Abstract
Animal studies suggest that the gut-brain peptide ghrelin plays an important role in the neurobiology of alcohol dependence (AD). Human studies show an effect of alcohol on ghrelin levels and a correlation between ghrelin levels and alcohol craving in alcoholics.
This investigation consisted of two studies. Study 1 was a 12-week study with alcohol-dependent subjects, where plasma ghrelin determinations were assessed four times (T0-T3) and related to alcohol intake and craving [Penn Alcohol Craving Score (PACS) and Obsessive Compulsive Drinking Scale (OCDS)]. Serum growth hormone (GH) levels and assessment of the nutritional/metabolic status were also performed. Study 2 was a pilot case-control study to assess ghrelin gene polymorphisms (Arg51Gln and Leu72Met) in alcohol-dependent individuals. Study 1 showed no significant differences in ghrelin levels in the whole sample, while there was a statistical difference for ghrelin between non-abstinent and abstinent subjects. Baseline ghrelin levels were significantly and positively correlated with the PACS score at T1 and with all craving scores both at T2 and T3 (PACS, OCDS, obsessive and compulsive OCDS subscores). In Study 2, although there was a higher frequency of the Leu72Met ghrelin gene polymorphism in alcohol-dependent individuals, the distribution between healthy controls and alcohol dependent individuals was not statistically significant.
This investigation suggests that ghrelin is potentially able to affect alcohol-seeking behaviors, such as alcohol drinking and craving, representing a new potential neuropharmacological target for AD.
Keywords: ghrelin, alcohol dependence, alcohol drinking, craving
INTRODUCTION
There exist commonalities between over-eating and over-consumption of alcohol (Leggio, 2009). Like alcoholism, obesity and binge eating are complex genetic traits determined by several genes, and interacting with the environment (Leggio et al, 2011). For example, sweet liking has been proposed as a possible endophenotype for alcohol dependence (AD) (for review: Kampov-Polevoy et al, 2003) and a link between glucose levels and alcohol-seeking behavior has been suggested both in animals (Connelly et al, 1983; Zito et al, 1984) and in alcohol-dependent individuals (Leggio et al, 2009a). Furthermore, it has been suggested that feeding-related peptides, such as leptin (Kiefer et al, 2005; Hillemacher et al, 2007), orexin-1/hypocretin-1 (Richards et al, 2008), insulin (Leggio et al, 2008a), thyroid hormones (Leggio et al, 2008b) and adiponectin (Hillemacher et al, 2009) are related to alcohol-seeking behavior. In recent years there has been a growing interest in the possible role of the feeding-related peptide ghrelin in AD.
Ghrelin is a 28-amino acid peptide acting as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R), a G-protein coupled receptor that induces growth hormone (GH) release from the pituitary (Kojima et al, 1999). Ghrelin was first isolated from the stomach (Kojima et al, 1999), but there may be a central production of ghrelin, e.g. in the hypothalamus (Nakazato et al, 2001). Ghrelin stimulates appetite by acting on the hypothalamic arcuate nucleus (ARC), a region that controls the intake of food and other substances, including alcohol. The expression of the GHS-R in the mesolimbic dopamine (DA) pathway suggests that ghrelin could also modulate the so-called reward system (Jerlhag et al, 2006, 2007).
Administration of ghrelin, either centrally into the Ventral Tegmental Area (VTA) (Jerlhag et al, 2008) or peripherally (Jerlhag, 2008), activates brain reward parameters, such as locomotor activity, accumbal-DA release and conditioned place preference (CPP). Another study demonstrated that ghrelin regulates alcohol consumption via the perioculomotor urocortin population of neurons (pIIIu) (Kaur and Ryabinin, 2010). In addition, genetic (i.e., GHS-R1A knockout mice) and pharmacological (i.e., GHS-R1A antagonists) models of suppressed ghrelin signaling demonstrate that the central ghrelin action not only stimulates the reward system, but also is required for stimulation of that system by alcohol (Jerlhag et al, 2009).
Human studies also support the hypothesis that the ghrelin system plays a role in AD. In healthy controls, alcohol, compared to water, significantly reduces ghrelin levels (Calissendorff et al, 2005, 2006; Zimmermann et al, 2007). Both our group (Addolorato et al, 2006) and others (Badaoui et al, 2008) reported that active drinking alcohol-dependent patients (with an alcohol intake within 24 hrs) have lower plasma ghrelin levels compared to controls. A significant increase of ghrelin in abstinent alcoholics was also described (Kim et al, 2005; Kraus et al, 2005). Together, these studies suggest that ghrelin is suppressed by acute alcohol intake and increases during abstinence. In addition, our group first demonstrated a significant positive correlation between plasma ghrelin levels and subjective measurements of alcohol craving [Obsessive-Compulsive Drinking Scale (OCDS)] in active drinking alcoholics (Addolorato et al, 2006). Two subsequent human studies have confirmed, at least partially, the relationship between plasma ghrelin levels and alcohol craving. In particular, Hillemacher et al. (2007) reported a significant relationship between ghrelin and OCDS score in an alcoholic subtype (Lesch type 1) characterized by a positive family history of alcoholism. Wurst et al. (2007) found a relationship between ghrelin levels and the compulsive subscore of the OCDS scale in female alcoholics. As further evidence of the role of the ghrelin system in AD, a study demonstrated that the SNP rs2232165 of the GHS-R1A gene was associated with heavy alcohol consumption and SNP rs2948694 of the same gene was associated with BMI in heavy alcohol drinkers (Landgren et al, 2008). In a more recent study, haplotypes TAACGT and TAACGG of the pro-ghrelin gene (GHRL) were associated with paternal AD and withdrawal symptoms, respectively; and the haplotype CCGG of the GHSR gene was associated with type 2 AD (Landgren et al, 2010). Together, these studies suggest that the ghrelin system plays an important role in alcohol craving and dependence.
The goal of the present investigation was to further substantiate and extend our knowledge of the role of the ghrelin system in AD. This investigation consisted of: 1) a longitudinal study where repeated plasma ghrelin determinations were assessed and were related to alcohol craving and intake in alcohol-dependent individuals; and 2) a pilot case-control study, whose aim was to assess ghrelin gene polymorphisms in alcohol-dependent individuals.
Both studies were performed at the Alcoholism Treatment Unit of the Institute of Internal Medicine, Catholic University of Rome, Italy. Both studies were approved by the local Ethics Committee of the Catholic University of Rome and were carried out in accordance with the ethical standards Declaration of Helsinki of 1975, as revised in 1983. All participants gave their written informed consent.
