Abstract
ARAP3 (Arf-GAP with Rho-GAP domain, ANK repeat, and PH domain-containing protein 3) is unique for its dual specificity GAPs (GTPase-activating protein) activity for Arf6 (ADP-ribosylation factor 6) and RhoA (Ras homolog gene family member A) regulated by phosphatidylinositol 3,4,5-trisphosphate and a small GTPase Rap1-GTP and is involved in regulation of cell shape and adhesion. However, the molecular interface between the ARAP3-RhoGAP domain and RhoA is unknown, as is the substrates specificity of the RhoGAP domain. In this study, we solved the crystal structure of RhoA in complex with the RhoGAP domain of ARAP3. The structure of the complex presented a clear interface between the RhoGAP domain and RhoA. By analyzing the crystal structure and in combination with in vitro GTPase activity assays and isothermal titration calorimetry experiments, we identified the crucial residues affecting RhoGAP activity and substrates specificity among RhoA, Rac1 (Ras-related C3 botulinum toxin substrate 1), and Cdc42 (cell division control protein 42 homolog).
Keywords: complex; crystal structure; GTPase-activating protein (GAP); Ras homolog gene family, member A (RhoA); Rho (Rho GTPase); substrate specificity; ARAP3; GTPase activity assay; RhoGAP
Introduction
The small GTPase Ras superfamily in human consists of at least 154 members divided into five principal families: the Ras, Rho, Rab, Arf, and Ran families (1). These proteins act as molecular switches, cycling between an inactive GDP-bound form and an active GTP-bound form, transducing signals from plasma membrane receptors to downstream signal molecules. These small GTPases generally have low intrinsic GTPase hydrolytic activity, and efficient hydrolysis requires interaction with family-specific groups of GAPs (GTPase-activating proteins) which can accelerate the cleavage step by several orders of magnitude. Arf6, an Arf (ADP-ribosylation factor) family member, is involved in endocytic pathways, endosomal recycling, cell migration, exocytosis, actin reorganization, plasma membrane reorganization, and cytokinesis (2). RhoA belongs to the Rho (Ras homologous) family, which primarily regulates cell shape, polarity, and motility by inducing stress fibers in cells that provides intracellular contractility (3–6). In addition, Arf6 plays a role in cancer cell invasion, and RhoA regulates cancer cell morphology and migration (7, 8).
ARAP3, a multidomain protein, belongs to the ARAP superfamily proteins and contains both functional ArfGAP and RhoGAP domains, a sterile α motif domain, five pleckstrin homology (PH)3 domains and an Ras-associating (RA) domain (see Fig. 1a). ARAP3 was originally identified as a PI3K effector in a screening for PtdIns(3,4,5)P3 binding proteins (9). ARAP proteins are regulated at different levels. It has been demonstrated that the ArfGAP activity of ARAP3 is induced by the phosphoinositols PtdIns(3,4,5)P3 and phosphatidylinositol 3,4-bisphosphate in vivo and in vitro, whereas its RhoGAP activity is unaffected in vitro (9, 10). Moreover, the RhoGAP activity of ARAP3 is up-regulated by direct binding of Rap1-GTP to the RA domain in vivo and in vitro (10). ARAP3 is unique because of its two different functional GAPs, ArfGAP and RhoGAP, which act specifically toward Arf6 and RhoA, respectively (9, 10). This property most likely provides an efficient way to coordinate the two signaling processes. However, the mechanisms underlying the specificity of ARAP3 for Rho and Arf GTPases have not been thoroughly characterized.
FIGURE 1.
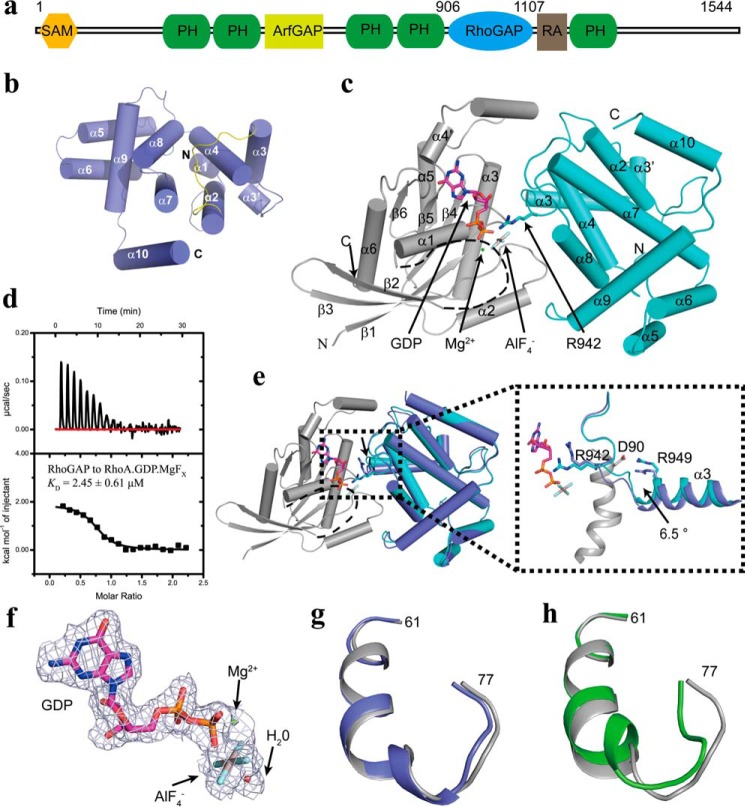
Overall structure of the ARAP3-RhoGAP domain in complex with RhoA·GDP·AlF4−. a, schematic diagram of the domain arrangement of ARAP3. b, overall structure of one molecular ARAP3-RhoGAP domain is represented in cylindrical helix cartoon and colored in slate. The 11 helical segments of the ARAP3-RhoGAP domain are labeled as α1–α10. The α2-α3 loop containing the arginine finger is colored in yellow. c, overall structure of one molecule of RhoA·GDP·AlF4− in complex with the ARAP3-RhoGAP domain (cyan) shown in cartoon representation. RhoA is colored in gray, and the switch I region, without interpretable electron density in our structure, is represented by a black dashed line. GDP and AlF4− are shown as sticks, and the Mg2+ ion is shown as a green ball. d, ITC fitting curves of the ARAP3 RhoGAP domain to RhoA·GDP·MgFx. e, superimposition of the structure of the ARAP3-RhoGAP domain in apo (slate) and complexed state (cyan). f, the 2Fo − Fc electron density map around the GDP, contoured at level 1.0 sigma, modeled as GDP·Mg2+·AlF4−·H2O. g and h, superimposition of the switch II region of RhoA in the ARAP3-RhoGAP·RhoA complex (gray) with that of RhoA-GTPγS (PDB code 1A2B; colored in slate, g) and RhoA-GDP (PDB code 1FTN; colored in green, h).
