Abstract
WHEP domains exist in certain eukaryotic aminoacyl-tRNA synthetases and play roles in tRNA or protein binding. We present evidence herein that cytoplasmic and mitochondrial forms of Caenorhabditis elegans glycyl-tRNA synthetase (CeGlyRS) are encoded by the same gene (CeGRS1) through alternative initiation of translation. The cytoplasmic form possessed an N-terminal WHEP domain, whereas its mitochondrial isoform possessed an extra N-terminal sequence consisting of an mitochondrial targeting signal and an appended domain. Cross-species complementation assays showed that CeGRS1 effectively rescued the cytoplasmic and mitochondrial defects of a yeast GRS1 knock-out strain. Although both forms of CeGlyRS efficiently charged the cytoplasmic tRNAsGly of C. elegans, the mitochondrial form was much more efficient than its cytoplasmic counterpart in charging the mitochondrial tRNAGly isoacceptor, which carries a defective TψC hairpin. Despite the WHEP domain per se lacking tRNA binding activity, deletion of this domain reduced the catalytic efficiency of the enzyme. Most interestingly, the deletion mutant possessed a higher thermal stability and a somewhat lower structural flexibility. Our study suggests a role for the WHEP domain as a regulator of the dynamic structure and activity of the enzyme.
Keywords: aminoacyl tRNA synthetase, Caenorhabditis elegans (C. elegans), neurodegenerative disease, neurological disease, protein domain, protein synthesis
Introduction
Aminoacyl-tRNA synthetases (aaRSs)3 belong to a ubiquitous and ancient family of enzymes that establish their genetic codes by attaching specific amino acids to their cognate tRNAs. The resultant aminoacyl-tRNAs are then delivered to ribosomes to decipher mRNA codons through base pairing with the anticodon of the aminoacyl-tRNA (1). Because protein translation takes place in both the cytoplasm and mitochondria in eukaryotes, two distinct sets of aaRSs are required: one functioning in the cytoplasm and the other functioning in mitochondria (1–4). In most cases, the cytoplasmic and mitochondrial forms of an aaRS are encoded by two different nuclear genes. Occasionally, both isoforms of an aaRS are encoded by the same nuclear gene through alternative initiation of translation, examples of which include genes encoding yeast alanyl-, glycyl-, histidyl-, and valyl-tRNA synthetases (5–9). As a result, cytoplasmic and mitochondrial forms of an aaRS, for example yeast glycyl-tRNA synthetase (GlyRS), possess essentially the same polypeptide sequence, except for a cleavable mitochondrial targeting signal (MTS) attached at the N terminus of the mitochondrial precursor form.
GlyRS is one of the most intriguing aaRSs because of its divergent quaternary structure and evolutionary origin. Two distinct oligomeric structures of GlyRS exist: one with an α2 structure and the other with an α2β2 structure (10, 11). These two forms are divergent not only in subunit composition but also in molecular size and protein sequence (12–14). Even so, they possess the same signature motifs and are thus assigned to the same class (class II). To date, α2β2 enzymes exist only in bacteria and chloroplasts, but α2 enzymes are spread over all three domains of life. The major identity elements of tRNAGly reside in the discriminator base (N73), the top three base pairs of the acceptor stem (1:72, 2:71, and 3:70), and C35/C36 in the anticodon loop (4). Among these identity elements, the discriminator base is of particular interest. It is a U in bacteria and an A in eukaryotic cytoplasm. In general, U73-containing tRNAGly pairs with an α2β2-type GlyRS enzyme, whereas A73-containing tRNAGly pairs with an α2-type GlyRS enzyme (15). However, this once-tight rule was broken by the discovery that Thermus thermophilus possesses a U73/α2 pair (15).
GlyRS has attracted enormous attention over the past decade because of its implication in Charcot-Marie-Tooth (CMT) disease, one of the most common inherited neurological disorders (16). To date, 13 missense mutations of human GlyRS were shown to cause a dominant axonal form of CMT, also known as CMT type 2D (17, 18). However, there is no direct causal relationship between loss of the primary function of the enzyme and CMT disease, because not all CMT-causing mutants possess impaired aminoacylation activity (19). Recent studies suggested that GlyRS mutations also cause a CMT-like syndrome in other animals such as mice (20) and flies (21).
There is only one GlyRS gene in the nuclear genome of Caenorhabditis elegans, namely, CeGRS1. Because mitochondrial tRNAs in this organism typically possess a defective TψC hairpin, the question arose as to how enzyme(s) encoded by this gene can efficiently charge such a tRNA species. Our study shows that CeGlyRS acquired two functional domains, a mitochondrial appended domain (MAD) and a WHEP domain, during evolution. The MAD enabled the enzyme to charge its cognate tRNA (containing a defective TψC hairpin) with a higher efficiency, whereas the WHEP domain contributed to regulating the dynamic structure of the enzyme. As a result, deletion of the WHEP domain altered the thermal stability, structural flexibility, and catalytic rate of the enzyme. These results provide new insights into understanding the structure-function relationships of CeGlyRS and its homologues.