STUDY 1. PLASMA GHRELIN LEVEL, ALCOHOL DRINKING AND CRAVING IN ALCOHOL-DEPENDENT SUBJECTS: A LONGITUDINAL STUDY
The main goal of Study 1 was to assess repeated plasma ghrelin levels in alcohol-dependent subjects during a 12-week period and investigate the relationship between ghrelin levels and alcohol drinking, as well as the relationship between ghrelin levels and alcohol craving. Since ghrelin stimulates GH release (Kojima et al, 1999), GH levels were also determined. In addition, since it has been suggested that ghrelin might help defend against symptoms of stress-induced depression and anxiety (Lutter et al, 2008) and both depressive and anxiety symptoms may often occur in alcoholics (Schuckit, 2009), these symptoms were assessed. Finally, since ghrelin plays an important regulatory role in metabolism (van der Lely, 2009) and metabolic changes have been reported in alcoholics (Addolorato et al, 1998a,b; Leggio et al, 2009b), metabolic parameters were assessed as well.
Subjects
Subjects of this study were outpatients who took part in a research project, named the Psychoneuroendocrinology Project (PNP), planned as a parallel addendum study of the Baclofen Intervention Study (BIS) (EudraCT Number: 2006-000713-37), a 12-week double-blind placebo-controlled study aimed at testing baclofen in the treatment of alcohol-dependent individuals (Addolorato and Leggio, 2010). Ninety-four patients with a diagnosis of AD according to Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 2000) were assessed for eligibility. Inclusion and exclusion criteria are reported in the Table 1. Of 94 initially screened for the study, 42 patients met inclusionary/exclusionary criteria and were enrolled in the main BIS study (and randomized to receive either baclofen 30 mg/day or baclofen 60 mg/day or placebo for 12 weeks) as well as in the parallel PNP study. All patients were enrolled into the study (baseline: T0) after 72 hours of total alcohol abstinence. During this 72-hour period before baseline, patients were monitored on a daily basis and those patients with significant withdrawal symptoms (i.e. Clinical Institute Withdrawal Assessment of Alcohol Scale-Revised score ≥ 10) received benzodiazepine treatment as needed. Starting from baseline (T0), no benzodiazepines were administered.
Table 1.
Inclusion and exclusion criteria (Study 1).
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; HDD, Heavy Drinking Days (five or more drinks per day for men and of four or more drinks per day for women); AST, aspartate aminotransferase; ALT, alanine aminotransferase; UNL, Upper Normal Limit.
Methods
Hormones
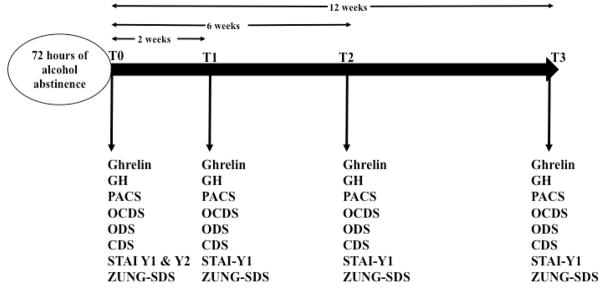
Hormonal determinations, craving and psychometric measurements were performed at baseline (T0), and then 2 weeks (T1), 6 weeks (T2) and 12 weeks (T3) after baseline. Timeline of the study is outlined in the Figure 1. Blood samples were collected at about 8 a.m., after an overnight food fast. Since alcohol suppresses acutely ghrelin levels (Calissendorff et al, 2005, 2006; Zimmermann et al, 2007; Addolorato et al, 2006; Badaoui et al, 2008), baseline (T0) ghrelin levels were determined after 3 days (72 hours) of alcohol abstinence. As for ghrelin determinations at T1, T2 and T3, none of the subjects had any recent alcohol consumption (i.e. after they woke up), as confirmed by the blood alcohol concentrations (data not shown). The blood collection was performed together with the administration of the questionnaires reported below to assess alcohol craving and other psychometric variables. Blood samples were collected, immediately centrifuged at 1500 × g for 15 min at 25°C, and stored at −20°C until analyzed. Plasma immunoreactive ghrelin levels were measured with a commercial radio-immunoassay kit (Linco, St. Charles, MO); the intra- and inter-assay variability was less than 6% and 9%, the sensitivity was 100 pg/ml; spike and recovery experiments, 91–96% recovery; serial dilutions yielded 99–146% of expected values; 100% specificity for human ghrelin, ghrelin-(9-16), and des-octonylghrelin with no cross-reactivity with human leptin or insulin. Serum GH levels were measured with a commercial chemiluminescence assay kit.
Figure 1.
Timeline of Study 1. GH: Growth Hormone; PACS: Penn Alcohol Craving Scale; OCDS: Obsessive Compulsive Drinking Scale; ODS: obsessive subscale of the OCDS; CDS: compulsive subscale of the OCDS; STAI: State and Trait Inventory; Zung-SDS: Zung Self-Rating Depression Scale.
Craving
The Penn Alcohol Craving Scale (PACS) and the Obsessive Compulsive Drinking Scale (OCDS) were used to assess alcohol craving at each time point (T0-T3). The PACS reflects the “appetitive urge” and the “emotional-motivational state” definitions of craving (Flannery et al, 2003). The OCDS consists of 2 subscales evaluating both the obsessive (OB subscale) and compulsive (CP subscale) components of craving (Anton et al, 1995; Janiri et al, 2004). Both the total score and OB and CP subscores were evaluated.
Anxiety and Depression
The State and Trait Inventory (STAI) test was used to assess the state (STAI-Y1) and trait (STAI-Y2) anxiety (Spielberg et al, 1983). Current depression was assessed by the Zung Self-Rating Depression Scale (Zung-SDS) (Zung et al, 1965). Both STAI and Zung-SDS questionnaires were administered at each time point (T0-T3).
Alcohol Drinking
Self reported alcohol use was determined by the timeline follow-back (TLFB) method (Sobell and Sobell, 1992) at each time point (T0-T3).
Metabolic Measurements
Body mass index (BMI), fat mass (FM), fat-free mass (FFM), total body water (TBW), resting energy expenditure (REE), non-protein respiratory quotient (npRQ) and basal metabolic rate (BMR) were measured with standardized techniques, as previously described (Addolorato et al, 1998a,b, 1999; Leggio et al, 2008a, 2009b). Since a 3-month period has been suggested as the minimum time to observe significant changes in nutritional and metabolic parameters in alcoholics (Addolorato et al, 1998a; Leggio et al, 2009b), these parameters were only assessed at T0 and T3.