In this study, we focused on the structure and function of RhoGAP in ARAP3. It is known that ARAP3 regulates many cell functions through its RhoGAP activity, such as formation of lamellipodia upon growth factor stimulation (11), development of angiogenesis in PI3K signaling pathway (12), and inhibition of peritoneal dissemination of scirrhous gastric carcinoma cells (13). Because Rap1-GTP can up-regulate the RhoGAP activity of ARAP3, the role of ARAP3 in downstream Rap1-GTP cell processes is RhoGAP-dependent. In neutrophils, ARAP3 regulates chemotaxis and adhesion-dependent processes (14). In macrophages, following long term treatment by TGF-β1, ARAP3 down-regulates the production of chemokines and slows cell migration (15). ARAP3 mediates the neurite outgrowth from PC12 cells in response to treatment by basic fibroblast growth factors, nerve growth factor, and cAMP, all of which can up-regulate Rap1-GTP level in PC12 cells (16–18). However, the nature of the ARAP3-RhoGAP·RhoA interaction was still unknown.
To determine the structure and function of the ARAP3-RhoGAP domain, we solved the x-ray crystal structures of the RhoGAP domain and of the complexed RhoA·RhoGAP domain with a substrate transition state analog GDP·AlF4−. Through these structures, we mapped the interface between RhoA and the ARAP3-RhoGAP domain. Using the complexed structure as a guide, we performed site-directed mutagenesis to analyze the role of interface residues on complex formation and on GTP hydrolysis. Meanwhile, we also found the residues responsible for the substrate specificity of ARAP3 among RhoA, Rac1, and Cdc42 in vitro.
Results
Overall Structure of the ARAP3-RhoGAP Domain in Complex with RhoA in the Transition State
To investigate the structure and function of the ARAP3-RhoGAP domain, we constructed different fragments according to the secondary structure prediction. The most promising construct is the fragment containing ARAP3 residues 906–1107, which binds RhoA with a dissociation constant (KD) of 2.45 μm (Fig. 1d; see Table 2). After extensive crystallization trials, we determined the 2.30 Å crystal structure of the ARAP3-RhoGAP domain (Table 1). Although we failed to obtain crystals by mixing the ARAP3-RhoGAP domain with different RhoA constructs, we successfully obtained crystals by expressing a fusion protein of the ARAP3-RhoGAP domain and RhoA, using a 28-residue flexible linker (19, 20), which was purified in buffer containing GDP, Mg2+, Al3+, and F− to mimic the transition state (21, 22).
TABLE 2.
Thermodynamic parameters of the wild type and mutants of the ARAP3-RhoGAP domain to different small GTPases by ITC experiments
The ITC experiments were carried out at 293 K, and all proteins were in buffer conditions: 20 mm Tris (pH 7.5), 200 mm NaCl, 5 mm MgCl2, and 10 mm NaF. ND, not detected. Errors in the table are standard errors.
| RhoGAP | GTPases with GDP·MgFx | ΔH | ΔS | KD | N |
|---|---|---|---|---|---|
| kcal/mol | cal/mol/K | μm | |||
| Wild type | RhoA | 1.87 ± 0.08 | 31.9 | 2.45 ± 0.61 | 0.74 |
| F972A | RhoA | 4.23 ± 0.19 | 37.7 | 4.37 ± 0.52 | 0.55 |
| R949E | RhoA | ND | |||
| R982E | RhoA | ND | |||
| R985E | RhoA | ND | |||
| Wild type | Cdc42 | 10.33 ± 2.21 | 54.5 | 59.88 ± 11.31 | 0.67 |
| Wild type | Cdc42 (S88D) | 5.50 ± 0.23 | 42.9 | 5.41 ± 0.95 | 0.73 |
| Wild type | Rac1 | ND | |||
| Wild type | Rac1 (A88D,A95E) | 14.13 ± 0.50 | 69.8 | 18.87 ± 1.56 | 0.79 |
TABLE 1.
Data collection and refinement statistics
| Data collection statistics | RhoGAPARAP3 | RhoGAPARAP3/RhoA·GDP·AlF4− |
|---|---|---|
| Protein Data Bank code | 5JD0 | 5JCP |
| Data collection | ||
| Wavelength (Å) | 0.9795 | 0.9795 |
| Space group | P43212 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 108.86, 108.86, 94.46 | 56.79, 93.21, 164.28 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| Resolution range (Å) | 38.36–2.30 (2.42–2.30)a | 47.22.00–2.10 (2.18–2.10)a |
| Rmerge (%) | 13.8 (64.2) | 10.8 (50.6) |
| I/σI | 11.1 (3.8) | 15.9 (4.1) |
| Completeness (%) | 99.1 (100) | 99.8 (99.9) |
| Redundancy | 9.1 (9.4) | 6.0 (6.1) |
| Refinement | ||
| Number of reflections (overall) | 25,540 | 51,660 |
| Number of reflections (test set) | 1300 | 2645 |
| Rwork/Rfree (%) | 22.0/26.0 | 18.6/22.7 |
| Number of atoms | ||
| Protein | 3088 | 5581 |
| Ligands | 0 | 68 |
| Water | 89 | 192 |
| B-factors (Å2) | ||
| Protein | 44.8 | 38.3 |
| Ligands | 0 | 27.8 |
| Water | 43.6 | 36.8 |
| RMSDs | ||
| Bond length (Å) | 0.008 | 0.007 |
| Bond angles (°) | 0.916 | 0.910 |
| Ramachandran plot (%) | ||
| Favored | 97.0 | 98.0 |
| Allowed | 3.0 | 2.0 |
| Outlier | 0 | 0 |
a The values in parentheses are for the highest resolution shell.
Crystals of the ARAP3-RhoGAP domain belong to space group P43212 with two molecules in the asymmetric unit. The two ARAP3-RhoGAP domain molecules in the asymmetric unit are similar to one another with a Cα atom root mean square deviation (RMSD) of 0.375 Å calculated by PyMOL. The overall structure of the ARAP3-RhoGAP domain exhibits a canonical RhoGAP domain structure, which consists of nine long α-helices and two short α-helices packed in an anti-parallel way, labeled as α1–α10, with an extra α-helix at the C terminus (Fig. 1b). Most RhoGAP domains have a conserved motif in the loop between the α2 and the α3 helices. In this motif, a central arginine is the primary element required for GTPase rate enhancement and is called the arginine finger. The sequence of this motif in the ARAP3-RhoGAP domain is GVYRKGG (residues 939–945; Fig. 1b). The ARAP3-RhoGAP domain structure exhibits structural similarity to many other RhoGAP structures with an RMSD from 1.4 to 2.4 Å and identity from 17 to 30%, analyzed by the Dali server (23).
Crystals of RhoA·GDP·AlF4− complexed with the ARAP3-RhoGAP domain belong to space group P212121, and the asymmetric unit contains two molecules. The structure was solved by molecule replacement, refined to 2.10 Å (Table 1), and the overall structure of one complex in the asymmetric unit is shown in Fig. 1c. The two complex molecules in the asymmetric unit are similar to one another with a Cα atom RMSD of 0.4 Å calculated by PyMOL. Residues 28–40 in the switch I region of RhoA, which cannot be observed because of missing electron density, are shown by the dotted line in Fig. 1c. In this final refined complex structure, the electron density map next to the GDP in the active center is almost square planar, modeled as AlF4− (Fig. 1f). The structures of the ARAP3-RhoGAP domain in the apo and the complexed state show no significant difference with a Cα atom RMSD of 0.523 Å calculated by PyMOL. The main differences between the apo and the complexed state structures of the ARAP3-RhoGAP domain are found in the α2-α3 loop and the α3 helix (Fig. 1e). The Arg-942 residue in the α2-α3 loop is moved closer to interact with and stabilize the orientation of GDP·AlF4−. The α3 helix of ARAP3 RhoGAP domain has a 6.5° rotation between the apo and the complexed state, driving Arg-949ARAP3 closer to Asp-90RhoA to form tight salt bridges (Fig. 1e). RhoA·GDP·AlF4− superimposes well with the structure of RhoA-GTPγS (PDB code 1A2B; Fig. 1g) but has a conformational difference at the switch II region compared with RhoA-GDP (PDB code 1FTN; Fig. 1h). However, we could not compare the conformation of switch I from these structures because the electron density map of this region was missing from the structure we solved.
