Key Clinical Message
Paraneoplastic pemphigus (PNP) is an autoimmune blistering disease associated with neoplasms. The disease is most commonly of lymphoproliferative origin and presents high mortality. We describe a patient with PNP and myasthenia gravis associated with inflammatory pseudotumor‐like follicular dendritic cell sarcoma, as well as the response to rituximab.
Keywords: Inflammatory pseudotumor‐like follicular dendritic cell sarcoma, myasthenia gravis, paraneoplastic pemphigus, rituximab
Introduction
Paraneoplastic pemphigus (PNP) is an extremely severe autoimmune blistering disease associated with neoplasms; the disease is most commonly of lymphoproliferative origin and presents a high mortality 1. Follicular dendritic cell (FDC) sarcoma is rare and classified either as a conventional‐type or as an inflammatory pseudotumor (IPT)‐like variant. IPT‐like FDC sarcoma is a rare neoplasm with different histologic appearance and behavior from those of the classical FDC sarcoma 2. A review of 38 cases of IPT‐like FDC sarcoma reveals that this tumor occurs predominantly in females with a predilection for liver and spleen, with a strong association with Epstein–Barr virus (EBV), and generally presents an indolent course 2. Less than 30 cases of FDC sarcoma have been reported, in which the patients were diagnosed with myasthenia gravis (MG) or PNP 3, 4. Kim et al. 5 described a 68‐year‐old man with FDC sarcoma associated with MG and PNP. Moreover, two cases of Castleman's disease were associated with MG and PNP, suggesting a putative alternative linkage of PNP, MG, and the associated neoplasms 6, 7. To date, we have not found any reports on IPT‐like FDC sarcoma associated with MG or PNP.
Case Report
A 60‐year‐old Chinese woman presented a 6‐month history of recurrent weakness and increasing upper extremity fatigue, fluctuating diplopia, and ptosis of both eyes, finally showing dysarthria and dysphagia. During 6 months, she lost 10 kg weight. Jolly and neostigmine tests were positive. She was clinically suspected to be suffering from MG. Several masses had been found in her left axillary region and neck for 15 years, and the masses were resected every 4 years without radiotherapy or chemotherapy. The last surgery was 2 years prior to the study. After admission, thoracic computed tomography (CT) showed several masses in the left axillary and neck, and the diameter of the maximal tumor was 6.4 cm. However, the evidence of thymoma was not found by mediastinal CT scan.
Core needle biopsy revealed spindle tumor cells arranged in a storiform pattern with scattered lymphocytes (Fig. 1A). Spindle tumor cells contained vesicular chromatin, small nucleoli, and thin smooth nuclear membranes (Fig. 1B). Immunohistochemical studies revealed that the tumor cells were positive for Ki‐67 (+20%) (Fig. 2A), vimentin, CD21, CD35, CD20, EGFR, CD68, S‐100 (Fig. 2B), CD1a, CD3, and CD79a and negative for EMA, CK, CD23, CD34, HMB‐45, CD30, MPO, and lysozyme. However, EBV‐encoded RNA was detected by in situ hybridization and was negative. Final pathological examination demonstrated IPT‐like FDC sarcoma.
Figure 1.
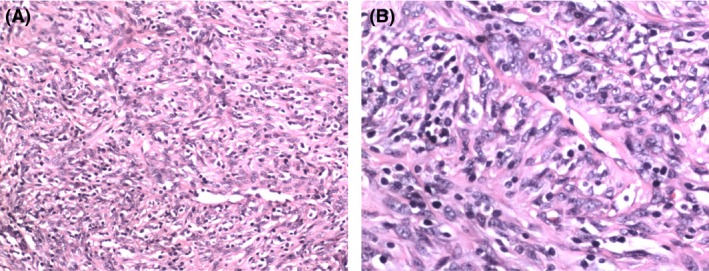
(A) Microphotograph showing spindle tumor cells arranged in a storiform pattern with scattered lymphocytes (×10). (B) Spindle tumor cells contained vesicular chromatin, small nucleoli, and thin smooth nuclear membranes (×20).
Figure 2.
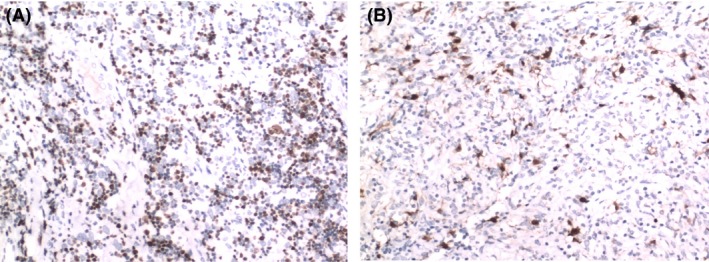
Immunohistochemistry of the tumor. (A) Ki‐67 is diffusely positive in the spindle cells (×10). (B) Some of the spindle cells are positive for S‐100 (×10).
After admission, treatment was immediately started with pyridostigmine at a dose of 60 mg three times per day following a subtle improvement of muscle weakness. Five days later, the patient developed severe dyspnea, and then, an emergency intubation and mechanical ventilation were administered. Immunoglobulin (0.4 g/kg/day ×5 days) and steroid pulse therapy (methylprednisolone sodium succinate, 1000 mg daily × 3 days, gradually tapered off) were given. Mucocutaneous lesions appeared at 10 days after admission. Physical examination showed erythemas involving back and right hypochondrial region, and stomatitis. The patient was diagnosed with drug‐induced dermatitis by dermatologists. The symptoms were remitted after treatment with immunoglobulin and steroids. Sixteen days after treatment with immunoglobulin and steroids, the patient was successfully detached from the respirator. However, eight days later, her myasthenia symptoms worsened. Mechanical ventilation was again administered. After steroid and immunoglobulin treatment failure, rituximab at a dose of 500 mg (330 mg/m²/week) was given to the patient. From the second week after rituximab treatment, the myasthenia symptoms progressively improved. After four courses of rituximab treatment, the skin lesions and muscle weakness were almost recovered, and ventilator weaning was successful. Then, the patient asked to be discharged. Three weeks after discharge, she was hospitalized with severe generalized blistering skin eruptions and polymorphic erythemas and mucosal ulcerations (Fig. 3), which were considerably worse than those in the previous occasion but without myasthenia symptoms. She was admitted to the dermatological department and diagnosed with PNP. Then, she was treated with immunoglobulin (1 mg/kg per day) without improvement of mucocutaneous lesions. After 2 months, the patient died because of multiple organ dysfunction syndrome.