Statistical Analysis
All data are expressed as mean ± SD. Normal distribution of data was tested with the Kolmogoroff–Smirnow test. Data collected over the course of the study were analyzed using analysis of variance (ANOVA) for repeated measures with drinking and baclofen use as between subjects factor and for the 4 different time intervals. Assumption of sphericity was tested by Mauchly’s test and because this was violated, multivariate test was used to evaluate the data analyzed. Correlations were analyzed using Pearson’s correlation coefficients. Statistical significance was accepted if a p-value less than 0.05 (two-sided) was obtained.
Results
Description of the sample
The 42 enrolled subjects had a median age of 44 years (range 23 to 60 years) and a BMI of 23.3±3.5 kg/m2. Out of the 42 subjects, 32 (76%) were males and 27 (64%) had a positive family history of alcoholism. During the 12-week period of the study, 10 patients (24%) dropped-out (i.e. drop-outs were defined as those patients who missed 3 or more consecutive visits). The remaining 32 patients completed all visits and all outcomes measured at every time-point.
Ghrelin and Alcohol Drinking
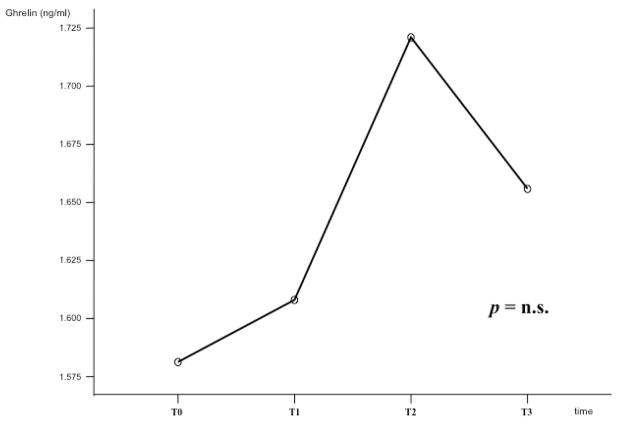
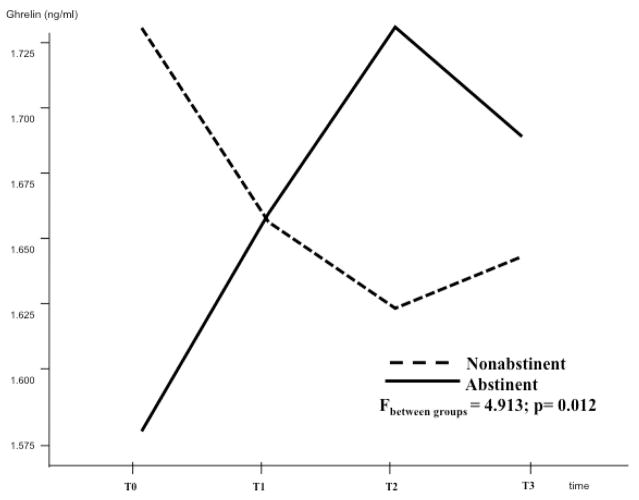
Figure 2 shows ghrelin changes during the four determinations (T0-T3). There was no statistical difference for ghrelin during the four different time intervals when we considered the total group of subjects (F=0.162, p=0.921 - Figure 2). Figure 3 shows ghrelin changes when we analyzed those subjects who were abstinent vs. those who were not abstinent during the 12-week period. Non-abstinent was defined each participant who consumed any amount of alcohol during the 12-week period of the study, while abstinent was defined each participant who was completely abstinent during the 12-week period. Baseline ghrelin levels were higher in the non-abstinent subjects, than in the abstinent ones (p=0.031). Moreover, during the 12-week period, ghrelin levels decreased in the non-abstinent group and increased in the abstinent one, showing a statistical difference in the changes of ghrelin between the two groups (F=4.913, p=0.012 - Figure 3). There were no statistically significant differences in ghrelin levels when we considered subjects treated with baclofen vs. those treated with placebo during the 12-week period (data not shown).
Figure 2.
Ghrelin changes during the four determinations (T0-T3); there were no statistical difference for ghrelin during the four different time intervals when we considered the total group of subjects (F=0.162, p=0.921).
Figure 3.
Ghrelin changes of subjects who were abstinent vs. those who were not abstinent during the 12-week period were analyzed separately. There was a statistical difference in the changes of ghrelin between the two groups (F=4.913, p=0.012).
Ghrelin and Alcohol Craving
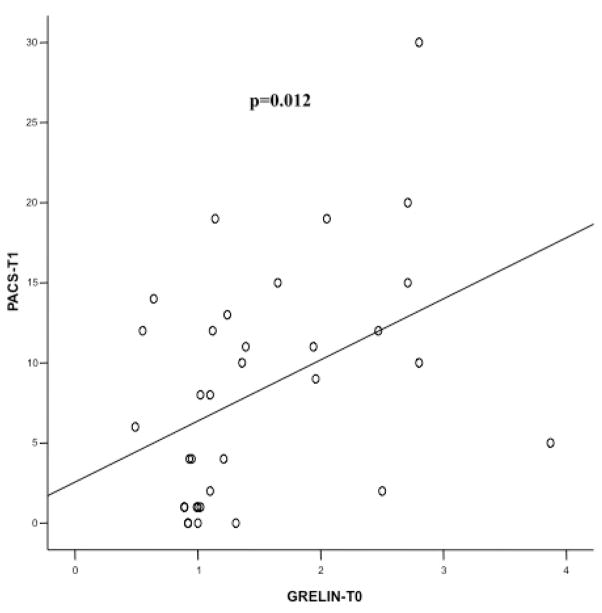
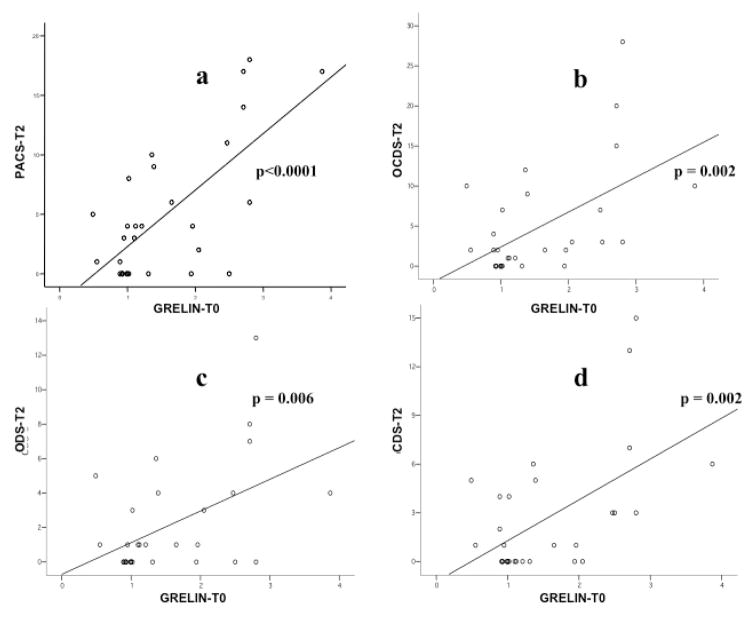
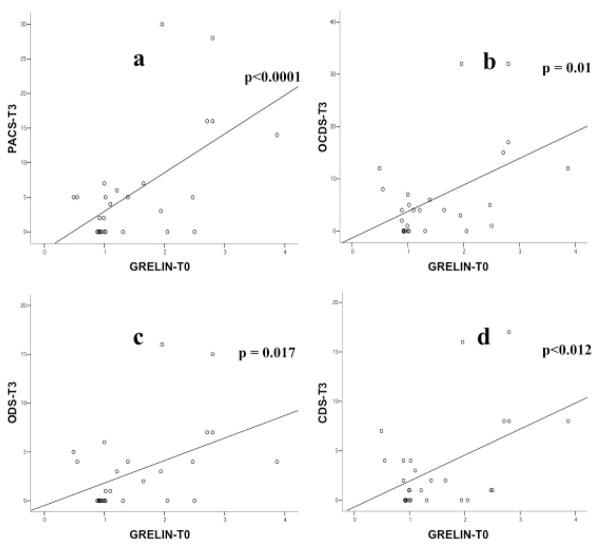
Baseline ghrelin levels (Ghrelin-T0) were significantly and positively correlated with the PACS score at T1 (r= 0.423, p=0.012 - Figure 4). Moreover, baseline ghrelin levels were significantly and positively correlated with all craving scores at T2, namely the PACS (r= 0.699, p<0.0001 - Figure 5a), the OCDS total score (r= 0.541, p=0.002 - Figure 5b), the obsessive OCDS subscore (r= 0.494, p=0.006 - Figure 5c) and the compulsive OCDS subscore (r=0.553, p=0.002 - Figure 5d). Finally, baseline ghrelin levels were significantly and positively correlated with all craving scores at T3, namely the PACS (r= 0.579, p<0.0001 - Figure 6a), the OCDS total score (r= 0.488, p=0.01 - Figure 6b), the obsessive OCDS subscore (r= 0.454, p=0.017 - Figure 6c) and the compulsive OCDS subscore (r= 0.479, p=0.012 - Figure 6d). Ghrelin-T1, Ghrelin-T2 and Ghrelin-T3 did not correlate with any of the alcohol craving scores. Correlations between plasma ghrelin levels and alcohol craving scores did not change when we analyzed separately non-abstinent and abstinent participants, but significantly higher craving levels were found in non-abstinent vs. abstinent participants (data not shown).
