnology). Rabbit polyclonal antibodies against murine SCIMP were described earlier (24). For the flow cytometry analysis, antibodies against the following mouse antigens conjugated to the indicated fluorophores were used: CD3-PE-Cy7, CD5-PE, CD11b-FITC, CD11b-PE, CD11b-APC, CD19-eFluor 660, CD19-FITC, CD23-Dye 647, F4/80-FITC, Gr1-PB, Ly6G-FITC, CD49b (DX5)-APC, c-kit-PE, Gr1-PE, Ly6C-PE-Cy7, B220-FITC, IgM-FITC, MHCII-FITC, FcϵRIα-PB, and CD86-APC (Biolegend); CD1d-FITC, CD4-PE, CD8-e450, CD11c-APC, CD43-PE, GL7-FITC, B220-e450, IgD-APC, and CD80-APC (eBiosciences); and CD3-APC and CD8-FITC (EXBIO). The anti-IgM-Dy547 antibody was conjugated in-house. For MACS purification, anti-CD43, anti-CD11b, anti-CD19, anti-FITC, and anti-mPDCA-1 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used. Other antibodies used in this study were anti-mouse I-A/I-E-biotin (Biolegend), HRP-conjugated goat anti-mouse IgG (Sigma-Aldrich), HRP-conjugated goat anti-rabbit IgG Fc fragment-specific, goat anti-mouse IgM F(ab)2 (Jackson ImmunoResearch Laboratories), Fc Bloc (2.4G2) (BD Biosciences), and HRP-conjugated goat anti-rabbit polyclonal antibody (Bio-Rad).
Other Reagents
We also used mannan from S. cerevisiae (Sigma-Aldrich), zymosan A from S. cerevisiae (Sigma-Aldrich), streptavidin (Jackson ImmunoResearch Laboratories), CFSE (5-(and 6)-carboxyfluorescein diacetate succinimidyl ester; eBioscience), and NP(14)-ovalbumin (Bioresearch Technologies, Inc.). Soluble β-glucan (Wellmune Soluble) was a kind gift from Bengt Hansson (Biogredia AB, Sandefjord, Norway). The Src family kinase inhibitor PP2 and Syk inhibitor IV (BAY 61-3606) were from Calbiochem/Merck (Darmstadt, Germany).
Mice
The SCIMP-deficient mouse strain Scimptm1a(KOMP)Wtsion the C57Bl/6J genetic background (throughout this article labeled as Scimp−/−) was generated at the Wellcome Trust Sanger Institute (Cambridge, UK) within International Knockout Mouse Consortium Project 24100 and was directly obtained from the Wellcome Trust Sanger Institute. B1–8i B cell antigen receptor-transgenic mice (44), the OTII transgenic mouse strain (C57BL/6-Tg(TcraTcrb)425Cbn/Crl), and the Myd88-deficient mouse strain (B6.129P2(SJL)-Myd88tm1.1Defr/J) derived from Myd88fl mice (45) were obtained from The Jackson Laboratory (Bar Harbor, ME). C57Bl/6J for comparative experiments originated from crossing Scimp+/− heterozygotes. Other C57Bl/6J mice were from the animal facility of the Institute of Molecular Genetics of the Academy of Sciences of the Czech Republic (IMG ASCR) (Prague, Czech Republic). All experiments in this work conducted on animals were approved by the Animal Care and Use Committee of the Institute of Molecular Genetics and were in agreement with local legal requirements and ethical guidelines.
Cell Lines and Primary Cells
Primary mouse B cells were isolated from spleens of Scimp−/− and C57Bl/6J mice by negative selection using anti-CD43 and anti-CD11b MicroBeads (Miltenyi Biotec) on an AutoMACS magnetic cell sorter (Miltenyi Biotec) and subsequently cultured in Iscove's modified Dulbecco's medium (IMDM). BMMFs were differentiated from mouse bone marrow cells in DMEM conditioned with 10% L929 culture supernatant containing M-CSF for 7–9 days. BMDCs were differentiated in DMEM conditioned with 3% LUTZ cell culture supernatant containing GM-CSF for 10–12 days. Ftl3L-generated dendritic cells were cultured in 100 ng/ml rmFLT3L (Peprotech) and RPMI medium supplemented with stem cell factor (supernatant from HEK293 cells transfected with a stem cell factor-coding construct) for 8 days, and then plasmacytoid dendritic cells were removed using anti-mPDCA-1 microbeads on an AutoMACS, and the rest of the dendritic cells were used further.
