Abstract
Ejaculates are fundamental to fitness in sexually-reproducing animals: males gain all their direct fitness via the ejaculate and females require ejaculates to reproduce. Both sperm and non-sperm components of the ejaculate (including parasperm, seminal proteins, water and macromolecules) play vital roles in post-copulatory sexual selection and conflict, processes that can potentially drive rapid evolutionary change and reproductive isolation. Here, we assess the increasing evidence that considering ejaculate composition as a whole – and potential trade-offs among ejaculate components – has important consequences for predictions about male reproductive investment and female responses to ejaculates. We review current theory and empirical work, and detail how social and environmental effects on ejaculate composition have potentially far-reaching fitness consequences for both sexes.
Keywords: sexual selection, ejaculate, sexual coevolution, sexual conflict, sperm competition, condition
The mix matters
Male animals typically transfer complex ejaculates that contain much more than just sperm. Semen components can include seminal fluid proteins or peptides (Sfps), salts and sugars, defensive compounds, lipids, water and microbes [1]. Despite this complexity, sperm have been the major focus of many studies of strategic male investment in the ejaculate [2], and ‘ejaculate’ and ‘sperm’ are frequently used synonymously in the literature. However, non-sperm components of the ejaculate perform a vast array of functions: they are often essential for fertility, regulating sperm storage and usage, and they can strongly impact fitness in both sexes both through effects on female physiology, behaviour, immunity, and life history, and through biasing the outcome of competition between rival males [1, 3–5] (Figure 1). Thus, to make biologically realistic predictions about the resources males should invest in ejaculates, and how females should respond to them, we need to understand the causes and consequences of variation not only in overall ejaculate investment, but also in the component parts of the ejaculate. That is, we need to understand ejaculate composition.
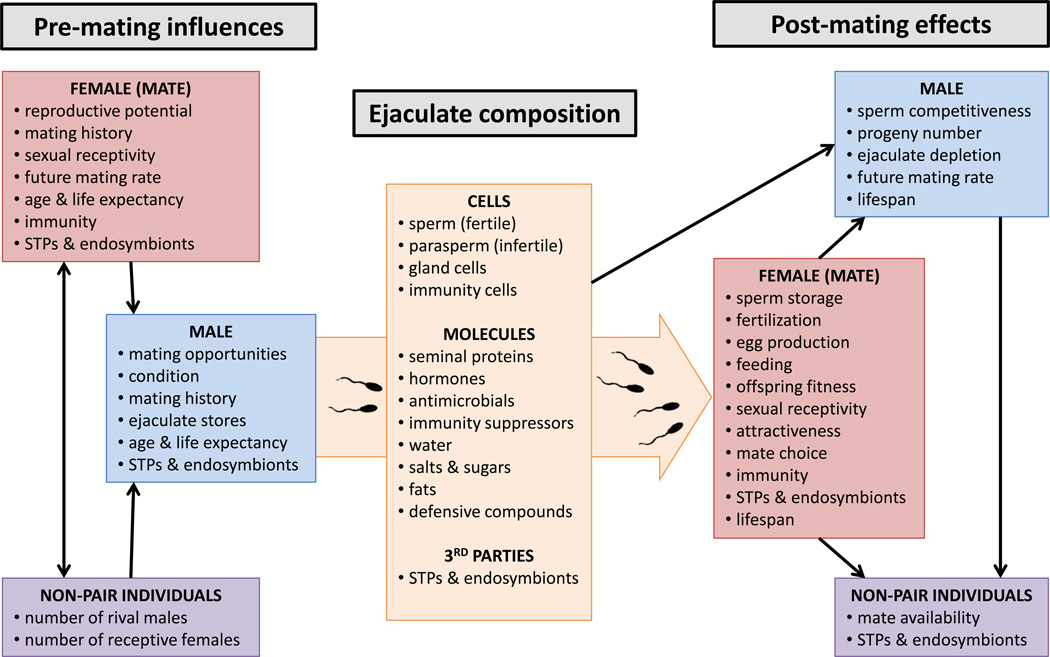
Figure 1.
Differential male allocation to ejaculate components can be influenced by, and have influences upon, a wide array of factors [1, 4]. The large arrow, containing the list of ejaculate components, represents the transfer of ejaculate from male to female; black arrows indicate relationships between factors and individuals. In composing ejaculates, males are likely to be sensitive to their own state as well as that of their mate, and individuals in the wider socio-sexual environment. These factors provide information about the reproductive value of their mate, future mating opportunities and reproductive competition, and theory predicts that males should strategically adjust ejaculate composition to maximize reproductive returns in response to these variables (Box 1). The resulting composition of the ejaculate can, in turn, influence the fitness of the mating male and female, other individuals in the population, and even sexually transmitted parasites and pathogens (STPs) and endosymbionts. Many effects on male fitness, such as progeny production and sperm competition success, occur via the female. The particular ejaculate components that influence each female response vary among taxa; for example, both sperm and Sfps can influence female fecundity, remating, sperm storage and sperm competitiveness [1].
Ejaculate composition has the potential to play a key role in the evolutionary ecology of mating and reproduction. This is because ejaculate components are likely to differ in their costs to males, and might trade-off against one another, and because females are likely to display different responses to variation in specific ejaculate components [3]. Moreover, the sex-specific costs and benefits associated with differently composed ejaculates are likely to vary with the social and ecological context, creating selection for plasticity in ejaculate composition. Thus, the composition of the ejaculate might have profound effects on both male and female fitness, and variation in ejaculate composition at different levels – between copulations, between males, between environments, and between populations and species – could play an important role in shaping mating behaviour, reproductive physiology and the evolutionary dynamics of these traits.
In this review, we bring together data from a range of fields to assess the importance of ejaculate composition in evolutionary ecology, considering both theoretical (Box 1) and empirical studies, and reviewing new technologies and practical methods (Box 2), as well as potential applications (Box 3). We evaluate the evidence that i) males are capable of adaptively modifying ejaculate composition, ii) male and female condition shapes ejaculate composition, and iii) ejaculate composition and female mating patterns coevolve and shape mating systems (Box 4). Many of the examples we use are drawn from insects, reflecting the taxonomic bias in empirical studies of ejaculates, but the hypotheses apply broadly.
BOX 1: Ejaculate composition in theory.
Three recent models investigate how selection should act on ejaculate composition, given distinct functions for different ejaculate components and production trade-offs among them [20, 70, 71]. These models assume that greater investment in sperm yields greater paternity (i.e., by ‘purchasing tickets’ in a sperm competition raffle), whereas increased investment in non-sperm ejaculate stimulates female fecundity. Given these assumptions, as sperm competition increases, a male should invest more in sperm and less in fecundity-enhancing ejaculate components.