Figure 4.
Relationship between baseline ghrelin levels (Ghrelin-T0) and Penn Alcohol Craving Scale (PACS) score at T1. Ghrelin-T0 was significantly and positively correlated with the PACS score at T1 (r= 0.423, p=0.012).
Figure 5.
Relationship between baseline ghrelin levels (Ghrelin-T0) and craving scores at T2. Ghrelin-T0 was significantly and positively correlated with all craving scores at T2, namely the Penn Alcohol Craving Scale (PACS; r= 0.699, p<0.0001 - Figure 5a), the Obsessive Compulsive Drinking Scale total score (OCDS; r= 0.541, p=0.002 - Figure 5b), the obsessive OCDS subscore (ODS; r= 0.494, p=0.006 - Figure 5c) and the compulsive OCDS subscore (CDS; r=0.553, p=0.002 - Figure 5d).
Figure 6.
Relationship between baseline ghrelin levels (Ghrelin-T0) and craving scores at T3. Ghrelin-T0 was significantly and positively correlated with all craving scores at T3, namely the Penn Alcohol Craving Scale (PACS; r= 0.579, p<0.0001 - Figure 6a), the Obsessive Compulsive Drinking Scale total score (OCDS; r= 0.488, p=0.01 - Figure 6b), the obsessive OCDS subscore (ODS; r= 0.454, p=0.017 - Figure 6c) and the compulsive OCDS subscore (CDS; r= 0.479, p=0.012 - Figure 6d).
Ghrelin, Anxiety and Depression
No correlation between ghrelin and anxiety scores (STAI-Y1; STAI-Y2) nor between ghrelin and depressive scores (ZUNG-SDS) was found at any time point of the study (T0-T3).
Ghrelin and GH
No significant changes in GH levels were found in the whole sample, nor when we analyzed separately non-abstinent vs. abstinent alcoholics. No correlations were found between GH levels and ghrelin levels at each time point of the study. No correlations were found between GH levels and craving scores, nor between GH levels and either STAI or ZUNG-SDS scores at each time point of the study.
Ghrelin and Metabolism
During the 12-week period there was a significant change of the nutritional parameters, namely a significant increase of BMI (p<0.0001), FM (p<0.0001), TBW (p<0.0001) and RQ (p=0.024), and a significant decrease of FFM (p<0.0001) and EE (p<0.025). At T0, no correlations were found between ghrelin levels and nutritional parameters. At T3, ghrelin only correlated with the RQ (r= −0.49, p=0.028).
STUDY 2. GHRELIN GENE POLYMORPHISMS IN ALCOHOL-DEPENDENT SUBJECTS: A CASE-CONTROL STUDY
Consistent with the hypothesis that ghrelin is involved in the neurobiology of alcohol craving and dependence, genes involved in the ghrelin physiology may contribute to the biological vulnerability to alcoholism. Previously, two polymorphisms of the ghrelin gene, the G152A (Arg51Gln) and C214A (Leu72Met) were studied in subjects with binge eating disorder. That study showed that the Leu72Met ghrelin gene variant was associated with a moderate, but significant risk for developing binge eating disorder (Monteleone et al, 2007). There exist an overlap between over-eating and over-consumption of alcohol. Both binge eating and alcoholism represent complex genetic traits determined by several genes, and interacting with the environment. Thus, the aim of this preliminary study (Study 2) was to explore the same two polymorphisms of the ghrelin gene, Arg51Gln and Leu72Met, in subjects with a diagnosis of AD, compared to control subjects.