Interface between ARAP3-RhoGAP Domain and RhoA
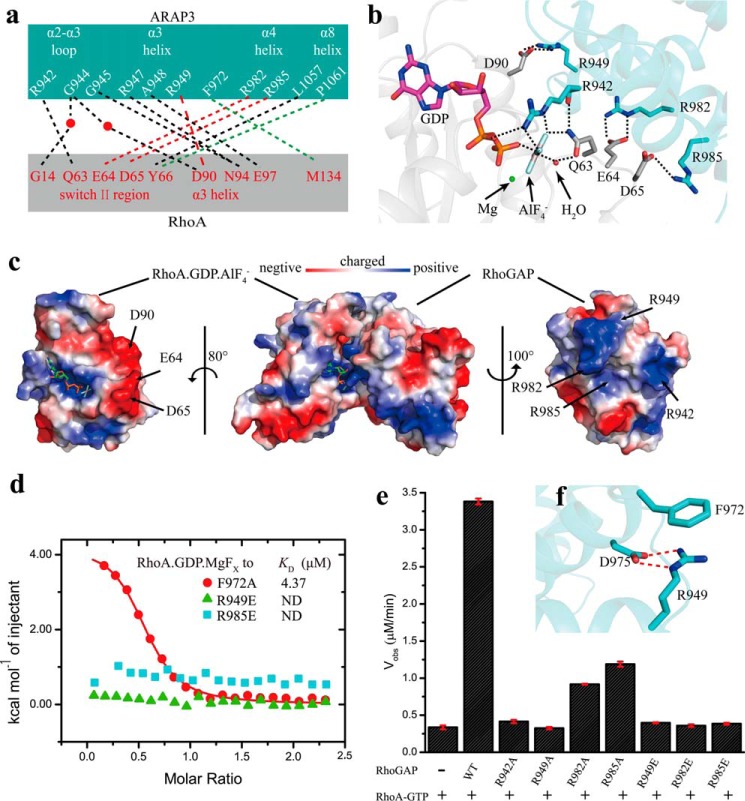
ARAP3-RhoGAP domain complexed with RhoA in the transition state buries 2092 Å2 of solvent-accessible surface. The interaction surface of the ARAP3-RhoGAP domain involves the α2-α3 loop and the α3, α4, and α8 helices. Residues contributing to the interaction interface from RhoA are located at the switch II region, the α3 helix, and the insert region (Fig. 2a). The complexed structure shows the conserved active center as reported previously. In the complexed structure, residue Arg-942 is pointed into the nucleotide binding pocket; the guanidinium group of Arg-942 interacts with the α-oxygen of GDP and two fluorides of AlF4−, and the main chain oxygen of Arg-942 forms a hydrogen bond with the side chain amide group of the RhoA catalytic residue Gln-63. Gln-63RhoA is thus stabilized and forms hydrogen bonds with a fluoride of AlF4− and a water molecule through its side chain (Fig. 2b). Through these conserved interactions, GTP is positioned in line with the water molecule, because the aluminum ion of the γ-phosphate analog AlF4− is located in line with the water molecule and the β-oxygen of GDP, with distances of 2.0 and 1.9 Å (Fig. 2b), respectively. In addition, the positively charged arginine finger of the ARAP3-RhoGAP neutralizes negative charge at the γ-phosphate, which makes the water molecule easier to conduct a nucleophilic attack on the γ-phosphate of GTP; thus the GTP hydrolysis rate of RhoA is accelerated. The RhoGAP domain of ARAP3 interacts with RhoA mainly through electrostatic interactions. Negatively charged GDP·AlF4− and negatively charged residues Glu-64 and Asp-65 of the switch II region and Asp-90 of the α3 helix of RhoA interact tightly with positively charged residues of the ARAP3-RhoGAP domain (Arg-942 of the α2-α3 loop, Arg-949 of the α3 helix, and Arg-982 and Arg-985 of the α4 helix) through salt bridges (Fig. 2b). We can observe the electrostatic interactions more clearly from the electrostatic representation of the complexed structure (Fig. 2c); residues Glu-64, Asp-65, and Asp-90 and GDP·AlF4− forming the negatively charged surface of RhoA directly bind to the positively charged surface of the ARAP3-RhoGAP domain formed by residues Arg-942, Arg-949, Arg-982, and Arg-985. In addition to the interactions shown above, there are several hydrogen bonds formed between the ARAP3-RhoGAP domain and RhoA: the main chain oxygen of residue Gly-945ARAP3 and the main chain nitrogen of residue Arg-947ARAP3 form hydrogen bonds with residue Asn-94RhoA (see Fig. 4b); the main chain nitrogen of residue Ala-948ARAP3 forms a hydrogen bond with residue Glu-97RhoA (see Fig. 4b); and the main chain oxygen of residue Leu-1057ARAP3 forms a hydrogen bond with residue Tyr-66RhoA. There are also water-mediated contacts between the main chain nitrogen of residue Gly-944ARAP3 and Gly-14RhoA and between the main chain oxygen of residue Gly-944ARAP3 and Asp-90RhoA. In addition to electrostatic and hydrogen bonding interactions, hydrophobic interactions are also observed; residue Phe-972ARAP3 of the α3′-α4 loop and residue Pro-1061ARAP3 of the α8 helix make hydrophobic contacts with residue Met-134RhoA and residue Tyr-66RhoA in the switch II region.
FIGURE 2.
Interaction surface between the ARAP3-RhoGAP domain and RhoA. a, schematic representation of interacting residues. Hydrogen bonds, salt bridges, and hydrophobic interactions are shown with black, red, and green dashed lines, respectively. Water is shown as a red ball. b, zoomed-in view of the electrostatic interaction surface with interacting residues displayed as sticks. c, structure of ARAP3-RhoGAP·RhoA·GDP·AlF4− is shown with electrostatic potential surface, in which blue, red, and white represent positively charged, negatively charged, and neutral areas, respectively. d, ITC fitting curves of different point mutants of the ARAP3-RhoGAP domain to RhoA·GDP·MgFx. e, GTPase assay results of 20 nm wild type ARAP3-RhoGAP domain and its mutants to 35 μm RhoA-GTP at 293 K, measured by MESG/PNP system. f, Arg-949 stabilizes the structure of the ARAP3 RhoGAP domain though salt bridges (red dashed lines) interactions with Asp-975 and cation-π interactions with Phe-972. The error bars represent the standard deviation from three independent experiments.
FIGURE 4.