To prepare primary classical dendritic cells, splenocytes from C57Bl/6J mice were incubated with Fc bloc, CD3-FITC, IgM-FITC, and Ter119-FITC and precleared on an AutoMACS using anti-FITC magnetic beads (Miltenyi Biotec). The negative fraction was further stained with B220-e450, Ly6C-PE_Cy7, and CD11c-APC and sorted by BD Influx FACS (BD Biosciences) for classical DCs (CD11c+, B220−, Ly6C−) (46). For GL7+ germinal center B cell purification, all splenic B cells were first purified from mice immunized intraperitoneally with sheep red blood cells by negative selection using CD11b and CD43 microbeads on an AutoMACS magnetic cell separator. The obtained B cells were then stained with GL-7-FITC antibody, followed by positive selection with anti-FITC magnetic beads using the same AutoMACS separator. Phoenix Eco cells were obtained from Origene and cultured in DMEM. All media were supplemented with 10% fetal calf serum and antibiotics. The cells were cultured at 37 °C in 5% CO2.
DNA Constructs, Transfection, and Transduction
The mouse SCIMP-YFP construct coding for full-length mouse SCIMP tagged at the C terminus with yellow fluorescent protein was cloned into the pMSCV-LNGFR retroviral vector. Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific) was used according to the protocol of the manufacturer for the transfection of SCIMP-YFP/pMSCV-LNGFR construct to Phoenix Eco cells to produce viral particles. Retrovirus-containing supernatants were then harvested, supplemented with Polybrene (10 mg/ml, Sigma-Aldrich), and added to the freshly isolated bone marrow cells. The cells were then centrifuged at 1250 × g for 90 min at 30 °C and differentiated into BMDCs or BMMFs.
Cell Activation, Lysis, and Immunoprecipitation
To test the effect of zymosan and other activators on BMDCs, fully differentiated BMDCs were plated on 6-well plates (2 × 106 cells/well) in culture medium and allowed to adhere to the plastic overnight. Then the medium was changed for DMEM without GM-CSF. After 2 h, zymosan in DMEM was added to a final concentration of 300 μg/ml. The activation of cells was stopped after the indicated time periods by lysis in SDS-PAGE sample buffer. For immunoprecipitation, the cells (BMDCs or BMMFs) were plated on 10-cm dishes and activated as above or by biotinylated anti mouse I-A/I-E antibody (Biolegend, 10 μg/ml), followed by cross-linking with 10 μg/ml streptavidin (Jackson ImmunoResearch Laboratories). They were then lysed in lysis buffer containing 1% laurylmaltoside (Calbiochem/Merck), 20 mm Tris (pH 7.5), 150 mm NaCl, 5 mm iodoacetamide, 5 mm NaF, 1 mm Na3VO4, 2 mm EDTA, and 100× diluted protease inhibitor mixture set III (Calbiochem) for 30 min on ice. Postnuclear supernatants were incubated for 1–2 h with anti-SCIMP rabbit antiserum followed by 1–2 h of incubation with protein A/G Plus-agarose beads (Santa Cruz Biotechnology) at 4 °C. After washing, the immunoprecipitates were eluted with 40 μl of SDS-PAGE sample buffer.
B cell stimulations were carried out in suspension. B cells were resuspended in a concentration of 5 × 107/ml in IMDM and activated by labeling with 10 μg/ml biotinylated anti-mouse I-A/I-E antibody, followed by cross-linking with 10 μg/ml streptavidin in IMDM at 37 °C for the indicated time intervals. The activation of cells was stopped by addition of an equal volume of 2× concentrated SDS-PAGE sample buffer. Western blotting quantification was performed by densitometry of scanned films using AIDA image analyzer software (Elysia-raytest, Straubenhardt, Germany), and the obtained values were inserted into the figures below the individual blots.
Flow Cytometry
Single cell suspensions were prepared from spleens, lymph nodes, bone marrow, and peripheral blood from 6- to 8-week-old mice. Erythrocytes were lysed with ACK buffer (150 mm NH4Cl, 0.1 mm EDTA (disodium salt), and 1 mm KHCO3). The remaining cells were incubated with Fc-bloc and fluorophore-conjugated antibodies and analyzed on a BD LSRII Flow cytometer. For calcium response measurement, a single cell suspension of splenocytes (after erythrocyte lysis in ACK buffer) was loaded with 2 μm calcium indicator Fura Red (Invitrogen) and subsequently stained with anti-CD3-APC and anti-CD11b-APC. Samples were analyzed using a BD LSRII flow cytometer for 30 s at rest and then another 150 s after activation (either with 10 μg/ml anti-mouse IgM F(ab)2 antibody (Jackson ImmunoResearch Laboratories) or with 10 μg/ml streptavidin, which cross-linked MHCIIgp molecules on B cells preincubated with biotinylated anti-I-A/I-E antibody (Biolegend). The relative calcium concentration was measured as a ratio of the Fura Red fluorescence intensity elicited by excitation wavelengths of 405 nm (emission measured at 635–720 nm) and 488 nm (emission measured at 655–695 nm). Data were analyzed using FlowJo software (TreeStar).