Subjects
Inclusion criteria included diagnosis of AD according to DSM-IV criteria (American Psychiatric Association, 2000). Exclusion criteria were: clinically significant psychiatric illness including any psychotic disorders, bipolar disorder, or severe depression, suicidal ideation, substance use disorders other than alcohol and nicotine dependence or cannabis abuse; BMI ≥ 30 Kg/m2. Matched healthy controls were enrolled, provided that they satisfied the same exclusion criteria and provided that they did not satisfy a DSM-IV diagnosis of alcohol abuse or dependence.
Methods
Clinical and demographic characteristics
Information on average alcohol drinking, age of onset of AD, years of addiction and family history of alcoholism was collected for alcohol-dependent subjects. Nicotine smoking behavior and BMI were collected for alcohol-dependent subjects and healthy controls.
Genotyping
Ghrelin gene polymorphisms were analyzed as previous described (Monteleone et al, 2006, 2007). Genomic DNA was collected from nucleated white blood cells and target DNA was amplified by polymerase chain reaction (PCR). Primers were designed from a genomic sequence (accession no.: AF296558; NCBI), as follows: GHR-F=50-TGACCTCACTGTTTCTGCAAG-30; GHR-R=50-GCACCCTGTTCACTGCCAC-30. Every cycle of the PCR except the first one (961C for 4.30min) was performed as follows: 961C for 30 s, 641C for 30 s and 721C for 30 s. This procedure generated a 278-bp fragment that was digested with AvaI (Arg51 allele: fragments of 168 and 110 bp) and BsrI (Leu72 allele: fragments of 172 and 106 bp). Restriction patterns were visualized on 2% agarose gels stained with ethidium bromide.
Statistical analysis
Values were expressed as mean ± standard deviation and categorical data as percentages. The chi square test was performed to compare the genotype and allele frequencies between patients and healthy controls. In order to test whether the BMI between the two groups were influenced by ghrelin genotype, a two-way analysis of variance (ANOVA) was performed with diagnosis (patients and control) and ghrelin genotype variant as independent variables. A p value of 0.05 or less was considered statistically significant. All statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
A sample of 70 alcohol-dependent subjects [52(74%) males; age: 42.8±9.8 years; BMI of 24.3±3.3 kg/m2] and 68 healthy controls [40(59%) males; age: 31.7±5.9 years; BMI of 23.8±3.4 kg/m2] were enrolled in Study 2. The frequencies of the L and M alleles were 85.3% and 14.7% in healthy controls, and 78.6% and 21.4% in alcohol dependent individuals, respectively (Table 2). The distribution of the Leu72Met variant between healthy controls and alcohol dependent individuals was not statistically significant (p=0.421).
Table 2.
Genotype and allele frequencies (percentages in parenthesis) of two polymorphisms within the ghrelin precursor gene in subjects with alcohol dependence and healthy controls.
| Leu72Met genotypes | Arg51Gln genotypes | |||
|---|---|---|---|---|
|
| ||||
| L | M | Arg51Arg | Arg51Gln | |
| Subjects with alcohol dependence (n=70) | 55 (78.6%) | 15 (21.4%) | 70 (100%) | 0 (0%) |
| Healthy Controls (n=68) | 58 (85.3%) | 10 (14.7%) | 68 (100%) | 0 (0%) |
The frequencies of the Arg and Gln alleles were respectively 100% and 0%, both in healthy controls and in alcohol dependent individuals (Table 2). Therefore, subsequent analyses were performed only for the Leu72Met genetic variant.
Clinical and demographic characteristics of the two study groups according to Leu72Met genotype are shown in Table 3. Statistical comparisons of BMI values by means of two-way ANOVA revealed the main effects of genotype (F =7.013, p=0.009) with no main effect of diagnosis (F=0.705, p=0.402) and no significant diagnosis_ genotype interactions (F=0.096, p=0.0757). No significant differences in the presence of nicotine smoking emerged among Leu72 Met and Leu72Leu genotypes in both healthy controls (p=0.546) and alcohol dependent individuals (p=1).
Table 3.
Clinical characteristics of healthy controls and of subjects with alcohol dependence according to Leu72Met ghrelin genotypes.
| Subjects with alcohol dependence (n=70) | Healthy Controls (n=68) | |||
|---|---|---|---|---|
|
| ||||
| L (n=55) | M (n=15) | L (n=57) | M (n=9) | |
| Body Mass Index (BMI) | 24.6±3.1 | 22.9±3.7 | 24.2±3.4 | 22±2.3 |
| Nicotine Smoking | 37 (67.3%) | 10 (67.1%) | 16 (28.1%) | 4 (44.4%) |
| Drinks/Day | 18.3±7.8 | 21.4±8.3 | - | - |
| Age of Onset | 31.4±10.1 | 32.4±7.8 | - | - |
| Years of Addiction | 16.2±8.9 | 17.3±12.8 | - | - |
| Family History of Alcoholism | 25 (45.5%) | 8 (53.3%) | - | - |
Finally, analysis of the alcohol dependent group revealed no differences between alcohol dependent individuals carrying the Leu72Met variant and those carrying the Leu72Leu genotype in drinks/day (p=0.192), age of onset (p=0.736), years of addiction (p=0.688) and family history of alcoholism (p=0.803).
DISCUSSION
Study 1 showed a significant relationship between plasma ghrelin levels, alcohol intake and alcohol craving. In the Study 1, determinations of ghrelin levels were repeated over the time. No significant changes in ghrelin levels were observed in the sample as a whole. Previous human studies have demonstrated that alcohol intake suppresses plasma ghrelin levels (Calissendorff et al, 2005, 2006; Zimmermann et al, 2007; Addolorato et al, 2006; Badaoui et al, 2008) and that plasma ghrelin levels increase during abstinence (Kim et al, 2005, Kraus et al, 2005). Therefore, we also considered abstinent vs. non-abstinent alcohol-dependent subjects. Interestingly, in the latter case, ghrelin’s patterns were inverse between the two groups and there was a significant difference in the changes of ghrelin between the two groups (Figure 3). In the non-abstinent subjects, ghrelin levels went down during the 12-week period, consistent with the inhibitory effect of alcohol on ghrelin (Calissendorff et al, 2005, 2006; Zimmermann et al, 2007; Addolorato et al, 2006; Badaoui et al, 2008). On the contrary, in the abstinent subjects, ghrelin levels increased during the 12-week period, consistent with the observation that ghrelin levels increase during alcohol abstinence (Kim et al, 2005; Kraus et al, 2005). In other words, ghrelin levels went down when alcohol consumption persisted, while ghrelin levels went up when subjects stopped drinking.