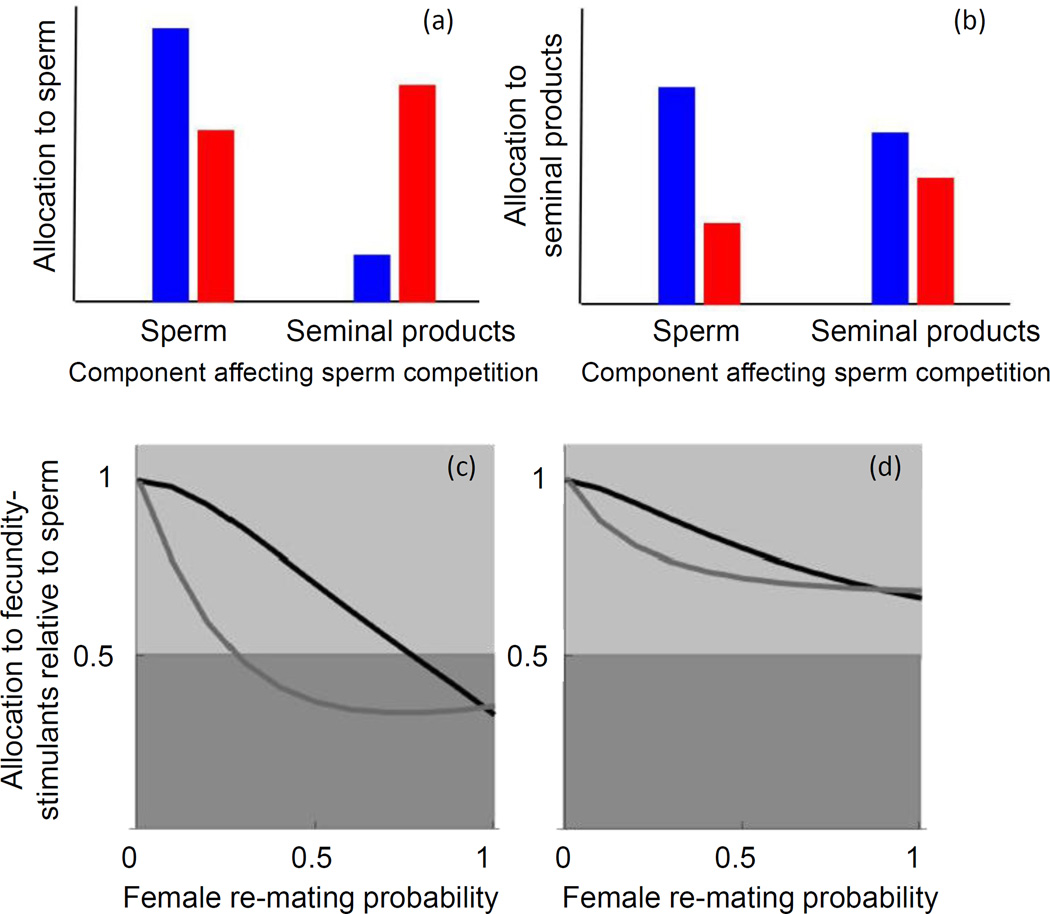
However, optimal ejaculate composition depends strongly on how ejaculate components function [70]. When ejaculate components function not only in the above ways but also influence success in sperm competition (i.e., by influencing the degree of bias in sperm competition raffles) [70], the predictions for overall ejaculate investment and composition depend strongly on which ejaculate components influence sperm competitiveness (Figure I a, b). If sperm numbers increase sperm competitive success, then males who already have an advantage in sperm competition (e.g., last males to mate in species with last-male sperm precedence) should invest more in all ejaculate components, compared to disadvantaged males. However, when non-sperm components enhance sperm competitiveness, favoured males should invest less in sperm and more in non-sperm [70].
Optimal ejaculate composition also depends on mating order [20]. When males are able to detect their place in the sequence of a female’s mates, later males can take advantage of an earlier male’s investment in fecundity-stimulation and transfer ejaculates containing relatively more sperm and less fecundity-stimulants [20] (Figure I c, d).
Changes in ejaculate composition are expected to have consequences for optimal female remating rate that in turn influence optimal ejaculate composition [20]. When ejaculates greatly stimulate female fecundity, females experience selection to increase remating, which feeds back on optimal ejaculate composition: sperm competition is intensified and generates selection for investment in sperm rather than fecundity-stimulants [20] (see also [71]). This prediction results from a model in which male investment per ejaculate is fixed and relative allocation to sperm and non-sperm evolve, and ejaculate investment does not entail costs for males. The field would benefit from future models that integrate these approaches to incorporate male-male competition and male-female coevolution, life history trade-offs, and the evolution of both overall ejaculate investment and ejaculate composition.
Figure I (Box 1)
The optimal ejaculate composition for males varies with ejaculate component function, male advantage in sperm competition, female remating rate and male mating order. (a, b) Males at an advantage in sperm competition (blue) invest more in sperm (a) than non-sperm seminal products (b) when sperm investment enhances sperm competitiveness, but vice versa when seminal products enhance sperm competitiveness. In contrast, males at a disadvantage in sperm competition (red) invest more in sperm than in seminal products regardless of sperm competitiveness function. (c, d) Males should allocate less to fecundity-stimulating ejaculate components (1) as the probability that females re-mate increases, (2) when these components increase fecundity only weakly (c) compared to strongly (d), and (3) when a male is second to mate with a female (grey lines) compared to first (black lines; no sperm precedence is assumed) Light grey shading indicates greater investment in fecundity-stimulation than in sperm; dark grey shading indicates the reverse. Reprinted with permission from [70] (a, b) and [20] (c, d).
BOX 2: Methods for measuring ejaculate composition.
Ejaculate quantity
Gross ejaculate composition can be assessed by combining ejaculate mass or volume with measures of at least one ejaculate component. In some species, whole ejaculates can be collected to measure [30, 72], but the collection method itself can affect composition [73, 74]. Ejaculates collected directly from males might not be identical to transferred ejaculates because males modify ejaculates in response to mating circumstances, and ejaculates collected from females can be modified in the female reproductive tract [4]. When ejaculate collection is impractical, indirect measures of ejaculate quantity include weighing males or measuring the volume of male organs before and after mating [40, 75], though these methods should ideally use control males that are capable of mating but not transferring ejaculate.
Quantifying sperm vs. non-sperm
Quantifying both sperm and overall ejaculate quantity yields an estimate of relative allocation to sperm vs. non-sperm. This is a potentially powerful approach, given that techniques for sperm quantification are well developed, and recent methods (real-time quantitative PCR, spectrophotometry) promise to further ease sperm quantification [30, 76].