Results
C. elegans GRS1 Is a Dual Functional Gene
Analysis of the ORF of CeGRS1 suggested that this gene is dual functional, with ATG1 and ATG65 being the respective initiator codons of the mitochondrial and cytoplasmic forms of CeGlyRS (Fig. 1A). The mitochondrial form of CeGlyRS (CeGlyRSm) possessed a 64-residue polypeptide extension with a mitochondrial matrix-processing peptidase cleavage site in between N-terminal residues 20 and 21. The sequence containing N-terminal residues 1–20 was rich in positively charged and hydroxylated residues and was devoid of acidic residues, a feature characteristic of an MTS, and the sequence containing N-terminal residues 21–64 was specific to the mitochondrial form; this sequence is herein referred to as the MAD. The MAD was absent from all other eukaryotic GlyRS homologues shown herein. In contrast, the N-terminal domain (containing residues 1–66) of the cytoplasmic form of CeGlyRS (CeGlyRSc) shared high sequence identity (57–70% identity) with the WHEP domains of GlyRSs of Drosophila melanogaster, Bombyx mori, and Homo sapiens (Fig. 1B).
FIGURE 1.
C. elegans glycyl-tRNA synthetase (CeGlyRS) and tRNAGly. A, schematic representation of CeGlyRS. The matrix-processing peptidase (MPP) cleavage site is indicated with ▾ above the sequence. Relative positions of CeGlyRS are shown: MTS (amino acid residues 1–20), MAD (amino acid residues 21–64), and WHEP (amino acid residues 65–130). M1 and M65, respectively, denote initiating methionines for the mitochondrial and cytoplasmic forms of CeGlyRS. B, the WHEP domains of various eukaryotic GlyRSs. DmGlyRS, D. melanogaster GlyRS; BmGlyRS, B. mori GlyRS; HsGlyRS, H. sapiens GlyRS. C, S. cerevisiae and C. elegans tRNAGly isoacceptors. Nucleotides and base pairs that are presumably important for recognition of tRNAGly by GlyRS are boxed. The normal and defective TψC hairpins in CetRNAsGly are marked with dashed circles.
Because only one GlyRS gene exists in the genome of C. elegans, one would normally assume that the identity elements must be strictly conserved between its nuclear and mitochondrial encoded tRNAsGly (CetRNAnGly and CetRNAmGly, respectively) so that CeGlyRS can efficiently charge both tRNA isoacceptors. As expected, the discriminator base A73 and C35/C36 in the anticodon loop were conserved in yeast and C. elegans tRNAnGly and tRNAmGly (Fig. 1C). However, contrary to our anticipation, the first three base pairs in the acceptor stem highly diverged among these tRNA isoacceptors, suggesting that they are not critical for recognition in these two eukaryotic organisms. In addition to differences identified in the acceptor stem, CetRNAmGly possessed a defective TψC hairpin, which prompted us to ask how CeGlyRS can effectively charge this tRNA.
C. elegans GRS1 Can Rescue Growth Defects of a Yeast GRS1 Knock-out Strain
To test the functional potential of CeGRS1, we cloned the ORF of this gene or its derivatives into pADH and tested the ability of the resultant construct to rescue the growth defects of a yeast grs1− strain. As shown in Fig. 2, CeGlyRSm conferred a positive growth phenotype to the null allele on both 5-fluoroorotic acid (5-FOA) and YPG, suggesting that this construct can functionally substitute for the yeast GRS1 gene (row 3 in Fig. 2, A and B). This result also implied that a minor portion of the CeGlyRSm protein was retained in the cytoplasm for functioning, a scenario often seen in yeast aaRS complementation (22). Deletion of the MTS alone or both the MTS and MAD specifically impaired its mitochondrial rescue activity; MTS-deleted mutants restored the growth phenotype of the knock-out strain on 5-FOA but not YPG (rows 4 and 5). Thus, the MTS was required for mitochondrial targeting of CeGlyRSm, and the MAD was dispensable for efficient aminoacylation of yeast tRNAnGly by CeGlyRS. Further deletion of the WHEP domain impaired both the cytoplasmic and mitochondrial rescue activities (rows 6 and 8). As expected, attaching a heterologous MTS to CeGlyRSc yielded a fusion construct that could restore the growth phenotype of the knock-out strain on YPG (row 7), lending further support to the observation that the MAD is not required for efficient aminoacylation of yeast tRNAmGly by CeGlyRS.
FIGURE 2.
Cross-species rescue activities of C. elegans glycyl-tRNA synthetase (CeGlyRS) variants. Constructs encoding the WT and truncated forms of CeGlyRS were transformed into a yeast grs1− strain, and the ability of the resultant transformants to grow on 5-FOA and YPG was tested. A, summary of the constructs and their rescue activities. The symbols + and −, respectively, denote positive and negative complementation. Mit, mitochondrial; Cyt, cytoplasmic. B, complementation assays on 5-FOA and YPG. C, Western blotting. Upper panel, GlyRS; lower panel, phosphoglycerate kinase (PGK; as a loading control). Indicated at the bottom of the blots are the amounts of protein extracts loaded into each well. Rows 1–8 (circled) in B and C represent constructs shown in A. D, CHX chase assay. T0–T16 denote 0–16 h after induction. Quantitative data for relative levels of CeGlyRS variants are shown in a separate diagram next to the Western blots.