Figure 3 also shows how baseline ghrelin levels were higher in those subjects who were non-abstinent during the 12-week period, than in those who were abstinent during the same period of time. Thus, those subjects with higher baseline ghrelin levels were those who continued drinking alcohol during the 12-week period, despite of the treatment program (pharmacological and behavioral) provided. On the contrary, those subjects with lower baseline ghrelin levels were those who stopped drinking alcohol during the 12-week period. These results lead to speculate that baseline ghrelin may represent a marker of alcohol drinking and relapse. Higher baseline (pre-treatment) ghrelin levels reflect higher subsequent (during treatment) alcohol drinking, thus representing a potential marker of risk of relapse. These observations are consistent with preclinical experiments, where genetic (GHS-R1A knockout mice) and pharmacological (two GHS-R1A antagonists: BIM28163, delivered i.c.v; and JMV2959, i.p.) models of suppressed ghrelin signaling were employed (Jerlhag et al, 2009). These preclinical experiments demonstrated that central administration of ghrelin into either the VTA or the LDTg significantly increased alcohol consumption compared to vehicle treatment in a 2-bottle (alcohol/water) free choice limited access paradigm. Consistent with these preclinical observations, here we demonstrated that higher baseline ghrelin levels were associated with subsequent alcohol consumption in subjects with a diagnosis of AD.
Although the lack of data on brain ghrelin concentrations could represent a limitation of this study due to the ethical limitations to collect it in a human study, we note that preclinical experiments were replicated in rats with identical results by using peripheral ghrelin administration (Jerlhag, 2008), thus further confirming the similarities between those preclinical studies (Jerlhag et al, 2007, 2008) and our clinical study. Additional preclinical studies have also demonstrated that both central and peripheral (i.p.) ghrelin administration increases extracellular concentrations of accumbal DA in NMRI mice (Jerlhag et al, 2006). Based on these (Jerlhag, 2008; Jerlhag et al, 2006, 2007, 2008, 2009) and previous studies (Asakawa et al, 2001; Date et al, 2002, 2006), it has been hypothesized that the effects of peripheral ghrelin on brain reward are mediated via nicotinic acetylcholine receptors and/or via the vagal nerve. Consistent with all of these preclinical observations, here we have demonstrated a potential key role of peripheral ghrelin at baseline in the subsequent alcohol drinking. However, it should be kept in mind that ‘baseline’ was related to a specific period of time (i.e. 12 weeks) during which participants received a treatment, thus precluding the possibility to generalize these findings.
Participants of this study were enrolled in a research treatment trial, consisting of a behavioural intervention (i.e. BRENDA) and a placebo-controlled pharmacological intervention (i.e. baclofen). While BRENDA was provided to all participants, only 2/3 of the participants received baclofen, whereas 1/3 received placebo. However, an analysis of baclofen-treated subjects vs. placebo-treated subjects did not show any significant difference in plasma ghrelin levels at each time (T0-T3). This leaves us to assume that the pharmacological intervention did not represent a confounding factor.
This study also showed a significant positive correlation between baseline ghrelin (T0) and craving during the 12-week period (T1-T3). These findings further support our hypothesis that baseline ghrelin plays a key role in alcohol-seeking behavior. In fact, subjects with higher baseline ghrelin levels had higher subsequent alcohol craving scores. As previously stated, subjects with higher baseline ghrelin levels were those who drank alcohol subsequently. Thus, this study leads to conclude that i) subjects with higher ghrelin levels at baseline were more likely to drink alcohol during the subsequent 12-week period; and ii) this relationship was mediated by alcohol craving, so that subjects with higher ghrelin levels at baseline had higher alcohol craving scores during the subsequent 12-week period.
The relationship between ghrelin and alcohol craving might be explained by the key role of DA in both the central actions of ghrelin and in the neurobiology of alcohol craving. Ghrelin, administered either centrally or peripherally, increases extracellular concentrations of accumbal DA in animals (Jerlhag, 2008; Jerlhag et al, 2006, 2007; Naleid et al, 2005; Kawahara et al, 2009; Quarta et al, 2009). Dopamine plays a key role in the neurobiology of alcohol craving and dependence. Cortico-mesolimbic DA pathways may mediate alcohol’s rewarding effects (including craving) associated with its abuse liability (Wise, 1996; Tupala and Tiihonen, 2004; Addolorato et al, 2005; Koob, 2006). In other words, research demonstrates that ghrelin affects central levels of DA, a key neurotransmitter in the neurobiology of alcohol craving. In agreement with these concepts, the present study with alcohol-dependent individuals show a relationship between peripheral plasma ghrelin and alcohol craving and drinking.
Our findings on the link between baseline ghrelin and alcohol drinking and craving were not just coincidental, but strengthened by the following observations. First, this significant and positive correlation was present at each time point of the 12-week period (T1-T3) and almost with all scales and subscales. Second, out of all the psychometric assessments performed in this study, ghrelin was constantly correlated only with alcohol craving scales, while ghrelin never correlated with the other psychometric assessments (anxiety, depression), thus leading to conclude that the relationship was somewhat specific and unique between ghrelin and alcohol craving. Our study supports the hypothesis that ghrelin plays a role in alcohol drinking via a direct effect on alcohol craving, at least in alcohol-dependent individuals without psychiatric co-morbidities. In fact, we excluded alcohol-dependent subjects with a formal diagnosis of any anxiety disorder or major depression. Third, there were no significant changes in GH levels, nor correlations between GH and alcohol craving scores. These last findings suggest that ghrelin per se affects alcohol drinking and craving, and that ghrelin’s role in alcohol-seeking behavior is not related to the ghrelin-mediated release in GH. This is further supported by the dissociation between ghrelin and GH levels in our sample; in fact no correlations were found between GH levels and ghrelin levels at each time point of the study. This is consistent with the knowledge that circulating ghrelin and GH levels may not be interrelated in all metabolic situations (Mustonen et al, 2001). Fourth, while a significant improvement of the nutritional parameters was observed during the 12-week period (a finding consistent with previous observations, e.g. Addolorato et al, 1998a; Leggio et al, 2008a, 2009b), baseline ghrelin (T0) never correlated with these nutritional parameters, thus suggesting the lack of a relationship between ghrelin and the nutritional status in our sample. Thus, it may be speculated that the nutritional status did not act as a confounding factor.