Chemical composition
Biochemical techniques can determine the chemical composition of ejaculates, in terms of water, lipids, carbohydrates and proteins [24, 36, 77–80]. These measures can be used as proxies for ejaculate quality: cholesterol and sugar levels correlate with sperm function in some species [78, 80], and protein quantity is often correlated with ejaculate effectiveness [36, 77, 79].
Seminal fluid proteins (Sfps)
Gene expression studies have proved useful in identifying potential Sfps; for example, expressed sequence tag (EST) screens have identified highly expressed loci in male reproductive organs [81]. Once the Sfp transcriptome is characterized, Sfp expression can be tracked individually using qPCR [17], or en masse using microarrays or RNA sequencing. However, these approaches do not examine the proteins actually transferred to females, and mRNA levels may not correspond to protein quantity [82, 83]. To look directly at protein, mass spectrometry can be used to identify Sfps in any organism where genomic or transcriptomic data are available and sufficient ejaculate can be obtained [84, 85]; isotopic labeling can identify Sfps transferred to females [84]. Sfps can then be quantified with ELISAs for proteins with highly specific antibodies [24, 79, 86], and new quantitative proteomics methods promise protein quantification in non-model organisms [87]. However, mRNA and protein quantities do not always correspond directly to post-mating phenotypes [83], and functionally characterizing individual Sfps remains challenging, particularly for non-model organisms.
BOX 3: Practical applications of ejaculate composition research.
A key challenge is to move from basic research on ejaculate composition to applications that solve practical problems. Potential areas for practical applications include insect pest management [3, 4, 88], captive breeding of animals for aquaculture, agriculture and conservation [5, 89], assisted reproductive technology (ART) [5] and assisted insemination, reproductive health diagnostics [90], and contraception for humans and non-human animals. A first step is to identify specific components of the seminal fluid that could be manipulated to effect changes in sperm performance or in female reproductive physiology or behavior (in the case of insects). In mammals, specific Sfps are known to promote sperm storage and ovulation in the mated female [91], and in insect pests, specific Sfps are associated with regulating female sperm storage [92], egg production, feeding and mating activity [3, 4], and pheromone synthesis [88]. Increased functional understanding of specific components of the ejaculate will improve our ability to identify targets for practical applications.
Manipulation of ejaculate composition will be accomplished differently depending on the taxonomic group and the purpose. In captive breeding of mammalian species, storing and transferring sperm with specific fertility-promoting seminal plasma components could potentially increase the success of artificial insemination [89]. Similarly, ART might be more successful increase if women are exposed to specific seminal plasma components prior to or at the time of attempted conception [5, 89]. For insect pest management, the amount of specific Sfps that a male transfers to females can be manipulated in lab-reared insects through transgenesis [93]. Transgenic strains could potentially be used in conjunction with the sterile insect technique to suppress populations.
In addition to the above manipulations, the quality of individual ejaculates can be changed through altering a male’s actual or perceived environment, an effect that is potentially mediated by changes in ejaculate composition [2, 79]. For example, a protein rich diet can increase the effectiveness of male Queensland fruit flies at promoting sperm storage and inhibiting remating [94]. In humans, treatment of male infertility could potentially be improved by collecting ejaculates under conditions of perceived sperm competition [95]. Such manipulations could represent simple and inexpensive methods of applying basic ejaculate composition research to practical applications.
BOX 4: Ejaculate composition, female remating and mating system evolution.
Male ejaculate strategies and female remating are expected to co-evolve in ways that shape mating system evolution across populations and species. In particular, female remating rates should influence the evolution of ejaculate composition: as average remating rate increases, males mate more frequently, experience stronger sperm competition and might be selected to reduce allocation to fecundity-stimulating ejaculate components or to increase allocation to receptivity-inhibiting components (Box 1). Although this prediction has not been directly tested, there is evidence that female remating rate is correlated with male ejaculate investment across species in some insects [72, 96]. Moreover, experimental enforcement of monogamy in D. melanogaster results in males that are less able to inhibit female remating [97], suggesting that relaxed selection to inhibit female remating can result in reduced investment in receptivity-inhibiting ejaculate components.
In turn, variation in ejaculate composition and in female responses to ejaculate components could underlie divergence in female remating among populations and closely-related species. If this is the case, then genetic variation underlying ejaculate composition and female responses could provide the raw material for the rapid evolution of differences in remating among populations. Recent studies have begun to address this prediction by testing whether population-level variation in female mating frequency is related to variation in ejaculate size [98] or levels of individual ejaculate components [99]. However, at present we know very little about the extent of variation in ejaculate composition among populations, or how the balance of components within an ejaculate influences female remating propensity.
Variation in ejaculate composition might contribute to reproductive isolation through effects on mate choice. Variation in female remating rate has traditionally been interpreted without regard to the quality of potential male mates. However, it is plausible that changes in remating propensity reflect changes in female mate selectivity [100]. In other words, ejaculate components termed ‘receptivity-inhibiting’ might actually be ‘selectivity-enhancing’ by raising the threshold of male quality or stimulation that females require to mate. If this is the case, then variation in the amount of selectivity-enhancing ejaculate components might be associated with variation in the selection that female choice exerts on male traits. Over time, such ejaculate-induced selectivity could result in reproductive isolation among populations, and thus contribute to speciation.
Polyandry and ejaculate composition
Polyandry is pervasive among animals and sets the stage for selection on ejaculate composition through sperm competition, sexual conflict and cryptic female choice [6–8]. The risk of sperm competition selects for investment in ejaculate components that function to reduce competition either directly, through effects on sperm storage and usage and displacement of rival sperm, or indirectly, by inhibiting female remating or stimulating fecundity to maximize paternity before a female remates. Evolutionary conflict between the sexes might likewise shape the evolution of ejaculate function; for example, through components that advance female reproductive schedules or delay remating at the expense of female longevity or investment in future offspring. Although conflict over the effects of ejaculate components is possible, it is generally not known whether females experience a net fitness benefit or cost [6, 9–12]. Females might also create strong selection on ejaculate composition in polyandrous species depending on the extent to which fertilization is biased by composition; however, there is currently little known about how ejaculate composition might influence such female-mediated postcopulatory selection.
Sperm competition
Theory predicts that males should modify ejaculate composition in response to the risk or intensity of sperm competition (Box 1). Many studies have shown that males modify sperm allocation at mating in response to cues of sperm competition [2], with consistent patterns in vertebrate and invertebrate taxa [13, 14]. However, these studies generally report sperm numbers alone, and as a result it is typically not known whether males adjust the composition of their ejaculates or simply the overall volume. Intriguingly, a recent meta-analysis shows that although males transfer larger ejaculates to virgin compared to mated females, males do not consistently transfer more sperm to virgins [14]. This suggests that non-sperm ejaculate components, and overall composition, might often change in response to the perceived risk of sperm competition.