Figure 3.
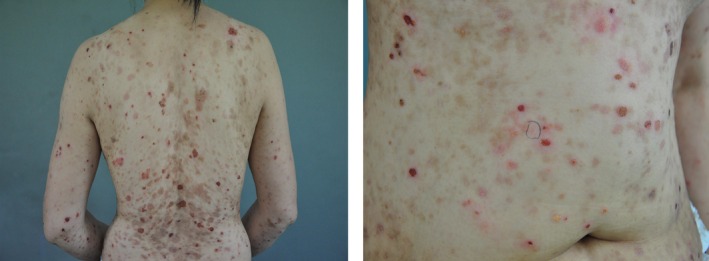
Skin lesions of the patient.
Discussion
We describe the extremely unusual association of an IPT‐like FDC sarcoma with PNP and MG. The pathogenesis is thought to be due to the production of antibodies against tumor‐derived antigens; the antibodies may attack components sharing the same or a similar epitope 8. Wang et al. 9 reported that MG is a complication of PNP. Ocampo–Candiani et al. 10 reported a case in which PNP associated with FDCS showed a good response to a treatment plan that included dexamethasone, surgery, and chemotherapy. Another case was successfully treated with rituximab 11. Our observations on this case indicate that rituximab could be an effective alternative therapy in the treatment of PNP and MG. However, PNP recurred catastrophically, perhaps because the standard treatment dose was not achieved and surgical resection of tumors was not performed. The patient presented multiple local recurrences without systemic dissemination for a long period, suggesting an indolent clinical course. Considering the high mortality of PNP, we should pay more attention on the diagnosis and treatment of IPT‐like FDC sarcoma.
Conflict of Interest
None declared.
References
- 1. Sinha, A. A. 2015. Paraneoplastic pemphigus: autoimmune‐cancer nexus in the skin. Anticancer Agents Med. Chem. 15:1215–1223. [DOI] [PubMed] [Google Scholar]
- 2. Ge, R. , Liu C., Yin X., Chen J., Zhou X., Huang C., et al. 2014. Clinicopathologic characteristics of inflammatory pseudotumor‐like follicular dendritic cell sarcoma. Int. J. Clin. Exp. Pathol. 7:2421–2429. [PMC free article] [PubMed] [Google Scholar]
- 3. Hwang, Y.‐Y. , Chan J. C. Y., Trendell‐Smith N. J., and Kwong Y.‐L.. 2014. Recalcitrant paraneoplastic pemphigus associated with follicular dendritic cell sarcoma:response to prolonged rituximab and ciclosporin therapy. Intern. Med. J. 44:1145–1146. [DOI] [PubMed] [Google Scholar]
- 4. Hsu, C. , Vega F., Grimes L. M., and Hunt K. K.. 2011. Follicular dendritic cell sarcoma and associated myasthenia gravis true, true, related. J. Clin. Oncol. 29:369–371. [DOI] [PubMed] [Google Scholar]
- 5. Kim, W. Y. , Kim H., Jeon Y. K., and Kim C. W.. 2010. Follicular dendritic cell sarcoma with immature T‐cell proliferation. Hum. Pathol. 41:129–133. [DOI] [PubMed] [Google Scholar]
- 6. Chorzelski, T. , Hashimoto T., Maciejewska B., Amagai M., Anhalt G. J., and Jablonska S.. 1999. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J. Am. Acad. Dermatol. 41:393–400. [DOI] [PubMed] [Google Scholar]
- 7. Jakubikova, M. , Pitha J., Latta J., Ehler E., and Schutzner J.. 2013. Myasthenia gravis, Castleman disease, pemphigus, and anti‐phospholipid syndrome. Muscle Nerve 47:447–451. [DOI] [PubMed] [Google Scholar]
- 8. Yong, A. A. , and Tey H. L.. 2013. Paraneoplastic pemphigus. Australas. J. Dermatol. 54:241–250. [DOI] [PubMed] [Google Scholar]
- 9. Wang, R. , Li J., Wang M., Hao H., Chen X., Li R., et al. 2015. Prevalence of myasthenia gravis and associated autoantibodies in paraneoplastic pemphigus and their correlations with symptoms and prognosis. Br. J. Dermatol. 172:968–975. [DOI] [PubMed] [Google Scholar]
- 10. Garza‐Chapa, J. , Ocampo‐Garza J., Vázquez‐Herrera N., and Miranda‐Maldonado I.. 2016. Paraneoplastic pemphigus associated with primary pulmonar follicular dendritic cell sarcoma showing good response to treatment. J. Eur. Acad. Dermatol. Venereol. 30:465–467. [DOI] [PubMed] [Google Scholar]
- 11. Aoi, J. , Makino K., Sakai K., and Masuguchi S.. 2013. Case of paraneoplastic pemphigus with follicular lymphoma treated with rituximab. J. Dermatol. 40:285–286. [DOI] [PubMed] [Google Scholar]