All three RhoGAP·RhoA complexes exhibit two interfaces in the same location. a, sequence alignment of the RhoGAP domains of ARAP3, ARHGAP1, and ARHGAP20 located at the two interfaces. The secondary elements of ARAP3 RhoGAP are shown on the top of the sequences. The residues on the interaction interface forming polar interactions with RhoA within 4 Å are highlighted in red, green, and blue, respectively. b and c, comparison of the differences of interfaces 1 (b) and 2 (c) in detail. The hydrogen bonds are shown as black dashed lines, and the salt bridges are highlighted with red dashed lines. RhoA is colored in gray. The RhoGAP domains of ARAP3, ARHGAP, and ARHGAP20 are colored in cyan, yellow, and orange, respectively.
Based on the complexed structure, we made site-directed mutations in ARAP3-RhoGAP to verify the importance of residues for the interaction between the ARAP3-RhoGAP domain and RhoA. We used ITC experiments to measure the binding affinity between RhoA and the wild type or mutants of the ARAP3-RhoGAP domain. The results indicated that substitution of residue Phe-972 in the RhoGAP domain by alanine reduced the binding affinity to a KD of 4.37 μm, approximately half of the binding ability of the wild type ARAP3-RhoGAP domain (Fig. 2d and Table 2). Moreover, a single mis-sense mutation of each of the residues Arg-949, Arg-982, or Arg-985 in the RhoGAP domain to glutamic acid was sufficient to abolish complex formation (Fig. 2d and Table 2). The GTPase assay results were consistent with the ITC findings. The GTP hydrolysis reaction rate, using 25 nm wild type ARAP3-RhoGAP domain and 35 μm RhoA-GTP, was 10-fold greater than that of the RhoA alone (Fig. 2e). A single point mutation of each of the residues R942A, R949A, R949E, R982E, and R985E in the RhoGAP domain dramatically reduced the GAP activity of the ARAP3-RhoGAP domain under the same conditions (Fig. 2e). Mutants R982A and R985A still retained slight GAP activity so they could weakly accelerate the GTP hydrolysis reaction rate of RhoA by ∼3-fold (Fig. 2e). Intriguingly, mutant R949A also dramatically reduced the GAP activity of the ARAP3-RhoGAP domain (Fig. 2e), indicating that Arg-949ARAP3 is important for the ARAP3-RhoGAP domain to perform its GAP activity. Indeed, Arg-949ARAP3 interacts tightly with Phe-972ARAP3 and Asp-975ARAP3 through cation-π and electrostatic interactions (Fig. 2f), thus stabilizing the RhoGAP structure. All of these results demonstrate that both the arginine finger and the residues responsible for binding to RhoA are crucial to maintain the GAP activity of the ARAP3-RhoGAP domain.
Comparison with Other RhoGAP·RhoA Complexes in the Transition State
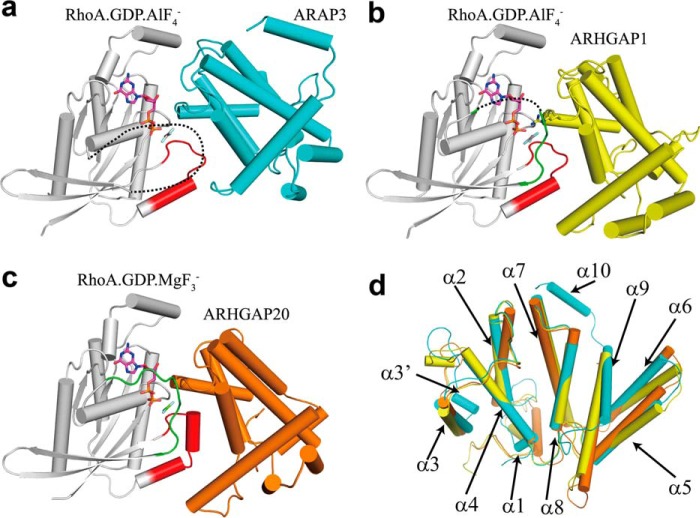
Although there are over 70 different RhoGAPs, only two other RhoGAPs in complex with RhoA in the transition state have been solved (Fig. 3, b and c): ARHGAP1, which is also called p50RhoGAP or Cdc42GAP (PDB codes 1TX4 and 1OW3), and ARHGAP20 (PDB code 3MSX). The sequence of the RhoGAP domain of ARAP3 shows identity with ARHGAP1 (26%) and ARHGAP20 (25%). The most remarkable difference was found at the interface involving the switch I region of RhoA; in the p50RhoGAP·RhoA complex residues Tyr-34RhoA, Pro-36RhoA, Val-38RhoA, and Phe-39RhoA make extensive hydrophobic contacts with RhoGAP of ARHGAP1 (24), whereas in the ARHGAP20·RhoA complex residues Tyr-34RhoA, Val-35RhoA, Pro-36RhoA, and Phe-39RhoA make extensive hydrophobic interactions with RhoGAP of ARHGAP20. However, we could not observe these hydrophobic interactions between RhoA and RhoGAP of ARAP3, because the switch I region in our structure is poorly ordered, and the electron density map of residues 28–40 is missing. The missing electron density in the switch I region of RhoA indicates that the switch I region may not be concerned with the ARAP3-RhoGAP binding. Another notable difference was found at the N terminus of the α9 helix. The α9 helix of ARAP3 RhoGAP is shorter than ARHGAP1 and ARHGAP20 (Fig. 3d), and the α8-α9 loop (residues 1067–1071) is poorly ordered, losing the hydrophobic interactions with residues Leu-69 and Leu-72 in the α2 helix of RhoA (24).
FIGURE 3.
Comparison of the RhoGAP domain of ARAP3, ARHGAP1, and ARHGAP20 in complex with RhoA in the transition state. a–c, structures of the RhoGAP domain of ARAP3 (cyan), ARHGAP1 (yellow, PDB code 1TX4), and ARHGAP20 (orange, PDB code 3MSX) binding to RhoA in the transition state. RhoA is colored in gray with the switch I region, and the switch II region is highlighted in green and red, respectively. Regions without interpretable electron density are represented by black dashed lines. d, superposition of the complexed state structures of ARAP3-RhoGAP (cyan), ARHGAP1-RhoGAP (yellow), and ARHGAP20-RhoGAP (orange).
Despite these differences, all three RhoGAP·RhoA complexes exhibit two interfaces in the same location. The first interface consists of the α3 helix of RhoGAP and the α3 helix of RhoA, and the second interface consists of the α4 helix of RhoGAP and the switch II region of RhoA. Residues in the first interface are less conserved than those in the second interface among ARAP3, ARHGAP1, and ARHGAP20 (Fig. 4a). There is a conserved alanine residue at the end of the α2-α3 loop of the RhoGAP domain (Fig. 4a), which interacts with Asn-94RhoA in ARHGAP1 and ARHGAP20 (Fig. 4b). However, in ARAP3, it is replaced by the Gly-945APAP3 (Fig. 4b). There is also a conserved arginine residue at the α3 helix (Fig. 4a), which interacts with Glu-97RhoA only in ARHGAP20 (Fig. 4b). In the first interface, ARHGAP1 only has interactions with Asn-94RhoA and Glu-97RhoA, whereas the ARAP3-RhoGAP domain has extra interactions with Asp-90RhoA through salt bridges, and ARHGAP20 has interactions with Asp-90RhoA, Glu-93RhoA, Asn-94RhoA, and Glu-97RhoA (Fig. 4b). In the α4 helix, there are two conserved residues, lysine and arginine (Fig. 4a). The conserved arginine in the ARAP3-RhoGAP domain (Arg-985) interacts with Asp-65RhoA, whereas in both ARHGAP1 and ARHGAP20, it interacts with both Glu-64RhoA and Asp-65RhoA (Fig. 4c). In ARHGAP1 and ARHGAP20, the conserved lysine interacts with Asp-65RhoA (Fig. 4c). In ARAP3, however, the conserved lysine is not on the interface and is replaced by Arg-982 that interacts tightly with Glu-64RhoA (Fig. 4c).