Antigen Presentation Assay
For the antigen-presentation assay, we modified a method published previously (47). CD4+ T cells isolated from lymph nodes of OTII mice using negative selection (CD11b, CD8, CD19-FITC) on AutoMACS were cultured for 2 days in RPMI medium supplemented with 100 ng/ml anti-CD3 antibody and 2 units/ml IL2 for 48 h. B cells negatively selected on AutoMACS were fed for 1 h with 10 mg/ml NP-ovalbumin at 37 °C, washed with PBS, and cultured in a ratio of 1:1 with CFSE-stained T cells. After 48 h, cell proliferation was measured using a BD LSRII flow cytometer.
Immunization
For antibody detection, Scimp−/− and C57Bl/6J mice, 6–8 weeks old, were intradermally injected with 100 μg of ovalbumin/mouse in incomplete Freund adjuvant. The second immunization followed 21 days after the first immunization. Serum from mice was collected on day 10 after the second immunization. Antibody concentration was measured by ELISA.
Cytokine Detection
Concentrations of TNFα and IL-6 in BMDC culture supernatants were determined by Ready-SET-Go!® ELISA kits from eBioscience according to the instructions of the manufacturer.
Microscopy
For live cell microscopy, BMDCs and BMMFs expressing SCIMP-YFP were transferred into Lab-Tek chambered coverglass (Nunc, Thermo Fisher Scientific), and strongly autofluorescent zymosan particles were subsequently added. Cells were observed in a climate chamber (37 °C, 5% CO2) under a Leica TCS SP5 confocal microscope using a ×63 objective lens (Leica Microsystems). Data were analyzed using LAS AF software (Leica Microsystems).
Author Contributions
J. K. conducted the majority of the experiments, analyzed the results, and wrote most of the paper. M. F. was involved in the preparation of BMDCs and conducted the part of the experiments addressing the expression and function of SCIMP in BMDCs. J. P. performed the analysis of antibody production by Scimp−/− mice. T. S. performed part of the BMDC biochemical analysis. B. M. designed and conducted part of the flow cytometry analysis. T. B. conceptualized and supervised the project, conducted some of the biochemical experiments, and wrote the paper with J. K. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank the International Knockout Mouse Consortium and the Wellcome Trust Sanger Institute for providing us with Scimp−/− mice. We also thank Bengt Hansson from Biogredia AB (Sandefjord, Norway) for providing us with a generous amount of soluble β-glucan (Wellmune Soluble). We also acknowledge the Microscopy Centre, IMG ASCR (Prague, Czech Republic) for support with obtaining the scientific data presented in this paper.
This work was supported by Czech Science Foundation (GACR, Project P302/12/1712) and received institutional funding from IMG ASCR (RVO 68378050). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Movie 1.
- TLR
- Toll-like receptor
- gp
- glycoprotein
- SCIMP
- SLP76/SLP65-interacting membrane adaptor protein
- APC
- antigen-presenting cell
- CFSE
- 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester
- NP
- 4-hydroxy-3-nitrophenylacetyl
- BMDC
- bone marrow-derived dendritic cell
- BMMF
- bone marrow-derived macrophage
- IMDM
- Iscove's modified Dulbecco's medium.