The findings of the present study are consistent with our first case-control study, where we first demonstrated a significant positive relationship between plasma ghrelin levels and alcohol craving in 15 active drinking alcohol-dependent subjects (Addolorato et al, 2006). The present study not only confirms our first case-control study, but also expands our knowledge on the role of ghrelin in AD in several significant ways. In our first study, ghrelin was determined in active drinking alcohol-dependent subjects with an alcohol intake within the 24 hours before the blood draw. Since alcohol affects acutely ghrelin levels (Calissendorff et al, 2005, 2006; Zimmermann et al, 2007; Addolorato et al, 2006; Badaoui et al, 2008), in the present study we eliminated the acute effect of ethanol on ghrelin, as a confounding factor. Thus, baseline ghrelin was determined after a standardized 3-day (72 hours) period of alcohol abstinence. Additionally, in the present study, we employed a longitudinal design with repeated ghrelin determinations. We also enrolled a relatively larger sample, and craving was assessed not only with the OCDS scale but also with the PACS scale. Finally, the present study included the determination of GH levels and psychometric measurements of anxiety and depression.
In the Study 2, although a higher percentage of the Leu72Met ghrelin gene polymorphism in alcohol-dependent subjects than in the controls (21.4% vs. 14.7%, respectively), this difference was not statistically significant. Nevertheless, future larger studies are needed to further investigate a possible association between the Leu72Met ghrelin gene polymorphism and diagnosis of AD. Study 2 also showed a main effect of genotype (Leu72Met vs. Leu72Leu) on the BMI, both in alcohol-dependent individuals and in the controls. This information is of importance because ghrelin plays an important regulatory role in metabolism (van der Lely, 2009) and metabolic changes have been reported in alcoholics (Addolorato et al, 1998a,b; Leggio et al, 2008a, 2009b). In fact, these observations suggest that the role of ghrelin in affecting metabolism is not different between alcohol-dependent subjects and healthy controls. The findings in the Study 2 are consistent with those of Study 1, where ghrelin did not correlate with the majority of the metabolic parameters (e.g. BMI, FM, FFM). Moreover, although the preliminary nature, results of Study 2 are also consistent with the previous genetic analyses by Landgren et al. (2008, 2010).
This investigation has limitations, which need to be addressed. First, while we measured total ghrelin levels, future studies will have to determine both acetylated and non-acetylated ghrelin levels. Second, Study 1 did not include a control group. However, the main goal of the Study 1 was to investigate the relationship between ghrelin levels, alcohol drinking and alcohol craving. Thus, we preferred a longitudinal within-subject design in a specific population of alcohol-dependent individuals. Third, the relatively small sample size did not allow us to perform additional analyses, which should be addressed by future larger studies, i.e. the role of ghrelin according to possible alcoholic subtypes (see: Hillemacher et al, 2007; Leggio et al, 2009c; Leggio, 2010), as well as potential gender differences in the link between ghrelin and alcohol drinking and craving (see: Wurst et al, 2007). Additionally, the trends of ghrelin levels in the abstinent vs. non-abstinent subject, as outlined in the Figure 3 (see in particular T2), might suggest a potential tendency for a homeostatic stabilization of ghrelin levels, although this only remains speculative. Finally, we understand that correlation is not causation and have tried our best to control for several alternative hypotheses to our own. As for Study 2, we note the preliminary nature and the need of performing larger haplotype analyses, which should also include GHSR genes (see: Landgren et al. 2008, 2010).
In keeping with the preclinical evidence, this study suggests that ghrelin represents a gut-brain peptide potentially able to affect alcohol-seeking behaviors, such as alcohol drinking and craving. These findings lead to hypothesize that ghrelin represents a new neuropharmacological target for AD and antagonizing the ghrelin system (for an extensive review, see: Leggio, 2010) might lead to a new and innovative way to provide an effective treatment for AD.
Acknowledgments
The authors would like to thank Anna Caprodossi, B.S., for performing the laboratory testing.
Funding for this study was partially provided by a grant (L.L. & G.A.) and by an Exchange Award (L.L.), both from the European Foundation for Alcohol Research (ERAB); and a grant (G.A.) from the Italian Ministry for University, Scientific and Technological Research (MURST). Both ERAB and MURST had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORS CONTRIBUTION
LL thought the study rationale for the research and designed the protocols. LL, AF, SC, AN and GA did the research (both Study 1 and Study 2). NM and EC participated in the Study 1 (hormonal, nutritional and metabolic determinations). BC and PM participated in the Study 2 (genetic polymorphisms). LL, AF, SC, AN, NM, EC, BC, PM and GA did the acquisition of data. LL, PM, GAK, RMS and GA did the analysis and interpretation of data. AM participated in the statistical analysis and interpretation of the data and in the editing of the report. All authors provided administrative, technical, or material support. LL wrote the first draft of the report. All authors edited the report, contributed to and have approved the final manuscript.