ARAP3-RhoGAP Domain Specifically Recognizes RhoA through Negatively Charged Residues Asp-90 and Glu-97 in the α3 Helix
All of the residues involved in interacting with the ARAP3-RhoGAP domain are conserved among RhoA, Cdc42, and Rac1, except for the negatively charged residues Asp-90 and Glu-97 in the α3 helix (Fig. 5a). In Cdc42, Ser-88 replaces Asp-90, whereas in Rac1, Ala-88 and Ala-95 replace Asp-90 and Glu-97, respectively. We speculated that residues Asp-90 and Glu-97 of RhoA confer the substrate specificity of the ARAP3-RhoGAP domain over Cdc42 and Rac1. To test this hypothesis, we generated RhoA-like mutations in Cdc42 and Rac1 and tested the effects on binding by ITC and GTPase activity assays. The ITC results showed that the ARAP3-RhoGAP domain binds wild type Cdc42 and the RhoA-like Cdc42 mutant (S88D) with KD values of 59.88 and 5.41 μm, respectively (Fig. 5b and Table 2). The RhoA-like Cdc42 mutant (S88D) shows ∼11-fold stronger binding affinity than wild type Cdc42 to the RhoGAP domain of ARAP3. The ARAP3-RhoGAP domain shows very weak binding affinity to wild type Rac1 that could not be detected by ITC. Meanwhile, the ARAP3-RhoGAP domain binds the RhoA-like Rac1 mutant (A88D,A95E) with a KD of 18.87 μm (Fig. 5c and Table 2). Additionally, in GTPase activity experiments, the ARAP3-RhoGAP domain had greater efficiency in the presence of the RhoA-like Cdc42 or Rac1 mutants than with wild type Cdc42 and Rac1 (Fig. 5, d and e, Table 3). The best fit Km and Kcat/Km values of ARAP3-RhoGAP to RhoA-GTP examined by the 2-amino-6-mercapto-7-methylpurineriboside (MESG)/purine nucleoside phosphorylase (PNP) system are 11.08 μm and 16.93 min−1 μm−1, respectively. The Km values of the ARAP3-RhoGAP domain to Cdc42-GTP and Cdc42 (S88D)-GTP are ∼7.4- and 1.8-fold greater than that of RhoA-GTP, indicating that the binding affinity of the ARAP3-RhoGAP domain for the RhoA-like mutation of Cdc42 with ∼4.2-fold higher than for wild type Cdc42 and ∼1.8-fold weaker affinity for RhoA during GAP-stimulated GTP hydrolysis reactions. The catalytic efficiency of the ARAP3-RhoGAP domain with the RhoA-like Cdc42 mutant is ∼4.3-fold greater than that of wild type Cdc42 and is comparable with that of RhoA (only 1.5-fold weaker than that of RhoA). The kinetic parameters of the ARAP3-RhoGAP domain to wild type Rac1-GTP was not determined because of the very low binding affinity. However, the Km and Kcat/Km values of ARAP3-RhoGAP to Rac1 (A88D,A95E)-GTP are 69.40 μm and 5.68 min−1 μm−1, indicating that the ARAP3-RhoGAP domain binding affinity and catalytic efficiency toward the RhoA-like Rac1-GTP mutant is only ∼6.2- and ∼3-fold weaker than that of RhoA-GTP, respectively. Taken together, the specific GAP activity of the ARAP3 RhoGAP domain to RhoA over Cdc42 and Rac1 may be conferred by the interaction with the negatively charged residues Asp-90 and Glu-97 of RhoA.
FIGURE 5.
The ARAP3-RhoGAP domain shows higher binding affinity and GAP activity toward RhoA-like Cdc42, and Rac1 mutations in vitro. a, partial sequence alignment of RhoA, Cdc42, and Rac1. Interface residues that form hydrogen bonds with the ARAP3-RhoGAP domain based on the complexed crystal structure are marked with triangles below. The variable residues at the interface are colored in red. b and c, ITC fitting curves of the ARAP3-RhoGAP domain to Cdc42·GDP·MgFx (green triangles), Rac1·GDP·MgFx (cyan inverted triangles), RhoA-like mutants Cdc42S88D·GDP·MgFx (red squares), and Rac1A88D,A95E·GDP·MgFx (blue circles). d and e, the Km and Kcat/Km values of the ARAP3-RhoGAP domain toward wild type RhoA, Cdc42, and RhoA-like mutants of Cdc42 and Rac1, measured by MESG/PNP system. The error bars are standard errors generated by nonlinear fitted using Origin, same as errors shown in Table 3.
TABLE 3.
Kinetic parameters of the ARAP3-RhoGAP domain to different small GTPases
The GTPase activity assays were carried out at 293 K, using the MESG/PNP system. For the measurement of the kinetic parameters of the ARAP3-RhoGAP domain toward the RhoA, Cdc42 (S88D), Cdc42, and Rac1 (A88D,A95E), the concentrations of the ARAP3-RhoGAP domain were 20, 25, 60, and 30 nm, respectively. ND, not detected because of the very low binding affinity. Errors in the table are standard errors.
| Vmax | Km | Kcat | Kcat/Km | |
|---|---|---|---|---|
| μm/min | μm | min−1 | min−1 μm−1 | |
| RhoA | 3.75 ± 0.10 | 11.08 ± 0.94 | 187.52 ± 4.86 | 16.93 ± 1.88 |
| Cdc42 | 13.14 ± 0.40 | 81.79 ± 4.58 | 219.07 ± 6.74 | 2.68 ± 0.23 |
| Cdc42 (S88D) | 5.65 ± 0.14 | 19.56 ± 1.55 | 225.88 ± 5.58 | 11.55 ± 1.20 |
| Rac1 | ND | ND | ND | ND |
| Rac1 (A88D,A95E) | 11.84 ± 0.16 | 69.40 ± 1.62 | 394.50 ± 5.35 | 5.68 ± 0.21 |
Discussion
ARAP3 was first reported in 2002 (9), and since then the function of ARAP3 in different cells and tissues has been extensively investigated, and most of the ARAP3 functions are related to its RhoGAP activity. However, the molecular mechanism of the RhoA-specific GAP activity of the ARAP3-RhoGAP domain remained unclear. In this study, we solved the crystal structure of the ARAP3-RhoGAP domain in complex with RhoA·GDP·AlF4−. Based on the interaction surface, we found that single point mutations of Arg-942, Arg-949, Arg-982, or Arg-985 can dramatically reduce the binding affinity and the RhoGAP activity of the ARAP3-RhoGAP domain toward RhoA in vitro (Fig. 2, d and e, and Table 2). Over the years, researchers have made loss of function mutants of the ARAP3-RhoGAP domain by point mutation of Arg-942 or Arg-982 (9, 13, 16, 17); here we proved that residue Arg-942 is the catalytic arginine finger of RhoGAP and that residue Arg-982 is critical for the interaction between RhoGAP and RhoA (Fig. 2b). Only two other RhoGAP·RhoA complexed structures (ARHGAP1, PDB code ITX4 and 1OW3; ARHGAP20, PDB code 3MSX) have been solved (Fig. 3, b and c). The ARAP3-RhoGAP domain shows ∼25% sequence identity with ARHGAP1 and ARHGAP20, and the complexed structures show differences at the binding interfaces, especially at the first interface (Fig. 4, a and b). Our complexed structure is an important contribution toward understanding the interactions between RhoGAPs and RhoA.