A Western blot analysis using an anti-His6 tag antibody showed that except for MTS-ΔWHEP, which was poorly expressed, all other CeGlyRS constructs used for cross-species rescue assays were properly expressed in yeast (rows 3–8 in Fig. 2C). Thus, ΔWHEP was much more stable in the cytoplasm than in mitochondria. We also noted that the processed CeGlyRSm was somewhat larger in size than its cytoplasmic counterpart CeGlyRSc (rows 3–5), suggesting that the MAD was not cleaved away from CeGlyRSm following its import into mitochondria.
Deletion of the WHEP Domain Has Little Effect on the Half-life of CeGlyRSc
To check whether deletion of the WHEP domain affects the half-life of CeGlyRSc, we performed a cycloheximide (CHX) chase assay in yeast as previously described (23). Genes encoding CeGlyRSc and ΔWHEP were respectively cloned into pGAL1, and the resultant constructs were transformed into a S. cerevisiae strain, INVSc1. Cultures of the transformants were induced with galactose for 4 h, followed by the addition of CHX to terminate protein synthesis. CHX-treated cells were harvested at various intervals (0∼16 h) following induction. Protein extracts were prepared for Western blot analyses using an anti-His6 tag antibody. As shown in Fig. 2D, both proteins were fairly stable; less than 20% of the proteins were degraded throughout the time period tested. Thus, deletion of the WHEP domain did not impair the stability of the protein in vivo.
CeGlyRSm and CeGlyRSc Are Respectively Localized in the Mitochondria and Cytoplasm
To map the subcellular localization of CeGlyRS and its derivatives in yeast, we carried out a fluorescence microscopic analysis. A DNA sequence that encodes the GFP was amplified by a PCR and inserted in-frame into the 3′ end of CeGlyRSm, CeGlyRSm(ΔMTS), and CeGlyRSc. We then transformed these GFP fusion constructs into the yeast knock-out strain and examined their subcellular distributions via fluorescence microscopy. As shown in Fig. 3, CeGlyRSm was predominantly confined to mitochondria, whereas CeGlyRSc was evenly distributed in the cytoplasm. Deletion of the MTS from CeGlyRSm redirected the protein to the cytoplasm. Because of the imaging technology resolution constraints, we could not rule out the possibility that a minor portion of the GFP fusion proteins targeted other unintended cellular compartments.
FIGURE 3.
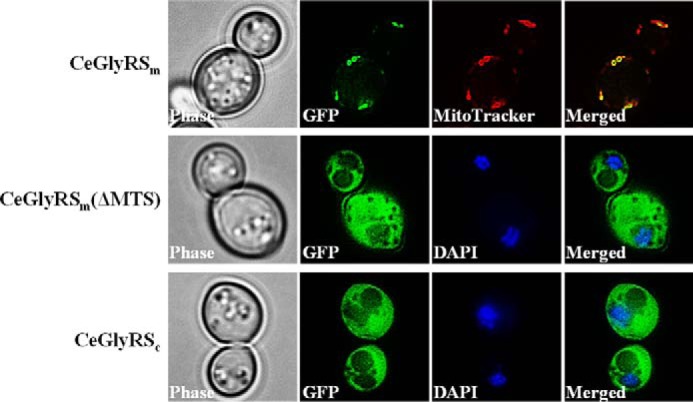
Cellular localization of C. elegans glycyl-tRNA synthetase (CeGlyRS) variants in yeast. Constructs encoding various GFP fusion proteins were individually transformed into a yeast GRS1 knock-out strain. The resultant transformants were treated with MitoTracker or DAPI and visualized under fluorescence microscopy. MitoTracker and DAPI were used to label mitochondria and nuclei, respectively.
The WHEP Domain-deleted CeGlyRS Variant Possesses Higher Thermal Stability
Because deletion of the WHEP domain impaired the cross-species rescue activity of CeGlyRSc (Fig. 2), we next investigated whether and to what extent this deletion alters the protein's thermal stability in vitro. Pursuant to this objective, genes encoding CeGlyRS variants were transformed into Escherichia coli, and His6-tagged CeGlyRS proteins were purified to homogeneity through nickel-nitrilotriacetic acid affinity chromatography (Fig. 4A). Purified CeGlyRSc and ΔWHEP proteins were then subjected to CD spectrometry at 200–250 nm. As shown in Fig. 4B, CeGlyRSc retained most of its secondary structure (with high molar ellipticity values at 222 and 208 nm) at temperatures below 40 °C but lost much of its secondary structure when the temperature reached 50 °C or higher. In contrast, ΔWHEP retained most of its secondary structure even when the temperature reached 50 °C. The deletion mutant did not lose much of its secondary structure until the temperature reached 60 °C or higher (Fig. 4B). As a result, CeGlyRSc and ΔWHEP. respectively. had melting temperatures of ∼60 and ∼68 °C (Fig. 4C). Thus, deletion of the WHEP domain increased the thermal stability of the protein.
FIGURE 4.
Thermal stability and structural flexibility of C. elegans glycyl-tRNA synthetase (CeGlyRS) variants. A, purification of CeGlyRS-His6 variants from E. coli transformants. B, circular dichroism spectra of CeGlyRSc and ΔWHEP. C, melting temperatures of CeGlyRSc and ΔWHEP determined at 222 nm. D, limited proteolysis of CeGlyRSc and ΔWHEP.