In fact, some case studies are consistent with this hypothesis. Male moths, Heliothis virescens, alter their ejaculate composition in response to female mating status, transferring larger spermatophores but equal sperm numbers, to previously-mated females, indicating greater allocation to non-sperm components [15]. Further indirect evidence comes from male voles, Myodes glareolus, which develop larger accessory glands (but not testes) in the presence of other males [16], and male fruit flies, Drosophila melanogaster, which alter the expression ratios of Sfp genes in response to the presence of rival males in the pre-mating environment [17]. In the black goby, Gobius niger, males adopt either a parental or sneaking behaviour and consequently face variable or consistently high sperm competition (respectively). Their investment in both ejaculate-producing tissues and ejaculates reflects this: parental males invest more in seminal vesicles and mesorchial glands and less in testes, relative to sneakers, and their ejaculates contain more mucins and less sperm [18].
These adjustments in ejaculate composition in response to cues of sperm competition might be adaptive if they result in male fitness benefits, such as increased paternity. However, there have not yet been direct tests of this hypothesis.
Ejaculate exploitation
An interesting consequence of female polyandry – and a potential driving force behind plasticity in ejaculate composition – is ejaculate exploitation. Theory predicts that, in sequentially mating internal fertilizers, male seminal fluids that remain in the female reproductive tract from previous matings should be exploited by later-mating males [19, 20] (Box 1). For example, when the seminal fluids of earlier-mating males protect the sperm of rival males [21], later-mating males can reduce allocation to sperm-protecting seminal components. Direct tests of this hypothesis are currently lacking. However, ejaculate exploitation might also play a key role in externally fertilizing species with alternative mating tactics. In the grass goby (Zosterisessor ophiocephalus), sneaker males benefit from the sperm-velocity and fertility-enhancing effects of territorial male seminal fluid [22]. Because sneaker males always encounter territorial male seminal fluid when ejaculating, sneaker males might exploit the seminal fluid of territorial males to achieve maximal sperm performance.
Ejaculate exploitation can also occur when ejaculate components ‘switch on’ female responses, such as egg production or feeding, such that subsequent ejaculates have no further effect [23]. Later-mating males should reduce investment in these components because female responses are already maximally stimulated. In female D. melanogaster, fecundity and refractoriness are elevated after one mating, and a second mating does not further increase fecundity, but does induce continued refractoriness [24]. Consistent with ejaculate exploitation, males transfer less ovulin (Acp26Aa) – a seminal protein that stimulates fecundity – to mated females than to virgins, but males transfer similar levels of sex peptide (Acp70A), a protein that induces refractoriness, to mated and virgin females [24]. These results are consistent with the idea that ejaculate components – even specific seminal proteins – come with production or transfer costs and that males carefully regulate investment in ejaculate components.
Condition and state
Female condition and state
The fitness returns to males from investment in different ejaculate components are likely to vary with female condition and state. In particular, female responses to fecundity stimulation and receptivity inhibition, as well as female sperm selectivity, can vary with female nutritional condition [12, 25, 26] and age [27]. Males should adjust ejaculate composition depending on the form of female response, and male investment in an ejaculate component should be positively associated with female sensitivity to that component and threshold for response [28].
Female condition or state might also influence the level of ejaculate competition that a male faces, with the implications for adaptive ejaculate composition discussed above. If a female’s mating rate depends on her condition or state – through changes in either receptivity or attractiveness – then females of different condition will vary in both mating history and the number of expected future matings. When females in good condition attract more mating attempts [25, 29], males mating with these females should invest more in receptivity-inhibiting components to avoid ejaculate competition. Although this prediction has yet to be directly tested, there is considerable evidence that males tend to invest more sperm in females of greater reproductive value [14]. In the fowl, Gallus gallus, dominant males transfer larger ejaculates with seminal fluid components that increase sperm velocity to more attractive females [30], strongly suggesting a difference in composition. Understanding similar effects in humans and other key species could play an important role in developing practical applications from ejaculate composition research (Box 4).
Male condition and state
Male condition and state should influence optimal ejaculate composition both directly through male ability to invest in costly ejaculate components, and indirectly through effects on future mating opportunities and the level of ejaculate competition.
In general, high-condition males should produce ejaculates enriched in components that are more costly to produce or store, when increased investment in these components yields fitness payoffs [31]. Although little is known about relative ejaculate component costs, a unique study in bed bugs (Cimex lectularius) demonstrates a productive approach [32]. The study established the minimum stores of sperm and seminal fluid that male bed bugs require to mate, and examined how both components became depleted in sequential matings. Seminal fluid limited remating before sperm did, suggesting that seminal fluid is more costly [32]. This study highlights the difficulty with using replenishment rate alone to assess costs: males replenished seminal fluid faster than sperm, but not fast enough to compensate for greater seminal fluid transfer at each mating.
Indirect evidence from D. melanogaster and the stalk-eyed fly, Cyrtodiopsis dalmanni, is also consistent with more rapid depletion of seminal fluid than sperm [33, 34], and in other insects, ejaculates become depleted of lipid or protein content after multiple mating [35, 36]. Male moths, Choristoneura rosaceana, transfer less refraction-inducing substance after recently mating than virgin males transfer, suggesting that these substances are costly [37]. Additional data on depletion rates of ejaculate components of known function would now be particularly useful to test whether ejaculate composition is best predicted by components costs versus function (Box 1). Data on relative depletion and replenishment rates of ejaculate components are also important for understanding which components males can rapidly alter in response to the socio-sexual environment. For example, spermatogenesis can take days or weeks depending on sperm length [38], whereas there is greater potential for rapid replenishment of non-sperm [39]. Furthermore, low-condition males often transfer ejaculates with relatively high water content [40, 41], consistent with the idea that water is a low-cost ejaculate component in non-arid environments.
Although males in good condition should invest more in ejaculates overall, better male condition will often increase mating success [42]. As a result, poor-condition males with fewer expected matings might transfer a greater quantity of costly ejaculate components in each mating compared to high-condition males, reversing the usual condition-dependent prediction and highlighting the need to also measure male mating opportunities. This prediction applies to any aspects of male state that influence future mating success (e.g., age [43], reproductive strategy [18], social status [42]).
Male condition can also influence adaptive ejaculate composition by modulating ejaculate competition; for example, low-condition males face increased ejaculate competition if their mates discriminate against their sperm or remate more quickly [44, 45]. Theory predicts that this should lead low-condition males to invest more in sperm and less in fecundity-enhancing ejaculate components (Box 1). Consistent with this prediction, male ladybird beetles, Adalia bipunctata, in poor nutritional condition transfer smaller ejaculates that nonetheless contain more sperm than males in good condition [40].