There are ∼22 mammalian genes encoding Rho family small GTPases, but ∼70 protein members of RhoGAPs have been found in mammals (25, 26). Most of the RhoGAPs show selectivity among the different Rho family small GTPases. ARAP1 and ARAP3 selectively use RhoA as their substrate (9, 27), but the ARAP2-RhoGAP domain lacks RhoGAP activity, because the catalytic arginine finger is replaced by glutamine. However, ARAP2 still has a direct interaction with RhoA-GTP (28). ARAP1 is an Arf1/Arf5 ArfGAP, whereas ARAP2 and ARAP3 are specific for Arf6 (9, 27, 28). The mechanism for the specificity of ARAP3 among Rho and Arf GTPases remains elusive. In this study, we have found that residues Asp-90 and Glu-97 in the α3 helix of RhoA are responsible for the substrate-specific GAP activity of the ARAP3-RhoGAP domain. In Cdc42, the related residues are Ser-88 and Glu-95, whereas in Rac1 they are Ala-88 and Ala-95 (Fig. 5a). The ARAP3-RhoGAP domain has interactions with both Asp-90 and Glu-97 (Fig. 4b) and exhibits higher binding affinity and GAP activity against a RhoA-like mutant Cdc42 (S88D) or Rac1 (A88D,A95E) than wild type Cdc42 and Rac1 (Fig. 5, b–e, and Tables 2 and 3). It is notable that the Kcat values are comparable (the biggest difference is 2-fold) and the Km values are the main factor for the differences of the catalytic efficiencies, further proving that ARAP3-RhoGAP chooses its specific target mainly through binding affinity. The same molecular mechanism for the specificity of RhoGAP activity was reported for the Myo9b-RhoGAP domain (29). As shown in Fig. 4b, ARHGAP1 interacts only via Glu-97 and ARHGAP20 interacts with both Asp-90 and Glu-97. Functionally, ARHGAP1 is a GAP with a preference for Cdc42 over Rho and Rac1 (30, 31), and ARHGAP20 is a GAP with a preference for RhoA (32), supporting our idea that RhoGAPs choose their specific target by recognizing differences in the α3 helix among RhoA, Cdc42, and Rac1. In addition, β2-chimaerin contains a Rac1-specific GAP domain, its specificity is conferred by interactions of Phe-315 and Glu-317 with Ala-88 and Ala-95 in the α3 helix of Rac1 (33). Glu-95 in Cdc42 and Glu-97 in RhoA would block that interaction and make them poor targets for β2-chimaerin. Thus, RhoGAPs may choose their specific target by the differences in the α3 helix of RhoA, Rac1, and Cdc42. Intriguingly, the GTPase substrate preference of p190-RhoGAP can be switched by phospholipids from RhoA and Rac1 to only Rac1 (34) and to only RhoA by phosphorylation (35). Phosphorylation can also switch the GTPase substrate preference of MgcRacGAP (male germ cell RacGAP, also named Rac GTPase-activating protein 1) from Rac1 and Cdc42 to RhoA (36). It is reasonable that there are other factors that regulate the substrate specificity of different RhoGAPs, and the detailed mechanisms need further investigation.
The ARAP3-ArfGAP activity can be activated by PtdIns(3,4,5)P3 (9); two tandem PH domains in the N terminus, especially the first PH domain, are required for the interaction with PtdIns(3,4,5)P3 (37). The mechanism of the regulation between PtdIns(3,4,5)P3, PH domains, and the ArfGAP remains unclear. β2-Chimaerin, a Rac1 specific GAP, contains SH2-C1-RacGAP tandem domain. The N-terminal peptide binds the DAG (diacylglycerol)-binding site in the C1 domain and partially occupies the GAP active site. By binding to DAG through the C1 domain, the N-terminal peptide is released from the GAP active site, and GAP activity is enhanced (33). Whether the activation of ARAP3-ArfGAP by binding to PtdIns(3,4,5)P3 adopts the same mechanism as β2-chimaerin requires further investigation. ARAP3 also contains an RA domain, and ARAP3 RhoGAP activity can be enhanced by direct binding of Rap1-GTP to the RA domain (10). ARHGAP20 has PH-RA-RhoGAP tandem domains; the RA domain can autoinhibit RhoGAP activity, and the autoinhibitory effect can be released by binding to Rap1-GTP (32). However, the mechanism of how Rap1-GTP regulates the ARAP3-RhoGAP activity remains obscure. ARAP3 has five PH domains, and the function of PH3 to PH5 needs further investigation. In summary, our work demonstrates the interface between the ARAP3-RhoGAP domain and RhoA at atomic resolution and is an important contribution to our understanding of the regulation and specific recognition of RhoA by ARAP3.
Experimental Procedures
Plasmid Constructions
Full-length ARAP3 cloned in a pEGFPC2 vector that was generously provided by Dr. Sonja Vermeren (Babraham Institute, Cambridge, UK). The ARAP3 RhoGAP domain (residues 906–1107) was cloned into a His6 tag (p28a) vector. Full-length RhoA, Cdc42, and Rac1 constructs were also cloned into the p28a vector. The linker sequence for the fusion protein was NLSSDSSLSSPSALNSTASNSPGIEGLS (19, 20). The RhoGAP (residues 906–1107)-linker-RhoA (residues 2–181, F25N) fusion protein was constructed by overlap extension PCR and also cloned in p28a. The fusion protein harbored the stabilizing point mutation F25N in RhoA (21). All mutants were generated using the MutanBEST kit (Takara) and verified by DNA sequencing.
Protein Expression and Purification
All proteins were expressed in Escherichia coli Bl21 DE3 (Gold) cells (Novagen) that were cultured in LB medium at 310 K to an A600 of 0.8–1.0, subsequently shifted to 289 K, and induced with 0.3–0.4 mm isopropyl β-d-thiogalactopyranoside for 24 h. RhoGAPs (wild type or mutant) were purified by nickel-nitrilotriacetic acid affinity chromatography after lysing cells in 20 mm Tris (pH 7.5) and 500 mm NaCl and eluted in buffer containing 30–500 mm imidazole (pH 7.5). The eluted His tag proteins were further purified using a Superdex 200 column (GE Healthcare) in buffer containing 20 mm Tris (pH 7.5) and 200 mm NaCl. The fusion protein was also purified by nickel-nitrilotriacetic acid affinity chromatography after lysing of cells in 20 mm Tris (pH 7.5), 500 mm NaCl, 5 mm MgCl2, and 1 mm GDP, and eluting with buffer containing 30–500 mm imidazole (pH 7.5). Further purification used a Superdex 200 column (GE Healthcare) in buffer containing 20 mm Tris (pH 7.5), 200 mm NaCl, 5 mm MgCl2, 1 mm AlCl3, and 10 mm NaF. For ITC experiments, small GTPases and RhoGAPs were purified as described for the fusion protein (without AlCl3). All purified proteins were concentrated and stored at 193 K. GDP and GTP were purchased from Sangon Biotech.