The WHEP Domain-deleted CeGlyRS Variant Possesses Slightly Lower Structural Flexibility
To further study the effect of the deletion on the structural flexibility of CeGlyRSc, limited proteolysis was carried out with CeGlyRSc/trypsin in a ratio of 100:1. Limited proteolysis is often used to probe the structure and dynamics of proteins. Exposed regions such as loops and other flexible regions are more susceptible to the prolific protease. As shown in Fig. 4D, ΔWHEP was somewhat more resistant to the protease than was the WT. Approximately 80% of the WT protein was hydrolyzed after 2 h of protease treatment, and only ∼10% protein remained after 4 h of treatment. In contrast, ∼40% of the ΔWHEP protein remained after 4 h of treatment. This result suggests that the WT protein possessed a slightly more-flexible structure than did the deletion mutant.
The MAD and WHEP Domain Respectively Play Roles in Aminoacylation of CetRNAmGly and CetRNAnGly
As mentioned above, CeGlyRS possesses two distinctive domains at its N terminus: MAD and WHEP. We were prompted to ask whether these two domains are involved in tRNA aminoacylation. Aminoacylation reactions were carried out at ambient temperature with 20 or 200 nm of enzyme and 5 μm of in vitro transcribed tRNAGly. Note that purified CeGlyRSm represents a mature form of CeGlyRSm (without MTS). As shown in Fig. 5A, CeGlyRSm and CeGlyRSc charged CetRNAnGly with a similarly high efficiency, suggesting that the MAD is dispensable for aminoacylation of CetRNAnGly. On the other hand, deletion of the WHEP domain from CeGlyRSc reduced its glycylation activity ∼3-fold, suggesting that the WHEP domain is somehow involved in this reaction (Fig. 5A). Although in vitro transcribed CetRNAmGly (lacking the entire T-arm) was a very poor substrate for glycylation (presumably because of a lack of modification at position 9 with 1-methyladenosine) (24), CeGlyRSm was more efficient (at least 5-fold) than CeGlyRSc (and ΔWHEP) in charging this T-armless tRNA (Fig. 5B). These results together suggest that the MAD enhances aminoacylation of CetRNAmGly by CeGlyRSm and that the WHEP domain contributes to aminoacylation of CetRNAnGly by CeGlyRSc. Relative aminoacylation activities of CeGlyRSc and ΔWHEP at various temperatures were determined and shown in Fig. 5C.
FIGURE 5.
Aminoacylation activities of C. elegans glycyl-tRNA synthetase (CeGlyRS) variants. A, aminoacylation of CetRNAnGly (5 μm) by CeGlyRS variants (20 nm). B, aminoacylation of CetRNAmGly (5 μm) by CeGlyRS variants (200 nm). C, aminoacylation activities of CeGlyRSc and ΔWHEP at various temperatures. Reactions were carried out under conditions similar to A for 6 min. D, polyacrylamide affinity co-electrophoresis. Affinities of CeGlyRSc and the WHEP domain for 32P-labeled CetRNAnGly were determined by polyacrylamide affinity co-electrophoresis. Indicated at the bottom of the gel are the protein concentrations used.
To take a closer look at the role of the WHEP domain in tRNA aminoacylation, kinetic parameters for aminoacylation of CetRNAnGly by CeGlyRSc and ΔWHEP were determined. As shown in Table 1, CeGlyRSc had a Km value of 0.24 μm for CetRNAnGly and a kcat value of 1.73 s−1, whereas ΔWHEP had a Km value of 0.21 μm for CetRNAnGly and a kcat value of 0.62 s−1. Thus, deletion of the WHEP domain had almost no effect on the apparent affinity of the enzyme for CetRNAnGly but reduced its catalytic rate 2.8 times. That is, CeGlyRSc possessed a catalytic efficiency (kcat/Km) 2.4 times higher than that of ΔWHEP. Similar results were obtained using yeast tRNAnGly as the substrate (Table 1).
TABLE 1.
Kinetic parameters for aminoacylation of tRNAnGly by C. elegans GlyRS variants
Each value is determined from a hyperbolic fit of three independent data sets.
| CeGlyRS variant | tRNA | Km | kcat | kcat/Km |
|---|---|---|---|---|
| μm | s−1 | μm−1 s−1 | ||
| CeGlyRSc | CetRNAnGly | 0.24 ± 0.09 | 1.73 ± 0.61 | 7.2 |
| ΔWHEP | CetRNAnGly | 0.21 ± 0.10 | 0.62 ± 0.28 | 3.0 |
| CeGlyRSc | SctRNAnGly | 0.39 ± 0.13 | 1.92 ± 0.75 | 4.9 |
| ΔWHEP | SctRNAnGly | 0.37 ± 0.14 | 0.51 ± 0.20 | 1.4 |
The WHEP Domain per se Does Not Bind tRNA
To test whether the WHEP domain itself binds tRNA, a DNA sequence encoding the WHEP domain was cloned into pET21, and the resultant construct was transformed into an E. coli strain, BL21(DE3), for expression. The recombinant WHEP domain (with a C-terminal His6 tag) was purified through nickel-nitrilotriacetic acid affinity chromatography, and an affinity co-electrophoresis assay was carried out using CetRNAnGly as the ligand. Affinity co-electrophoresis is particularly useful for determining weak protein-nucleic acid interactions. As shown in Fig. 5D, whereas CeGlyRSc effectively bound tRNAnGly (with a Kd value of ∼4 μm), the purified WHEP domain failed to bind the tRNA even at high protein concentrations (up to 64 μm). This result is in agreement with the findings of the kinetic study shown in Table 1.