A recent study provides intriguing evidence that male age and mating experience affect ejaculate composition in D. melanogaster [46]. As males age and accumulate matings, secondary cells from the accessory glands separate from and migrate along the epithelium. Remarkably, aged mated males – but not young, naïve males – transfer these cells to females in the ejaculate, demonstrating a change in ejaculate composition. A signalling pathway that is required for normal behaviour of these cells is also required for males to induce refractoriness in females, raising the interesting hypothesis that the transfer of these cells is adaptive.
It is likely that the effects of male and female condition on ejaculate composition will often be interdependent, such as when there is assortative mating by condition, or when females modulate their mating behaviour – and thus the level of ejaculate competition – based on their own or their mate’s condition. This highlights the need for theory that considers the allocation of ejaculate components in response to the condition of both sexes.
The evolution of ejaculate composition
Because ejaculate composition should impact female fitness, coevolution between ejaculate composition and female traits is expected (Boxes 1, 4). Such coevolution is expected through two pathways: ejaculate composition might track female reproductive traits evolving under natural selection, or the sexes might coevolve in response to each other. Several ejaculate traits (e.g., sperm traits [47–50]; ejaculate mass [51]) do coevolve with female traits in both vertebrates and invertebrates, making it likely that ejaculate composition also coevolves. In fact, surveys in mammals and birds have found that sperm concentration increases with indices of sperm competition [52, 53], which can be correlated with variation in female traits such as remating rate. This kind of sexual coevolution might contribute to evolutionary change in mating systems (Box 4).
An open question is the extent to which allocation to distinct ejaculate components is free to evolve independently. Strong genetic correlations among ejaculate traits would imply at least short term constraints on the evolution of ejaculate composition, with negative correlations implying trade-offs among components. Studies have begun to investigate genetic correlations among ejaculate traits, especially sperm characteristics [54, 55], and findings range from no detectable genetic correlations [56] to positive [57, 58] and negative correlations [57, 59, 60]. For example, spermatophore mass and sperm viability are positively genetically correlated in the cockroach Nauphoeta cinerea [57], whereas sperm velocity and length are negatively correlated in zebra finch Taeniopygia guttata [58]. These results suggest that studies investigating genetic and phenotypic correlations among ejaculate components would be worthwhile.
Future directions
The recent developments we have highlighted make it clear that there are many exciting questions to address about ejaculate composition, and that both theoretical and empirical efforts are required.
Theory
We identify three areas in which theoretical development would be particularly valuable. First, empirical studies show that ejaculate component function is complex and context-dependent, such that female responses to ejaculate vary with female mating history [23, 24] and nutritional state [12]. This sets the stage for adaptive plasticity in ejaculate composition in response to these contexts that will be difficult to interpret without formal modeling. A second and related need is for coevolutionary models that incorporate evolution in female responses to ejaculate components and subsequent changes in ejaculate composition. Finally, we need models that investigate condition-dependence in ejaculate composition both when ejaculate components differ in production costs and when female responses to ejaculate components are condition-dependent, particularly when condition is positively correlated between mates.
Empirical directions
Progress in understanding adaptive ejaculate composition requires studies in four areas.
First, very few studies examine how ejaculate component function changes with female state and condition. Yet, ejaculate components can have profoundly different effects depending on female state [12], suggesting that we need these data as well as data on the dose-dependence of female responses to understand adaptive ejaculate composition (Box 1).
A second gap is in the assessment of relative physiological costs of producing ejaculate components. As we have discussed, many predictions for adaptive ejaculate composition rely on these relative costs, and early attempts to measure them suggest that differences in costs can determine which ejaculate components limit male re-mating rate [32]. We therefore urge a more detailed empirical assessment of relative costs. Promising approaches include measuring the relative depletion and replenishment rates of ejaculate components [32], using calorimetry to measure energy expenditure on distinct components [61], and, when males produce components in discrete stages, measuring metabolic expenditure during each stage [62].
Third, we need studies that measure how variation in ejaculate composition affects male and female fitness [63]. We need direct tests of whether plasticity in ejaculate composition is adaptive for males, and to achieve this, we ideally need to measure the fitness of males that are experimentally prevented from altering their ejaculate composition. In turn, the sign of effects on female fitness can strongly influence predictions about adaptive ejaculate composition through sexual coevolution (Box 1).
Fourth, we currently do not know the extent of constraints on ejaculate composition, both in terms of adaptive plasticity and evolutionary change. Valuable data would include tests of adaptive predictions (Box 1), the strength of genetic correlations among components and the extent of variation in ejaculate composition among populations. The latter are needed to test the role of ejaculate composition in reproductive isolation among populations (Box 4), along with data on how composition influences postcopulatory selection.
Finally, a challenge in testing adaptive hypotheses about ejaculate composition is the need to separate male and female influence. Females can potentially control sperm storage and usage [7] and control might extend to ejaculate transfer and composition. For example, in species where ejaculate components are transferred in a fixed order, females can differentially accept components by strategically terminating copulation. Testing this hypothesis requires characterizing the sequence of ejaculate transfer, testing for female termination of copulation and measuring the effects of termination on ejaculate receipt and female fitness. Furthermore, the possibility of cryptic female choice based on ejaculate composition is an intriguing area for future study.
Third-party interests
Ejaculate composition might be shaped in part by the evolutionary interests of third parties. The fitness of sexually-transmitted parasites and pathogens, as well as endosymbionts, can potentially be influenced by ejaculate composition through effects on the likelihood of transmission, remating rates of their male host and his mates, and mate fecundity. There is abundant evidence that parasites and endosymbionts can influence host mating behaviour [64] and ejaculate properties [65–67], and the potential for effects on ejaculate composition is a promising area for future research.
Promising systems
An obvious starting point for investigating adaptive ejaculate composition is the classic systems of postcopulatory interactions (e.g., Drosophila spp. fruit flies, Gallus gallus fowl, Scathophaga stercoraria dungflies, Tribolium castaneum flour beetles). These species are well-characterized for patterns of sperm competition and the mechanisms underlying them, and in D. melanogaster there is already evidence for adaptive plasticity in ejaculate composition in response to female mating history [24]. For testing how sperm competition influences composition (Box 1), systems with alternative male mating strategies are ideal because they offer identifiable classes of males that consistently face different levels of sperm competition and are thus likely to face strong selection to adaptively adjust their ejaculates.
On a practical level, species with external ejaculate transfer are attractive because they avoid the potential problems of ejaculate collection associated with internal transfer (Box 2). These species include those in which males deposit ejaculates into the environment at large [68] or target ejaculates to specific females after pre-copulatory interactions. For example, male plethodontid salamanders deposit spermatophores onto the substrate after courting females [69], making it possible to test how manipulating female characteristics and the presence of rival males affects the entire ejaculate. Tettigoniid orthopterans represent another intriguing system because ejaculates are easily detached from the female immediately after copulation [9].