Crystallization and Structure Solution
RhoGAP was concentrated to ∼10 mg/ml, and the buffer was exchanged to 20 mm KH2PO4 (pH 6.5), 200 mm NaCl, and 10 mm DTT. Crystals of RhoGAP formed in mother liquor containing 100 mm HEPES (pH 6.9) and 10% PEG8000. The fusion protein was concentrated to ∼10 mg/ml, and the buffer was exchanged to 20 mm Tris (pH 7.5), 150 mm NaCl, 5 mm DTT, 5 mm MgCl2, 1 mm AlCl3, 10 mm NaF, and 2 mm GDP. Crystals of the fusion protein formed in the mother liquor containing 100 mm MES (pH 6.0), 15% (v/v) polyethylene glycol monomethyl ether 550, and 4% (v/v) acetone. All crystals were grown at 293 K via the hanging drop method. X-ray diffraction data of the crystals were collected on Beamline 17U1 of the Shanghai Synchrotron Radiation Facility. The data were processed with HKL2000 and programs in the CCP4 suite. The structure of the RhoGAP domain and the fusion protein (complex) were solved by molecular replacement using the program PHASER (38). All the structural models were subsequently refined by programs REFMAC5 (39), PHENIX (40), and COOT (41), and ligands (GDP, AlF4−, and Mg2+) were added manually. Crystallographic parameters are listed in Table 1. All structure figures were prepared with PyMOL (42). The transition state complex interface was calculated in PDBePISA (43).
Isothermal Titration Calorimetry
ITC assays were carried out on a MicroCal iTC200 calorimeter (GE Healthcare) at 293 K. The buffer for RhoGAPs (wild type or mutant) and full-length GTPases was 20 mm Tris (pH 7.5), 200 mm NaCl, 5 mm MgCl2, and 10 mm NaF. The titration protocol consisted of 20 injections of 0.8–1.0 mm RhoGAPs (wild type or mutant) into 0.06–0.08 mm GTPases. Curve fitting to a one-binding-site model was performed by ITC data analysis module of Origin7.5 (OriginLab) provided by the manufacturer. The parameters are summarized including the standard errors in Table 2.
GTPase Activity Assay
The initial rates and kinetic measurements of GAP-stimulated GTP hydrolysis were measured using the MESG and PNP system with a constant amount of RhoGAP and increasing concentrations of GTPases (44). MESG and PNP were purchased as part of the EnzChek® phosphate assay kit (Life Technologies, E6646). Briefly, the indicated amount of GTPase was incubated in buffer: 50 mm HEPES (pH 7.6), 0.1 mm EDTA, 400 μm GTP, 0.2 mm MESG, and 1 unit of PNP. The reactions were started by adding 10 μl a solution containing the indicated amounts of RhoGAP and MgCl2 to the mixture. The final concentration of MgCl2 was 5 mm. The time courses of absorbance change at 360 nm were recorded by DU 800 (Beckman Coulter) at 293 K. The kinetic parameters were nonlinear fitted by Origin 8.5.1 (OriginLab) using the modified Michaelis-Menten equation as described (44) and are summarized including the standard errors in Table 3.
Author Contributions
H. B., F. L., N. W., and Y. J. performed the experiments. H. B. and Y. S. designed the experiments. H. B. wrote the manuscript. H. B., C. W., Y. T., J. W., and Y. S. discussed the results and revised the paper.
Acknowledgments
We thank the staff of Beamline BL17U at Shanghai Synchrotron Radiation Facility for the assistance in data collection. We also thank Dr. Zhixin Wang for the advices on the MESG/PNP system GTPase activity assay experiments.
This work was supported by National Basic Research Program of China 973 Program Grant 2012CB917201; Chinese National Natural Science Foundation Grants 31330018 and 31170693; Hefei Center for Physical Science and Technology Grant 2012FXZY002; and Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB08010101. The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 5JD0 and 5JCP) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- PH
- pleckstrin homology
- RA
- Ras-associating
- RMSD
- root mean square deviation
- PDB
- Protein Data Bank
- PtdIns(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- ITC
- isothermal titration calorimetry
- MESG
- 2-amino-6-mercapto-7-methylpurineriboside
- PNP
- purine nucleoside phosphorylase.
References
- 1. Wennerberg K., Rossman K. L., and Der C. J. (2005) The Ras superfamily at a glance. J. Cell Sci. 118, 843–846 [DOI] [PubMed] [Google Scholar]
- 2. D'Souza-Schorey C., and Chavrier P. (2006) ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358 [DOI] [PubMed] [Google Scholar]
- 3. Bryan B. A., Li D., Wu X., and Liu M. (2005) The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell. Mol. Life Sci. 62, 1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ridley A. J. (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 [DOI] [PubMed] [Google Scholar]
- 5. Csépányi-Kömi R., Lévay M., and Ligeti E. (2012) Small G proteins and their regulators in cellular signalling. Mol. Cell. Endocrinol. 353, 10–20 [DOI] [PubMed] [Google Scholar]
- 6. Riching K. M., and Keely P. J. (2015) Rho family GTPases: making it to the third dimension. Int. J. Biochem. Cell Biol. 59, 111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sabe H. (2003) Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 134, 485–489 [DOI] [PubMed] [Google Scholar]
- 8. Ridley A. J. (2013) RhoA, RhoB and RhoC have different roles in cancer cell migration. J. Microsc. 251, 242–249 [DOI] [PubMed] [Google Scholar]
- 9. Krugmann S., Anderson K. E., Ridley S. H., Risso N., McGregor A., Coadwell J., Davidson K., Eguinoa A., Ellson C. D., Lipp P., Manifava M., Ktistakis N., Painter G., Thuring J. W., Cooper M. A., et al. (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol. Cell 9, 95–108 [DOI] [PubMed] [Google Scholar]
- 10. Krugmann S., Williams R., Stephens L., and Hawkins P. T. (2004) ARAP3 is a PI3K- and Rap-regulated GAP for RhoA. Curr. Biol. 14, 1380–1384 [DOI] [PubMed] [Google Scholar]
- 11. Krugmann S., Andrews S., Stephens L., and Hawkins P. T. (2006) ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J. Cell Sci. 119, 425–432 [DOI] [PubMed] [Google Scholar]
- 12. Gambardella L., Hemberger M., Hughes B., Zudaire E., Andrews S., and Vermeren S. (2010) PI3K signaling through the dual GTPase-activating protein ARAP3 is essential for developmental angiogenesis. Sci. Signal. 3, ra76. [DOI] [PubMed] [Google Scholar]
- 13. Yagi R., Tanaka M., Sasaki K., Kamata R., Nakanishi Y., Kanai Y., and Sakai R. (2011) ARAP3 inhibits peritoneal dissemination of scirrhous gastric carcinoma cells by regulating cell adhesion and invasion. Oncogene 30, 1413–1421 [DOI] [PubMed] [Google Scholar]