References
- Addolorato G, Capristo E, Caputo F, Greco AV, Ceccanti M, Stefanini GF, Gasbarrini G. Nutritional status and body fluid distribution in chronic alcoholics compared with controls. Alcohol Clin Exp Res. 1999;23:1232–1237. doi: 10.1111/j.1530-0277.1999.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Caputo F, Stefanini GF, Gasbarrini G. Three months of abstinence from alcohol normalizes energy expenditure and substrate oxidation in alcoholics: a longitudinal study. Am J Gastroenterol. 1998a;93:2476–2481. doi: 10.1111/j.1572-0241.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med. 1998b;244:387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–2117. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, Gasbarrini G. Neurobiochemical and clinical aspects of craving in alcohol addiction: a review. Addict Behav. 2005;30:1209–1224. doi: 10.1016/j.addbeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Stärkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38:397–403. doi: 10.1111/j.1365-2362.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152:743–747. doi: 10.1530/eje.1.01905. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism. 2006;55:1625–1629. doi: 10.1016/j.metabol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Connelly DM, Unwin JW, Taberner PV. The role of the blood glucose level in determining voluntary ethanol consumption in the LACG and diabetogenic C57BL strains of mice. Biochem Pharmacol. 1983;32:221–226. doi: 10.1016/0006-2952(83)90547-6. [DOI] [PubMed] [Google Scholar]
- Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli JR. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 2003;64:120–126. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- Harris JA, Benedict FG. Biometric Studies of BasalMetabolism in Man. Washington, DC: Carnegie Institute; 1919. Publication no. 279. [Google Scholar]
- Hillemacher T, Bleich S, Frieling H, Schanze A, Wilhelm J, Sperling W, et al. Evidence of an association of leptin serum levels and craving in alcohol dependence. Psychoneuroendocrinology. 2007;32:87–90. doi: 10.1016/j.psyneuen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Kraus T, Rauh J, Weiss J, Schanze A, Frieling H, et al. Role of appetite-regulating peptides in alcohol craving: an analysis in respect to subtypes and different consumption patterns in alcoholism. Alcohol Clin Exp Res. 2007;31:950–954. doi: 10.1111/j.1530-0277.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Weinland C, Heberlein A, Gröschl M, Schanze A, Frieling H, et al. Increased levels of adiponectin and resistin in alcohol dependence--possible link to craving. Drug Alcohol Depend. 2009;99:333–337. doi: 10.1016/j.drugalcdep.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Janiri L, Calvosa F, Dario T, Pozzi G, Ruggeri A, Addolorato G, et al. The Italian version of the obsessive-compulsive drinking scale: validation, comparison with the other versions and difference between type 1- and type 2-like alcoholics. Drug Alcohol Depend. 2004;74:187–195. doi: 10.1016/j.drugalcdep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin Stimulates Locomotor Activity and Accumbal Dopamine-Overflow via Central Cholinergic Systems in Mice: Implications for its Involvement in Brain Reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin-induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsychopharmacol. 2008;18:508–518. doi: 10.1016/j.euroneuro.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27:1743–1749. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Kaneko F, Yamada M, Nishi Y, Tanaka E, Nishi A. Peripherally administered ghrelin induces bimodal effects on the mesolimbic dopamine system depending on food-consumptive states. Neuroscience. 2009;161:855–864. doi: 10.1016/j.neuroscience.2009.03.086. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Demiralay C, Wolf K, Wiedemann K. Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J Psychiatr Res. 2005;39:545–551. doi: 10.1016/j.jpsychires.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Yoon SJ, Choi B, Kim TS, Woo YS, Kim W, et al. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40:76–79. doi: 10.1093/alcalc/agh108. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Gröschl M, Bayerlein K, Hillemacher T, Reulbach U, et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29:2154–2157. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- Landgren S, Jerlhag E, Hallman J, Oreland L, Lissner L, Strandhagen E, et al. Genetic variation of the ghrelin signaling system in females with severe alcohol dependence. Alcohol Clin Exp Res. 2010;34:1519–1524. doi: 10.1111/j.1530-0277.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- Landgren S, Jerlhag E, Zetterberg H, Gonzalez-Quintela A, Campos J, Olofsson U, et al. Association of pro-ghrelin and GHS-R1A gene polymorphisms and haplotypes with heavy alcohol use and body mass. Alcohol Clin Exp Res. 2008;32:2054–2061. doi: 10.1111/j.1530-0277.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- Leggio L. Understanding and treating alcohol craving and dependence: recent pharmacological and neuroendocrinological findings. Alcohol Alcohol. 2009;44:341–352. doi: 10.1093/alcalc/agp026. [DOI] [PubMed] [Google Scholar]
- Leggio L. Role of the ghrelin system in alcoholism: acting on the growth hormone secretagogue receptor (GHS-R) to treat alcohol-related disorders. Drug News & Perspectives. 2010;23(3):157–166. doi: 10.1358/dnp.2010.23.3.1429490. [DOI] [PubMed] [Google Scholar]
- Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov-Polevoi AB, Swift RM. Role of feeding-related pathways in alcohol dependence: a focus on sweet preference, NPY and ghrelin. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, et al. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients. A longitudinal study. Alcohol Clin Exp Res. 2008b;32:2047–2053. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Malandrino N, Miceli A, Capristo E, Gasbarrini G, Addolorato G. Insulin but not insulin growth factor-1 correlates with craving in currently drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2008a;32:450–458. doi: 10.1111/j.1530-0277.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev. 2009c;19:115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Leggio L, Malandrino N, Ferrulli A, Cardone S, Miceli A, Gasbarrini G, et al. Is cortisol involved in the alcohol-related fat mass impairment? A longitudinal clinical study. Alcohol Alcohol. 2009b;44:211–215. doi: 10.1093/alcalc/agn116. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ray LA, Kenna GA, Swift RM. Blood Glucose Level, Alcohol Heavy Drinking, and Alcohol Craving During Treatment for Alcohol Dependence: Results From the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Alcohol Clin Exp Res. 2009a;33:1539–1544. doi: 10.1111/j.1530-0277.2009.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Tortorella A, Castaldo E, Di Filippo C, Maj M. No association of the Arg51Gln and Leu72Met polymorphisms of the ghrelin gene with anorexia nervosa or bulimia nervosa. Neurosci Lett. 2006;398:325–327. doi: 10.1016/j.neulet.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Tortorella A, Castaldo E, Di Filippo C, Maj M. The Leu72Met polymorphism of the ghrelin gene is significantly associated with binge eating disorder. Psychiatr Genet. 2007;17:13–16. doi: 10.1097/YPG.0b013e328010e2c3. [DOI] [PubMed] [Google Scholar]
- Mustonen AM, Nieminen P, Hyvärinen H. Preliminary evidence that pharmacologic melatonin treatment decreases rat ghrelin levels. Endocrine. 2001;16:43–46. doi: 10.1385/ENDO:16:1:43. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009;54:89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa NJ: 1992. pp. 41–72. [Google Scholar]
- Spielberg CD, Gorsuch RL, Lushene RE. Manual for the State and Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto (CA): 1983. [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ. Ghrelin and new metabolic frontiers. Horm Res. 2009;71(Suppl 1):129–133. doi: 10.1159/000178055. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Graf I, Ehrenthal HD, Klein S, Backhaus J, Blank S, et al. Gender differences for ghrelin levels in alcohol-dependent patients and differences between alcoholics and healthy controls. Alcohol Clin Exp Res. 2007;31:2006–2011. doi: 10.1111/j.1530-0277.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12:17–21. doi: 10.1111/j.1369-1600.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Zito KA, Vickers G, Telford L, Roberts DC. Experimentally induced glucose intolerance increases oral ethanol intake in rats. Alcohol. 1984;1:257–261. doi: 10.1016/0741-8329(84)90046-6. [DOI] [PubMed] [Google Scholar]
- Zung WW, Richards CB, Short MJ. A self-rating depression scale in outpatient clinic. Further validation of SDS. Arch Gen Psychiatry. 1965;37:847–858. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]