Conclusions
Theory predicts that males should adjust the composition of their ejaculates to maximise reproductive gains at mating. The next steps for theory are to develop models predicting adaptive ejaculate composition in a wider range of contexts and incorporating costs to males and sexual coevolution. Empirically, there is already some direct evidence and a great deal of indirect evidence for adaptive adjustment in ejaculate composition. Perhaps the biggest challenge now is to provide direct tests of predictions from theory, and to assess the effects of plasticity in ejaculate composition on fitness, sexual coevolution, and divergence amongst populations and species. There are numerous biological systems and new technologies (Box 2) that provide exciting opportunities for investigating the evolutionary ecology of ejaculate composition, and the abundant sperm competition literature provides a valuable starting point for this work. The major effects that variation in ejaculate composition is likely to have on male and female fitness, sperm competition, sexual conflict and reproductive evolution make this endeavour worthwhile.
Acknowledgments
We thank Z. Lewis, T. Pizzari, T. Uller, M. Wolfner and two anonymous reviewers for helpful comments that improved the manuscript, and L. Rowe for discussion. SW is funded by a Natural Environmental Research Council fellowship. LS is supported by National Institutes of Health grants R01- AI095491 to Laura Harrington and Mariana Wolfner (Cornell University) and R15-ES020051 to Anthony Fiumera (Binghamton University). JCP is funded by a Natural Sciences and Engineering Research Council fellowship.
Glossary
- Assisted Reproductive Technology (ART)
techniques used in both humans and non-human animals to assist in achieving pregnancy through artificial means
- Condition
the pool of resources an individual has available to allocate to trait expression; influenced by both genetic and environmental variation
- Ejaculate
ejaculated seminal fluid
- Ejaculate composition
the relative abundance of components (e.g., sperm, Sfps, etc.) that make up the ejaculate
- Ejaculate exploitation
the process whereby males mating with a mated female can take advantage of her previous mates’ ejaculates, permitting a strategic reduction in one or more ejaculate components
- Nuptial gifts
material (usually edible) transferred from males to females before or during mating
- Parasperm
a morphologically distinct sperm caste that is incapable of fertilization
- Polyandry
a mating system whereby females mate with multiple males
- Receptivity
the willingness of an individual to mate
- Refractory period
a period after mating during which females are unreceptive to male mating attempts. Female refractoriness is often induced by Sfps in insects
- Seminal fluid
male-produced fluid, usually containing sperm, ejaculated during mating
- Sfps (Seminal Fluid Proteins)
proteins produced in male reproductive organs and transferred in the ejaculate
- Sperm competition
competition between the sperm of two or more males to fertilize ova
- State
an organism’s variable characteristics, including age, mating history, health and parasite infection status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poiani A. Complexity of seminal fluid: a review. Behav. Ecol. Soc. 2006;60:289–310. [Google Scholar]
- 2.Wedell N, et al. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. [Google Scholar]
- 3.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 4.Avila FW, et al. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J. Anim. Sci. 2007;85:E36–E44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
- 6.Arnqvist G, Rowe L. Sexual Conflict. Princeton University Press; 2005. [Google Scholar]
- 7.Eberhard WG. Female Control: Sexual Selection By Cryptic Female Choice. Princeton University Press; 1996. [Google Scholar]
- 8.Simmons LW. Sperm Competition And Its Evolutionary Consequences In The Insects. Princeton University Press; 2001. [Google Scholar]
- 9.Gwynne DT. Sexual conflict over nuptial gifts in insects. Annu. Rev. Entomol. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- 10.Vahed K. All that glisters is not gold: sensory bias, sexual conflict and nuptial feeding in insects and spiders. Ethology. 2007;113:105–127. [Google Scholar]
- 11.Stockley P. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 1997;12:154–159. doi: 10.1016/s0169-5347(97)01000-8. [DOI] [PubMed] [Google Scholar]
- 12.Fricke C, et al. Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster . J. Evol. Biol. 2010;23:157–165. doi: 10.1111/j.1420-9101.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- 13.Delbarco-Trillo J. Adjustment of sperm allocation under high risk of sperm competition across taxa: a meta-analysis. J. Evol. Biol. 2011;24:1706–14. doi: 10.1111/j.1420-9101.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelly CD, Jennions MD. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 2011;86:863–884. doi: 10.1111/j.1469-185X.2011.00175.x. [DOI] [PubMed] [Google Scholar]
- 15.LaMunyon CW, Huffman TS. Determinants of sperm transfer by males of the noctuid moth Heliothis virescens . J. Insect Behav. 2001;14:187–199. [Google Scholar]
- 16.Lemaître J-F, et al. Social cues of sperm competition influence accessory reproductive gland size in a promiscuous mammal. Proc. Roy. Soc. Lond. B. 2011;278:1171–1176. doi: 10.1098/rspb.2010.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedorka KM, et al. Perceived sperm competition intensity influences seminal fluid protein production prior to courtship and mating. Evolution. 2011;65:584–590. doi: 10.1111/j.1558-5646.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 18.Rasotto M, Mazzoldi C. Male traits associated with alternative reproductive tactics in Gobius niger . J. Fish Biol. 2002;61:173–184. [Google Scholar]
- 19.Hodgson DJ, Hosken DJ. Sperm competition promotes the exploitation of rival ejaculates. J. Theor. Biol. 2006;243:230–234. doi: 10.1016/j.jtbi.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Alonzo SH, Pizzari T. Male fecundity stimulation: conflict and cooperation within and between the sexes: model analyses and coevolutionary dynamics. Am. Nat. 2010;175:174–185. doi: 10.1086/649596. [DOI] [PubMed] [Google Scholar]
- 21.Holman L. Drosophila melanogaster seminal fluid can protect the sperm of other males. Func. Ecol. 2009;23:180–186. [Google Scholar]
- 22.Locatello L, et al. Tactic-specific differences in seminal fluid influence sperm performance. Proc. Roy. Soc. Lond. B. 2013;280:20122891. doi: 10.1098/rspb.2012.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry JC. Mating stimulates female feeding: testing the implications for the evolution of nuptial gifts. J. Evol. Biol. 2011;24:1727–36. doi: 10.1111/j.1420-9101.2011.02299.x. [DOI] [PubMed] [Google Scholar]