References
- 1. Hardison S. E., and Brown G. D. (2012) C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13, 817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seo B. S., Lee S. H., Lee J. E., Yoo Y. C., Lee J., and Park S. R. (2013) Dectin-1 stimulation selectively reinforces LPS-driven IgG1 production by mouse B cells. Immune Netw. 13, 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor P. R., Brown G. D., Reid D. M., Willment J. A., Martinez-Pomares L., Gordon S., and Wong S. Y. (2002) The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876–3882 [DOI] [PubMed] [Google Scholar]
- 4. Willment J. A., Marshall A. S., Reid D. M., Williams D. L., Wong S. Y., Gordon S., and Brown G. D. (2005) The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur. J. Immunol. 35, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 5. Brown G. D., and Gordon S. (2001) Immune recognition: a new receptor for β-glucans. Nature 413, 36–37 [DOI] [PubMed] [Google Scholar]
- 6. Barreto-Bergter E., and Figueiredo R. T. (2014) Fungal glycans and the innate immune recognition. Front. Cell. Infect. Microbiol. 4, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown G. D., Herre J., Williams D. L., Willment J. A., Marshall A. S., and Gordon S. (2003) Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown G. D., Taylor P. R., Reid D. M., Willment J. A., Williams D. L., Martinez-Pomares L., Wong S. Y., and Gordon S. (2002) Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saijo S., Fujikado N., Furuta T., Chung S. H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., Kinjo T., Nakamura K., Kawakami K., and Iwakura Y. (2007) Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8, 39–46 [DOI] [PubMed] [Google Scholar]
- 10. Marakalala M. J., Vautier S., Potrykus J., Walker L. A., Shepardson K. M., Hopke A., Mora-Montes H. M., Kerrigan A., Netea M. G., Murray G. I., Maccallum D. M., Wheeler R., Munro C. A., Gow N. A., Cramer R. A., et al. (2013) Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 9, e1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steele C., Rapaka R. R., Metz A., Pop S. M., Williams D. L., Gordon S., Kolls J. K., and Brown G. D. (2005) The β-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferwerda B., Ferwerda G., Plantinga T. S., Willment J. A., van Spriel A. B., Venselaar H., Elbers C. C., Johnson M. D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morré S. A., et al. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plantinga T. S., van der Velden W. J., Ferwerda B., van Spriel A. B., Adema G., Feuth T., Donnelly J. P., Brown G. D., Kullberg B. J., Blijlevens N. M., and Netea M. G. (2009) Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 49, 724–732 [DOI] [PubMed] [Google Scholar]
- 14. Sancho D., and Reis e Sousa C. (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 30, 491–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brubaker S. W., Bonham K. S., Zanoni I., and Kagan J. C. (2015) Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dambuza I. M., and Brown G. D. (2015) C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gantner B. N., Simmons R. M., Canavera S. J., Akira S., and Underhill D. M. (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., and Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 [DOI] [PubMed] [Google Scholar]
- 19. Goodridge H. S., Reyes C. N., Becker C. A., Katsumoto T. R., Ma J., Wolf A. J., Bose N., Chan A. S., Magee A. S., Danielson M. E., Weiss A., Vasilakos J. P., and Underhill D. M. (2011) Activation of the innate immune receptor Dectin-1 upon formation of a “phagocytic synapse.” Nature 472, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willment J. A., Lin H. H., Reid D. M., Taylor P. R., Williams D. L., Wong S. Y., Gordon S., and Brown G. D. (2003) Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 171, 4569–4573 [DOI] [PubMed] [Google Scholar]
- 21. Mantegazza A. R., Barrio M. M., Moutel S., Bover L., Weck M., Brossart P., Teillaud J.-L., and Mordoh J. (2004) CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood 104, 1183–1190 [DOI] [PubMed] [Google Scholar]
- 22. Meyer-Wentrup F., Figdor C. G., Ansems M., Brossart P., Wright M. D., Adema G. J., and van Spriel A. B. (2007) Dectin-1 interaction with tetraspanin CD37 inhibits IL-6 production. J. Immunol. 178, 154–162 [DOI] [PubMed] [Google Scholar]
- 23. Berditchevski F., and Odintsova E. (2007) Tetraspanins as regulators of protein trafficking. Traffic 8, 89–96 [DOI] [PubMed] [Google Scholar]