Overexpression of the WHEP Domain-deleted CeGlyRS Variant Can Rescue the Yeast Knock-out Strain
Because deletion of the WHEP domain only reduced the aminoacylation efficiency (kcat/Km) of CeGlyRS for yeast tRNAGly 3.5-fold (Table 1), we wondered whether the deletion mutant could be made a functional yeast enzyme in vivo by overexpression from a stronger promoter. To this end, a DNA sequence encoding ΔWHEP was cloned into pTEF1 (with a strong TEF1 promoter), and the ability of the resultant construct to rescue the growth defects of the yeast GRS1 knock-out strain was tested. As shown in Fig. 6A, ΔWHEP, overexpressed from a TEF1 promoter, successfully restored the growth phenotype of the knock-out strain on 5-FOA, but not on YPG (row 3). Attaching a heterologous MTS to ΔWHEP failed to yield a functional yeast mitochondrial enzyme (row 4). Western blotting results showed that MTS-ΔWHEP had an expression level much lower than that of its counterpart without an MTS, i.e. ΔWHEP, in yeast (Fig. 6B, left panel), regardless of the promoters used. This may have been the reason why MTS-ΔWHEP failed to provide sufficient glycylation activity in yeast mitochondria (Figs. 2, 6). We also found that the TEF1 promoter used was ∼12-fold stronger than the ADH promoter used (Fig. 6B, right panel).
FIGURE 6.
Cross-species rescue activities of the WHEP domain-deleted CeGlyRSc. Constructs encoding ΔWHEP and MTS-ΔWHEP were transformed into a yeast grs1− strain, and the ability of the resultant transformants to grow on 5-FOA and YPG was tested. A, summary of the constructs and their rescue activities on 5-FOA and YPG. B, Western blotting. Left panel, relative expression levels of ΔWHEP and MTS-ΔWHEP; right panel, relative expression levels of ΔWHEP driven by different promoters. Indicated at the bottom of the blots are the amounts of protein extracts loaded into each well. Rows 1–4 (circled) in B represent constructs shown in A.
Discussion
Based on the modular organization of aaRSs, it is widely accepted that modern aaRSs descended from successive additions of new domains to the catalytic core (25). According to this hypothesis, primitive aaRSs possessed only a minimal core capable of activating amino acids. New domains were later recruited to the catalytic core, forming a larger aaRS capable of recognizing an ancient tRNA with a structure mimicking the existing tRNA acceptor stem. These early tRNA-aaRS pairs continued to grow through additions of the anticodon stem-loop structure and anticodon-binding domain to yield contemporary tRNAs and aaRSs. Other functional domains, such as the editing, protein-interacting, and auxiliary tRNA-binding domains, were later recruited by aaRSs to facilitate their translation efficiencies (25). The MAD and WHEP domain of CeGlyRS are probably among such examples (Fig. 5). However, some domains recruited by aaRSs are unrelated to their primary functions. Such domains often confer noncanonical functions, such as transcriptional regulation, translational regulation, mitochondrial RNA splicing, and cytokine-like activity, to the aaRSs (26).
In addition to serving as an intracellular translation enzyme, human GlyRS also circulates in serum and acts as a component of the innate defense system against ERK-activated tumorigenesis (27). Moreover, mutations in human GlyRS are known to cause an axonal form of CMT disease. A recent study further demonstrated that several CMT-associated GlyRS mutants can competitively block binding of the VEGF to its membrane receptor, Nrp1, leading to impaired motor neuron migration (28).
WHEP domains exist as single domains in higher eukaryotic TrpRS, HisRS, GlyRS, and MetRS and as multiple repeated domains in Glu-ProRS, but they do not exist in any non-aaRS proteins (29). Human Glu-ProRS possesses three tandem WHEP domains that participate in regulating the noncanonical function of the enzyme through interactions with specific protein factors and the 3′-UTRs of interferon-γ-induced inflammatory genes (30). In contrast, the single WHEP domain of CeGlyRS appears to participate in regulating the dynamic structure of the enzyme, a feature somewhat similar to its human homologue (31). Deletion of this domain had no discernible effect on the tRNA binding of the enzyme (Table 1) but substantially affected its catalytic efficiency, thermal stability, and structural flexibility (Figs. 4 and 5). Because all WHEP-containing GlyRS enzymes possess a similar protein sequence and domain organization (Fig. 1), the WHEP domains may play a similar role in these homologues.