- 24.Sirot LK, et al. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster . Proc. Natl. Acad. Sci. U.S.A. 2011;108:9922–9926. doi: 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amitin EG, Pitnick S. Influence of developmental environment on male- and female-mediated sperm precedence in Drosophila melanogaster . J. Evol. Biol. 2007;20:381–391. doi: 10.1111/j.1420-9101.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 26.Aluja M, et al. Male and female condition influence mating performance and sexual receptivity in two tropical fruit flies (Diptera: Tephritidae) with contrasting life histories. J. Insect Physiol. 2009;55:1091–1098. doi: 10.1016/j.jinsphys.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Rogina B, et al. Distinct biological epochs in the reproductive life of female Drosophila melanogaster . Mech. Ageing Dev. 2007;128:477–485. doi: 10.1016/j.mad.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe L, et al. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 2005;165:S5–S18. doi: 10.1086/429395. [DOI] [PubMed] [Google Scholar]
- 29.Long TAF, et al. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 2009;7:e1000254. doi: 10.1371/journal.pbio.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornwallis CK, O’Connor EA. Sperm: seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc. Roy. Soc. Lond. B. 2009;276:3467–3475. doi: 10.1098/rspb.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. Roy. Soc. Lond. B. 1996;263:1415–1421. [Google Scholar]
- 32.Reinhardt K, et al. Male mating rate is constrained by seminal fluid availability in bedbugs, Cimex lectularius . PLoS ONE. 2011;6:e22082. doi: 10.1371/journal.pone.0022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers D, et al. Mating-induced reduction in accessory reproductive organ size in the stalk-eyed fly Cyrtodiopsis dalmanni . BMC Evol. Biol. 2005;5:37. doi: 10.1186/1471-2148-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linklater JR, et al. Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster . Evolution. 2007;61:2027–2034. doi: 10.1111/j.1558-5646.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 35.Marshall LD, McNeil JN. Spermatophore mass as an estimate of male nutrient investment: a closer look in Pseudaletia unipuncta (Haworth) (Lepidoptera: Noctuidae) Func. Ecol. 1989;3:605–612. [Google Scholar]
- 36.Wedell N, Ritchie MG. Male age, mating status and nuptial gift quality in a bushcricket. Anim. Behav. 2004;67:1059–1065. [Google Scholar]
- 37.Marcotte M, et al. Effects of different male remating intervals on the reproductive success of Choristoneura rosaceana males and females. J. Insect Physiol. 2007;53:139–145. doi: 10.1016/j.jinsphys.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Ramm SA, Stockley P. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol. Lett. 2010;6:219–221. doi: 10.1098/rsbl.2009.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirot LK, et al. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: an ELISA-based method for tracking individual ejaculates. Behav. Ecol. Soc. 2009;63:1505–1513. doi: 10.1007/s00265-009-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry JC, Rowe L. Condition-dependent ejaculate size and composition in a ladybird beetle. Proc. Roy. Soc. Lond. B. 2010;277:3639–3647. doi: 10.1098/rspb.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Z, et al. Nutritional condition influences investment by male katydids in nuptial food gifts. Ecol. Entomol. 2000;25:115–118. [Google Scholar]
- 42.Andersson M. Sexual Selection. Princeton University Press; 1994. [Google Scholar]
- 43.Pröhl H. Variation in male calling behaviour and relation to male mating success in the strawberry poison frog (Dendrobates pumilio) Ethology. 2003;109:273–290. [Google Scholar]
- 44.Pitcher TE, et al. Multiple mating and sequential mate choice in guppies: females trade up. Proc. Roy. Soc. Lond. B. 2003;270:1623–9. doi: 10.1098/rspb.2002.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dean R, et al. The risk and intensity of sperm ejection in female birds. Am. Nat. 2011;178:343–354. doi: 10.1086/661244. [DOI] [PubMed] [Google Scholar]
- 46.Leiblich A, et al. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proc. Natl. Acad. Sci. U.S.A. 2012;109:19292–19297. doi: 10.1073/pnas.1214517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitnick S, et al. Ejaculate-female coevolution in Drosophila mojavensis . Proc. Roy. Soc. Lond. B. 2003;270:1507–1512. doi: 10.1098/rspb.2003.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockley P, et al. Female reproductive biology and the coevolution of ejaculate characteristics in fish. Proc. Roy. Soc. Lond. B. 1996;263:451–458. [Google Scholar]
- 49.Gomendio M, Roldan ER. Coevolution between male ejaculates and female reproductive biology in eutherian mammals. Proc. Roy. Soc. Lond. B. 1993;252:7–12. doi: 10.1098/rspb.1993.0039. [DOI] [PubMed] [Google Scholar]
- 50.Higginson DM, et al. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4538–4543. doi: 10.1073/pnas.1111474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rönn JL, et al. Correlated evolution between male and female primary reproductive characters in seed beetles. Func. Ecol. 2011;25:634–640. [Google Scholar]
- 52.Soulsbury C, Iossa G. The impact of ovulation mode on sperm quantity and quality in mammals. Evol. Ecol. 2010;24:879–889. [Google Scholar]
- 53.Møller AP. Testes size, ejaculate quality and sperm competition in birds. Biol. J. Linn. Soc. 1988;33:273–283. [Google Scholar]
- 54.Simmons LW, Moore AJ. Evolutionary quantitative genetics of sperm. In: Birkhead T, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Elsevier; 2009. pp. 405–434. [Google Scholar]
- 55.Snook RR. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Simmons LW, Kotiaho JS. Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution. 2002;56:1622–1631. doi: 10.1111/j.0014-3820.2002.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 57.Moore PJ, et al. Constraints on evolution and postcopulatory sexual selection: trade-offs among ejaculate characteristics. Evolution. 2004;58:1773–1780. doi: 10.1111/j.0014-3820.2004.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 58.Mossman J, et al. Sperm morphology and velocity are genetically codetermined in the zebra finch. Evolution. 2009;63:2730–2737. doi: 10.1111/j.1558-5646.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- 59.Evans JP. Patterns of genetic variation and covariation in ejaculate traits reveal potential evolutionary constraints in guppies. Heredity. 2011;106:869–875. doi: 10.1038/hdy.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birkhead TR, et al. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. [DOI] [PubMed] [Google Scholar]
- 61.Thomsen R, et al. How costly are ejaculates for Japanese macaques? Primates. 2006;47:272–274. doi: 10.1007/s10329-005-0171-7. [DOI] [PubMed] [Google Scholar]
- 62.Olsson M, et al. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus . Proc. Roy. Soc. Lond. B. 1997;264:455–459. [Google Scholar]
- 63.Parker GA, Pizzari T. Sperm competition and ejaculate economics. Biol. Rev. 2010;85:897–934. doi: 10.1111/j.1469-185X.2010.00140.x. [DOI] [PubMed] [Google Scholar]