- 24. Draber P., Vonkova I., Stepanek O., Hrdinka M., Kucova M., Skopcova T., Otahal P., Angelisova P., Horejsi V., Yeung M., Weiss A., and Brdicka T. (2011) SCIMP, a transmembrane adaptor protein involved in major histocompatibility complex class II signaling. Mol. Cell. Biol. 31, 4550–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stepanek O., Draber P., and Horejsi V. (2014) Palmitoylated transmembrane adaptor proteins in leukocyte signaling. Cell. Signal. 26, 895–902 [DOI] [PubMed] [Google Scholar]
- 26. Di Carlo F. J., and Fiore J. V. (1958) On the composition of zymosan. Science 127, 756–757 [DOI] [PubMed] [Google Scholar]
- 27. Underhill D. M., Ozinsky A., Hajjar A. M., Stevens A., Wilson C. B., Bassetti M., and Aderem A. (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 [DOI] [PubMed] [Google Scholar]
- 28. Takeuchi O., Kaufmann A., Grote K., Kawai T., Hoshino K., Morr M., Mühlradt P. F., and Akira S. (2000) Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164, 554–557 [DOI] [PubMed] [Google Scholar]
- 29. Akira S. (2000) Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28, 551–556 [DOI] [PubMed] [Google Scholar]
- 30. Jia X. M., Tang B., Zhu L. L., Liu Y. H., Zhao X. Q., Gorjestani S., Hsu Y. M., Yang L., Guan J. H., Xu G. T., and Lin X. (2014) CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J. Exp. Med. 211, 2307–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. André P., Cambier J. C., Wade T. K., Raetz T., and Wade W. F. (1994) Distinct structural compartmentalization of the signal transducing functions of major histocompatibility complex class II (Ia) molecules. J. Exp. Med. 179, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGreal E. P., Rosas M., Brown G. D., Zamze S., Wong S. Y., Gordon S., Martinez-Pomares L., and Taylor P. R. (2006) The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16, 422–430 [DOI] [PubMed] [Google Scholar]
- 33. Xu S., Huo J., Lee K.-G., Kurosaki T., and Lam K.-P. (2009) Phospholipase Cγ2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J. Biol. Chem. 284, 7038–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koretzky G. A., Abtahian F., and Silverman M. A. (2006) SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat. Rev. Immunol. 6, 67–78 [DOI] [PubMed] [Google Scholar]
- 35. Jun J. E., Rubio I., and Roose J. P. (2013) Regulation of ras exchange factors and cellular localization of ras activation by lipid messengers in T cells. Front. Immunol. 4, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slack E. C., Robinson M. J., Hernanz-Falcón P., Brown G. D., Williams D. L., Schweighoffer E., Tybulewicz V. L., and Reis e Sousa C. (2007) Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur. J. Immunol. 37, 1600–1612 [DOI] [PubMed] [Google Scholar]
- 37. Zhu W., Downey J. S., Gu J., Di Padova F., Gram H., and Han J. (2000) Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J. Immunol. 164, 6349–6358 [DOI] [PubMed] [Google Scholar]
- 38. Wu Y.-J., Wu Y.-H., Mo S.-T., Hsiao H.-W., He Y.-W., and Lai M.-Z. (2015) Cellular FLIP inhibits myeloid cell activation by suppressing selective innate signaling. J. Immunol. 195, 2612–2623 [DOI] [PubMed] [Google Scholar]
- 39. Cenci E., Mencacci A., Casagrande A., Mosci P., Bistoni F., and Romani L. (2001) Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J. Infect. Dis. 184, 610–617 [DOI] [PubMed] [Google Scholar]
- 40. Romani L., Mencacci A., Cenci E., Spaccapelo R., Toniatti C., Puccetti P., Bistoni F., and Poli V. (1996) Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183, 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Enckevort F. H., Netea M. G., Hermus A. R., Sweep C. G., Meis J. F., Van der Meer J. W., and Kullberg B. J. (1999) Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med. Mycol. 37, 419–426 [DOI] [PubMed] [Google Scholar]
- 42. Filler S. G., Yeaman M. R., and Sheppard D. C. (2005) Tumor necrosis factor inhibition and invasive fungal infections. Clin. Infect. Dis. 41, S208–S212 [DOI] [PubMed] [Google Scholar]
- 43. Vallabhaneni S., and Chiller T. M. (2016) Fungal infections and new biologic therapies. Curr. Rheumatol. Rep. 18, 29. [DOI] [PubMed] [Google Scholar]
- 44. Sonoda E., Pewzner-Jung Y., Schwers S., Taki S., Jung S., Eilat D., and Rajewsky K. (1997) B cell development under the condition of allelic inclusion. Immunity 6, 225–233 [DOI] [PubMed] [Google Scholar]
- 45. Hou B., Reizis B., and DeFranco A. L. (2008) Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edwards A. D., Diebold S. S., Slack E. M., Tomizawa H., Hemmi H., Kaisho T., Akira S., and Reis e Sousa C. (2003) Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 [DOI] [PubMed] [Google Scholar]
- 47. Chatterjee P., Tiwari R. K., Rath S., Bal V., and George A. (2012) Modulation of antigen presentation and B cell receptor signaling in B cells of beige mice. J. Immunol. 188, 2695–2702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.