Experimental Procedures
Construction of Plasmids
Cloning of the CeGRS1 gene or its derivatives into pADH (a high copy number yeast shuttle vector with a constitutive ADH promoter), pTEF1 (a high copy number yeast shuttle vector with a constitutive TEF1 promoter), or pET21b (an E. coli expression vector with a T7 promoter) followed the protocol described earlier. Briefly, a cDNA sequence containing an ORF of CeGlyRSm (1–2226 bp) was amplified by RT-PCR as an EagI-NdeI fragment using a pair of gene-specific primers. The PCR-amplified fragment was digested with EagI and NdeI and then cloned into the EagI/NdeI sites of pADH. Cloning of genes encoding various truncated forms of CeGlyRSm followed a similar protocol. To fuse a heterologous MTS to CeGlyRSc (or its derivatives), a DNA sequence encoding amino acid residues 1–46 of the mitochondrial precursor form of yeast valyl-tRNA synthetase was amplified by a PCR as an EagI-EagI fragment and was inserted at the 5′ end of the ORF of CeGlyRSc. To fuse the GFP to CeGlyRSc (or its derivatives), a DNA sequence encoding the GFP was amplified by a PCR as an XhoI-XhoI fragment and then inserted at the 3′ end of the ORF of CeGlyRSc.
Complementation Assay for Cytoplasmic Activity
The yeast GRS1 knock-out strain RJT3/II-1 was previously described (32). We carried out a complementation assay for cytoplasmic GlyRS activity by introducing a test plasmid carrying the target gene and a LEU2 marker into RJT3/II-1 and determining the ability of the transformants to grow in the presence of 5-FOA. Starting from a cell density of 1.0 A600, cell cultures were 3-fold serially diluted, and 10-μl aliquots of each dilution were spotted onto designated plates containing 5-FOA. The plates were incubated at 30 °C for 3 days. Transformants ejected the maintenance plasmid (which carried the WT ScGRS1 gene) with a URA3 marker in the presence of 5-FOA and thus could not grow on 5-FOA plates unless the test plasmid encoded a functional cytoplasmic GlyRS.
Complementation Assay for Mitochondrial Activity
The yeast GRS1 knock-out strain RJT3/II-1 was cotransformed with a test plasmid (which carried a LEU2 marker) and a second maintenance plasmid (which carried a TRP1 marker and only expressed the cytoplasmic form of ScGlyRS). In the presence of 5-FOA, the first maintenance plasmid (carrying a URA3 marker) was evicted from the cotransformants, and the second maintenance plasmid was retained. Thus, all cotransformants could survive 5-FOA selection because of the presence of the cytoplasmic ScGlyRS derived from the second maintenance plasmid. The mitochondrial phenotypes of the cotransformants were further tested on YPG plates at 30 °C, with results documented on day 3 following plating. Because a yeast cell cannot survive on glycerol (a nonfermentable carbon source) without functional mitochondria, the cotransformants did not grow on the YPG plates unless the test plasmid encoded a functional mitochondrial GlyRS.
Fluorescence Microscopy
Yeast cells were grown to ∼0.6 A600 in SD/−Leu selective medium. We pretreated cells with DAPI (0.5 μg/ml) or MitoTracker (300 nm) for 30 min. Fluorescence microscopy (Axio observer.A1; Carl Zeiss, Oberkochen, Germany) was then used to examine samples with a 100× objective at 25 °C, and images were captured with a CCD camera (Axiocam MRm; Carl Zeiss). Nuclear and mitochondrial tracks and merged images were generated with AxioVision Rel. 4.8 software and then subjected to two-dimensional deconvolution with AutoQuant X2.
Aminoacylation Assay
Aminoacylation reactions were carried out at ambient temperature in a buffer containing 50 mm HEPES (pH 7.5), 50 mm KCl, 15 mm MgCl2, 5 mm dithiothreitol, 10 mm ATP, 0.1 mg/ml bovine serum albumin, 5 μm tRNAGly, and 20 μm glycine (2 μm [3H]glycine; Moravek Biochemicals, Brea, CA). The specific activity of [3H]glycine used was 35.0 Ci/mmol. The final concentration of the enzymes used in the reactions was 20 nm (unless otherwise indicated). The reactions were quenched by spotting 10-μl aliquots of the reaction mixture onto Whatman filters (Maidstone, Kent, UK) soaked in 5% TCA and 2 mm glycine. Filters were washed three times for 15 min each in ice-cold 5% TCA before liquid scintillation counting. The data were obtained from three independent experiments and averaged.
Kinetic parameters for aminoacylation of tRNA by purified enzymes were determined by directly fitting the data points to the Michaelis-Menten equation. Initial rates of aminoacylation were determined at 25 °C with tRNAGly concentrations ranging 1–20 μm and enzyme concentrations ranging 10–200 nm. The data were obtained from three independent experiments and averaged.
Polyacrylamide Affinity Co-electrophoresis
In vitro transcribed CetRNAnGly was labeled with 32P using polynucleotide kinase (New England Biolabs, Beverly, MA) after dephosphorylation with calf intestine phosphatase. The recombinant WHEP domain was 2-fold serially diluted and mixed with a 5% polyacrylamide solution, forming a mini gel matrix with a protein gradient of 1–64 μm. 32P-Labeled tRNA was loaded into each well at an estimated concentration of 1 nm in 2-μl aliquots. The gel was run in buffer containing 1× TBE (90 mm Tris borate and 2 mm EDTA) and 50 mm NaCl at 20 °C at 50 V for 1 h. After electrophoresis, the gel was vacuum-dried and then exposed to x-ray film.