- 64.Libersat F, et al. Manipulation of host behavior by parasitic insects and insect parasites. Annu. Rev. Entomol. 2009;54:189–207. doi: 10.1146/annurev.ento.54.110807.090556. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, et al. Do Ureaplasma urealyticum infections in the genital tract affect semen quality? Asian J. Androl. 2006;8:562–568. doi: 10.1111/j.1745-7262.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 66.Lewis Z, et al. Wolbachia infection lowers fertile sperm transfer in a moth. Biol. Lett. 2011;7:187–189. doi: 10.1098/rsbl.2010.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otti O, et al. In vitro antimicrobial sperm protection by an ejaculate-like substance. Func. Ecol. 2013;27:219–226. [Google Scholar]
- 68.Schaller F. Indirect sperm transfer by soil arthropods. Annu. Rev. Entomol. 1971;16:407–446. [Google Scholar]
- 69.Verrell P, Mabry M. The courtship of plethodontid salamanders: form, function and phylogeny. In: Bruce RC, Jaeger RG, Houck LD, editors. The Biology of Plethodontid Salamanders. Kluwer Academic/Plenum Publishers; 2000. pp. 371–380. [Google Scholar]
- 70.Cameron E, et al. Sperm competition and the evolution of ejaculate composition. Am. Nat. 2007;169:E158–E172. doi: 10.1086/516718. [DOI] [PubMed] [Google Scholar]
- 71.Kura T, Yoda K. Can voluntary nutritional gifts in seminal flow evolve? J. Ethol. 2001;19:9–15. [Google Scholar]
- 72.Vahed K. Larger ejaculate volumes are associated with a lower degree of polyandry across bushcricket taxa. Proc. Roy. Soc. Lond. B. 2006;273:2387. doi: 10.1098/rspb.2006.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerris J. Methods of semen collection not based on masturbation or surgical sperm retrieval. Hum. Reprod. Update. 1999;5:211–215. doi: 10.1093/humupd/5.3.211. [DOI] [PubMed] [Google Scholar]
- 74.Zambelli D, et al. Sperm evaluation and biochemical characterization of cat seminal plasma collected by electroejaculation and urethral catheterization. Theriogenology. 2010;74:1396–1402. doi: 10.1016/j.theriogenology.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Savalli UM, Fox CW. Sexual selection and the fitness consequences of male body size in the seed beetle Stator limbatus . Anim. Behav. 1998;55:473–483. doi: 10.1006/anbe.1997.0622. [DOI] [PubMed] [Google Scholar]
- 76.Doyle JM, et al. The quantification of spermatozoa by real-time quantitative PCR, spectrophotometry, and spermatophore cap size. Mol. Ecol. Resour. 2011;11:101–106. doi: 10.1111/j.1755-0998.2010.02892.x. [DOI] [PubMed] [Google Scholar]
- 77.Bissoondath CJ, Wiklund C. Protein content of spermatophores in relation to monandry/polyandry in butterflies. Behav. Ecol. Soc. 1995;37:365–371. [Google Scholar]
- 78.Fernández-Novell JM, et al. Glucose and fructose as functional modulators of overall dog, but not boar sperm function. Reprod. Fertil. Dev. 2011;23:468–480. doi: 10.1071/RD10120. [DOI] [PubMed] [Google Scholar]
- 79.Wigby S, et al. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 2009;19:751–757. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Máchal L. The relationship between plasma cholesterol and lipids and qualitative indicators of the ejaculate of Holstein and Czech Spotted bulls. J. Anim. Feed Sci. 2001;10:273–281. [Google Scholar]
- 81.Walters JR, Harrison RG. Combined EST and proteomic analysis identifies rapidly evolving seminal fluid proteins in Heliconius butterflies. Molecular Biology and Evolution. 2010;27:2000–2013. doi: 10.1093/molbev/msq092. [DOI] [PubMed] [Google Scholar]
- 82.Diz AP, et al. Proteomics in evolutionary ecology: linking the genotype with the phenotype. Mol. Ecol. 2012;21:1060–1080. doi: 10.1111/j.1365-294X.2011.05426.x. [DOI] [PubMed] [Google Scholar]
- 83.Smith DT, et al. The consequences of genetic variation in sex peptide expression levels for egg laying and retention in females. Heredity. 2012;109:222–225. doi: 10.1038/hdy.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Findlay GD, et al. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dean M, et al. Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics. 2011;12:306. doi: 10.1186/1471-2164-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dostàlovà Z, et al. Quantitation of boar spermadhesins in accessory sex gland fluids and on the surface of epididymal, ejaculated and capacitated spermatozoa. BBA-Gen. Subjects. 1994;1200:48–54. doi: 10.1016/0304-4165(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 87.Claydon AJ, et al. Heterogenous turnover of sperm and seminal vesicle proteins in the mouse revealed by dynamic metabolic labeling. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M111.014993. M111.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kingan TG, et al. The loss of female sex pheromone after mating in the corn earworm moth Helicoverpa zea: identification of a male pheromonostatic peptide. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5082–5086. doi: 10.1073/pnas.92.11.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 90.Tomar AK, et al. Differential proteomics of human seminal plasma: a potential target for searching male infertility marker proteins. Proteom. Clin. Appl. 2012;6:147–151. doi: 10.1002/prca.201100084. [DOI] [PubMed] [Google Scholar]
- 91.Ratto MH, et al. The nerve of ovulation-inducing factor in semen. Proc. Natl. Acad. Sci. U.S.A. 2012;109:15042–15047. doi: 10.1073/pnas.1206273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rogers DW, et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dottorini T, et al. Regulation of Anopheles gambiae male accessory gland genes influences postmating response in female. FASEB J. 2013;27:86–97. doi: 10.1096/fj.12-219444. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Staples D, et al. Potential for pre-release diet supplements to increase the sexual performance and longevity of male Queensland fruit flies. Agric. Forest Entomol. 2008;10:255–262. [Google Scholar]
- 95.Kilgallon SJ, Simmons LW. Image content influences men’s semen quality. Biol. Lett. 2005;1:253–255. doi: 10.1098/rsbl.2005.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katvala M, et al. Correlated evolution between male ejaculate allocation and female remating behaviour in seed beetles (Bruchidae) J. Evol. Biol. 2008;21:471–479. doi: 10.1111/j.1420-9101.2007.01494.x. [DOI] [PubMed] [Google Scholar]
- 97.Pitnick S, et al. Evolution of female remating behaviour following experimental removal of sexual selection. Proc. Roy. Soc. Lond. B. 2001;268:557–563. doi: 10.1098/rspb.2000.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Välimäki P, Kaitala A. Properties of male ejaculates do not generate geographical variation in female mating tactics in a butterfly Pieris napi . Anim. Behav. 2010;79:1173–1179. [Google Scholar]
- 99.Yamane T, Miyatake T. Evolutionary correlation between male substances and female remating frequency in a seed beetle. Behav. Ecol. 2012;23:715–722. [Google Scholar]
- 100.Eberhard WG, Cordero C. Sexual selection by cryptic female choice on male seminal products - a new bridge between sexual selection and reproductive physiology. Trends Ecol. Evol. 1995;10:493–496. doi: 10.1016/s0169-5347(00)89205-8. [DOI] [PubMed] [Google Scholar]