- 14. Gambardella L., Anderson K. E., Nussbaum C., Segonds-Pichon A., Margarido T., Norton L., Ludwig T., Sperandio M., Hawkins P. T., Stephens L., and Vermeren S. (2011) The GTPase-activating protein ARAP3 regulates chemotaxis and adhesion-dependent processes in neutrophils. Blood 118, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 15. Moon M. Y., Kim H. J., Kim J. G., Lee J. Y., Kim J., Kim S. C., Choi I. G., Kim P. H., and Park J. B. (2013) Small GTPase Rap1 regulates cell migration through regulation of small GTPase RhoA activity in response to transforming growth factor-beta1. J. Cell. Physiol. 228, 2119–2126 [DOI] [PubMed] [Google Scholar]
- 16. Jeon C. Y., Kim H. J., Lee J. Y., Kim J. B., Kim S. C., and Park J. B. (2010) p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells. Exp. Mol. Med. 42, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeon C. Y., Kim H. J., Morii H., Mori N., Settleman J., Lee J. Y., Kim J., Kim S. C., and Park J. B. (2010) Neurite outgrowth from PC12 cells by basic fibroblast growth factor (bFGF) is mediated by RhoA inactivation through p190RhoGAP and ARAP3. J. Cell. Physiol. 224, 786–794 [DOI] [PubMed] [Google Scholar]
- 18. Jeon C. Y., Moon M. Y., Kim J. H., Kim H. J., Kim J. G., Li Y., Jin J. K., Kim P. H., Kim H. C., Meier K. E., Kim Y. S., and Park J. B. (2012) Control of neurite outgrowth by RhoA inactivation. J. Neurochem. 120, 684–698 [DOI] [PubMed] [Google Scholar]
- 19. van Leeuwen H. C., Strating M. J., Rensen M., de Laat W., and van der Vliet P. C. (1997) Linker length and composition influence the flexibility of Oct-1. EMBO J. 16, 2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ismail S. A., Vetter I. R., Sot B., and Wittinghofer A. (2010) The structure of an Arf-ArfGAP complex reveals a Ca2+ regulatory mechanism. Cell 141, 812–821 [DOI] [PubMed] [Google Scholar]
- 21. Rittinger K., Walker P. A., Eccleston J. F., Smerdon S. J., and Gamblin S. J. (1997) Structure at 1.65 Å of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature 389, 758–762 [DOI] [PubMed] [Google Scholar]
- 22. Graham D. L., Lowe P. N., Grime G. W., Marsh M., Rittinger K., Smerdon S. J, Gamblin S. J., and Eccleston J. F. (2002) MgF3− as a transition state analog of phosphoryl transfer. Chem. Biol. 9, 375–381 [DOI] [PubMed] [Google Scholar]
- 23. Holm L., and Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dvorsky R., and Ahmadian M. R. (2004) Always look on the bright site of Rho: structural implications for a conserved intermolecular interface. EMBO Reports 5, 1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tcherkezian J., and Lamarche-Vane N. (2007) Current knowledge of the large RhoGAP family of proteins. Biol. Cell 99, 67–86 [DOI] [PubMed] [Google Scholar]
- 26. Cherfils J., and Zeghouf M. (2013) Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 [DOI] [PubMed] [Google Scholar]
- 27. Miura K., Jacques K. M., Stauffer S., Kubosaki A., Zhu K., Hirsch D. S., Resau J., Zheng Y., and Randazzo P. A. (2002) ARAP1: a point of convergence for Arf and Rho signaling. Mol. Cell 9, 109–119 [DOI] [PubMed] [Google Scholar]
- 28. Yoon H.-Y., Miura K., Cuthbert E. J., Davis K. K., Ahvazi B., Casanova J. E., and Randazzo P. A. (2006) ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. J. Cell Sci. 119, 4650–4666 [DOI] [PubMed] [Google Scholar]
- 29. Kong R., Yi F., Wen P., Liu J., Chen X., Ren J., Li X., Shang Y., Nie Y., Wu K., Fan D., Zhu L., Feng W., and Wu J. Y. (2015) Myo9b is a key player in SLIT/ROBO-mediated lung tumor suppression. J. Clin. Invest. 125, 4407–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barfod E. T., Zheng Y., Kuang W. J., Hart M. J., Evans T., Cerione R. A., and Ashkenazi A. (1993) Cloning and expression of a human CDC42 GTPase-activating protein reveals a functional SH3-binding domain. J. Biol. Chem. 268, 26059–26062 [PubMed] [Google Scholar]
- 31. Wang L., Yang L., Burns K., Kuan C. Y., and Zheng Y. (2005) Cdc42GAP regulates c-Jun N-terminal kinase (JNK)-mediated apoptosis and cell number during mammalian perinatal growth. Proc. Natl. Acad. Sci. U.S.A. 102, 13484–13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamada T., Sakisaka T., Hisata S., Baba T., and Takai Y. (2005) RA-RhoGAP, Rap-activated Rho GTPase-activating protein implicated in neurite outgrowth through Rho. J. Biol. Chem. 280, 33026–33034 [DOI] [PubMed] [Google Scholar]
- 33. Canagarajah B., Leskow F. C., Ho J. Y., Mischak H., Saidi L. F., Kazanietz M. G., and Hurley J. H. (2004) Structural mechanism for lipid activation of the Rac-specific GAP, beta2-chimaerin. Cell 119, 407–418 [DOI] [PubMed] [Google Scholar]
- 34. Ligeti E., Dagher M. C., Hernandez S. E., Koleske A. J., and Settleman J. (2004) Phospholipids can switch the GTPase substrate preference of a GTPase-activating protein. J. Biol. Chem. 279, 5055–5058 [DOI] [PubMed] [Google Scholar]
- 35. Lévay M., Settleman J., and Ligeti E. (2009) Regulation of the substrate preference of p190RhoGAP by protein kinase C-mediated phosphorylation of a phospholipid binding site. Biochemistry 48, 8615–8623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minoshima Y., Kawashima T., Hirose K., Tonozuka Y., Kawajiri A., Bao Y. C., Deng X., Tatsuka M., Narumiya S., May W. S. Jr., Nosaka T., Semba K., Inoue T., Satoh T., Inagaki M., et al. (2003) Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549–560 [DOI] [PubMed] [Google Scholar]
- 37. Craig H. E., Coadwell J., Guillou H., and Vermeren S. (2010) ARAP3 binding to phosphatidylinositol-(3,4,5)-trisphosphate depends on N-terminal tandem PH domains and adjacent sequences. Cell Signal. 22, 257–264 [DOI] [PubMed] [Google Scholar]
- 38. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murshudov G. N., Skubák P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., and Vagin A. A. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeLano W. L. (2002) PyMOL, DeLano Scientific, San Carlos, CA [Google Scholar]
- 43. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 44. Zhang B., Wang Z.-X., and Zheng Y. (1997) Characterization of the interactions between the small GTPase Cdc42 and its GTPase-activating proteins and putative effectors: comparison of kinetic properties of cDC42 binding to the Cdc42-interactive domains. J. Biol. Chem. 272, 21999–22007 [DOI] [PubMed] [Google Scholar]