Miscellaneous Methods
Western blotting (33), purification of His6-tagged proteins (34), the CHX chase assay (23), and limited proteolysis (35) followed previously described protocols.
Author Contributions
C.-C. W. designed the study and wrote the paper. B.-C. L. performed the CD assay. C.-P. C completed the limited proteolysis assay. C.-Y. C. and C.-I. C. performed other assays. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by Ministry of Science and Technology Grants MOST 103-2311-B-008-003-MY3, MOST 103-2923-B-008-001-MY3, and NSC 102-2311-B-008-004-MY3 (to C.-C. W.) and National Central University and Landseed Hospital Joint Research Program Grant NCU-LSH-103-A-003 (to C.-C. W.). The authors declare that they have no conflicts of interest with the contents of this article.
- aaRS
- aminoacyl-tRNA synthetase
- CHX
- cycloheximide
- DAPI
- 4′,6-diamidino-2-phenylindole
- 5-FOA
- 5-fluoroorotic acid
- GlyRS
- glycyl-tRNA synthetase
- MAD
- mitochondrial appended domain
- MTS
- mitochondrial targeting signal
- YPG
- yeast extract peptone glycerol
- CMT
- Charcot-Marie-Tooth.
References
- 1. Carter C. W., Jr. (1993) Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 62, 715–748 [DOI] [PubMed] [Google Scholar]
- 2. Burbaum J. J., and Schimmel P. (1991) Structural relationships and the classification of aminoacyl-tRNA synthetases. J. Biol. Chem. 266, 16965–16968 [PubMed] [Google Scholar]
- 3. Giegé R. (2006) The early history of tRNA recognition by aminoacyl-tRNA synthetases. J Biosci 31, 477–488 [DOI] [PubMed] [Google Scholar]
- 4. Giegé R., Sissler M., and Florentz C. (1998) Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 26, 5017–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang H. Y., Tang H. L., Chao H. Y., Yeh L. S., and Wang C. C. (2006) An unusual pattern of protein expression and localization of yeast alanyl-tRNA synthetase isoforms. Mol. Microbiol. 60, 189–198 [DOI] [PubMed] [Google Scholar]
- 6. Tang H. L., Yeh L. S., Chen N. K., Ripmaster T., Schimmel P., and Wang C. C. (2004) Translation of a yeast mitochondrial tRNA synthetase initiated at redundant non-AUG codons. J. Biol. Chem. 279, 49656–49663 [DOI] [PubMed] [Google Scholar]
- 7. Chang K. J., and Wang C. C. (2004) Translation initiation from a naturally occurring non-AUG codon in Saccharomyces cerevisiae. J. Biol. Chem. 279, 13778–13785 [DOI] [PubMed] [Google Scholar]
- 8. Natsoulis G., Hilger F., and Fink G. R. (1986) The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell 46, 235–243 [DOI] [PubMed] [Google Scholar]
- 9. Chatton B., Walter P., Ebel J. P., Lacroute F., and Fasiolo F. (1988) The yeast VAS1 gene encodes both mitochondrial and cytoplasmic valyl-tRNA synthetases. J. Biol. Chem. 263, 52–57 [PubMed] [Google Scholar]
- 10. Mazauric M. H., Reinbolt J., Lorber B., Ebel C., Keith G., Giegé R., and Kern D. (1996) An example of non-conservation of oligomeric structure in prokaryotic aminoacyl-tRNA synthetases. Biochemical and structural properties of glycyl-tRNA synthetase from Thermus thermophilus. Eur. J. Biochem. 241, 814–826 [DOI] [PubMed] [Google Scholar]
- 11. Ostrem D. L., and Berg P. (1970) Glycyl-tRNA synthetase: an oligomeric protein containing dissimilar subunits. Proc. Natl. Acad. Sci. U.S.A. 67, 1967–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nada S., Chang P. K., and Dignam J. D. (1993) Primary structure of the gene for glycyl-tRNA synthetase from Bombyx mori. J. Biol. Chem. 268, 7660–7667 [PubMed] [Google Scholar]
- 13. Shiba K., Schimmel P., Motegi H., and Noda T. (1994) Human glycyl-tRNA synthetase. Wide divergence of primary structure from bacterial counterpart and species-specific aminoacylation. J. Biol. Chem. 269, 30049–30055 [PubMed] [Google Scholar]
- 14. Chien C. I., Chen Y. L., Chen S. J., Chou C. M., Chen C. Y., and Wang C. C. (2015) Vanderwaltozyma polyspora possesses two glycyl-tRNA synthetase genes: One constitutive and one inducible. Fungal Genet. Biol. 76, 47–56 [DOI] [PubMed] [Google Scholar]
- 15. Mazauric M. H., Keith G., Logan D., Kreutzer R., Giegé R., and Kern D. (1998) Glycyl-tRNA synthetase from Thermus thermophilus: wide structural divergence with other prokaryotic glycyl-tRNA synthetases and functional inter-relation with prokaryotic and eukaryotic glycylation systems. Eur. J. Biochem. 251, 744–757 [DOI] [PubMed] [Google Scholar]
- 16. Krajewski K. M., Lewis R. A., Fuerst D. R., Turansky C., Hinderer S. R., Garbern J., Kamholz J., and Shy M. E. (2000) Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain 123, 1516–1527 [DOI] [PubMed] [Google Scholar]
- 17. Antonellis A., Ellsworth R. E., Sambuughin N., Puls I., Abel A., Lee-Lin S. Q., Jordanova A., Kremensky I., Christodoulou K., Middleton L. T., Sivakumar K., Ionasescu V., Funalot B., Vance J. M., Goldfarb L. G., et al. (2003) Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 72, 1293–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motley W. W., Talbot K., and Fischbeck K. H. (2010) GARS axonopathy: not every neuron's cup of tRNA. Trends Neurosci. 33, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nangle L. A., Zhang W., Xie W., Yang X. L., and Schimmel P. (2007) Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc. Natl. Acad. Sci. U.S.A. 104, 11239–11244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Achilli F., Bros-Facer V., Williams H. P., Banks G. T., AlQatari M., Chia R., Tucci V., Groves M., Nickols C. D., Seburn K. L., Kendall R., Cader M. Z., Talbot K., van Minnen J., Burgess R. W., et al. (2009) An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis. Model Mech. 2, 359–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grice S. J., Sleigh J. N., Motley W. W., Liu J. L., Burgess R. W., Talbot K., and Cader M. Z. (2015) Dominant, toxic gain-of-function mutations in gars lead to non-cell autonomous neuropathology. Hum. Mol. Genet. 24, 4397–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang C. P., Chang C. Y., Lee Y. H., Lin Y. S., and Wang C. C. (2015) Divergent alanyl-tRNA synthetase genes of Vanderwaltozyma polyspora descended from a common ancestor through whole-genome duplication followed by asymmetric evolution. Mol. Cell. Biol. 35, 2242–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao C. C., Lin C. H., Chen S. J., and Wang C. C. (2012) Trans-kingdom rescue of Gln-tRNAGln synthesis in yeast cytoplasm and mitochondria. Nucleic Acids Res. 40, 9171–9181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakurai M., Ohtsuki T., and Watanabe K. (2005) Modification at position 9 with 1-methyladenosine is crucial for structure and function of nematode mitochondrial tRNAs lacking the entire T-arm. Nucleic Acids Res. 33, 1653–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakubowski H. (2012) Quality control in tRNA charging. Wiley Interdiscip Rev. RNA 3, 295–310 [DOI] [PubMed] [Google Scholar]
- 26. Martinis S. A., Plateau P., Cavarelli J., and Florentz C. (1999) Aminoacyl-tRNA synthetases: a family of expanding functions. EMBO J. 18, 4591–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park M. C., Kang T., Jin D., Han J. M., Kim S. B., Park Y. J., Cho K., Park Y. W., Guo M., He W., Yang X. L., Schimmel P., and Kim S. (2012) Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 109, E640–E647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He W., Bai G., Zhou H., Wei N., White N. M., Lauer J., Liu H., Shi Y., Dumitru C. D., Lettieri K., Shubayev V., Jordanova A., Guergueltcheva V., Griffin P. R., Burgess R. W., et al. (2015) CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature 526, 710–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ray P. S., and Fox P. L. (2014) Origin and evolution of glutamyl-prolyl tRNA synthetase WHEP domains reveal evolutionary relationships within Holozoa. PLoS One 9, e98493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jia J., Arif A., Ray P. S., and Fox P. L. (2008) WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol. Cell 29, 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He W., Zhang H. M., Chong Y. E., Guo M., Marshall A. G., and Yang X. L. (2011) Dispersed disease-causing neomorphic mutations on a single protein promote the same localized conformational opening. Proc. Natl. Acad. Sci. U.S.A. 108, 12307–12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turner R. J., Lovato M., and Schimmel P. (2000) One of two genes encoding glycyl-tRNA synthetase in Saccharomyces cerevisiae provides mitochondrial and cytoplasmic functions. J. Biol. Chem. 275, 27681–27688 [DOI] [PubMed] [Google Scholar]
- 33. Chang C. P., Tseng Y. K., Ko C. Y., and Wang C. C. (2012) Alanyl-tRNA synthetase genes of Vanderwaltozyma polyspora arose from duplication of a dual-functional predecessor of mitochondrial origin. Nucleic Acids Res. 40, 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang C. P., Lin G., Chen S. J., Chiu W. C., Chen W. H., and Wang C. C. (2008) Promoting the formation of an active synthetase/tRNA complex by a nonspecific tRNA-binding domain. J. Biol. Chem. 283, 30699–30706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ladror U. S., Egan D. A., Snyder S. W., Capobianco J. O., Goldman R. C., Dorwin S. A., Johnson R. W., Edalji R., Sarthy A. V., McGonigal T., Walter K. A., and Holzman T. F. (1998) Domain structure analysis of elongation factor-3 from Saccharomyces cerevisiae by limited proteolysis and differential scanning calorimetry. Protein Sci. 7, 2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]







