Abstract
Single words have affective and aesthetic properties that influence their processing. Here we investigated the processing of a special case of word stimuli that are extremely difficult to evaluate, bivalent noun-noun-compounds (NNCs), i.e. novel words that mix a positive and negative noun, e.g. ‘Bombensex’ (bomb-sex). In a functional magnetic resonance imaging (fMRI) experiment we compared their processing with easier-to-evaluate non-bivalent NNCs in a valence decision task (VDT). Bivalent NNCs produced longer reaction times and elicited greater activation in the left inferior frontal gyrus (LIFG) than non-bivalent words, especially in contrast to words of negative valence. We attribute this effect to a LIFG-grounded process of semantic integration that requires greater effort for processing converse information, supporting the notion of a valence representation based on associations in semantic networks.
Seek the positive, avoid the negative. A simple formula and useful basis for decision and action. The outcome of a positive/negative evaluation, a valence judgment, is therefore of utmost importance and integral to many theories of emotion. Most dimensional emotion theories starting with Wundt1,2,3,4,5 incorporate valence as the primal dimension together with a dimension for emotional activation or intensity (arousal). High valence words show systematic processing differences at the behavioral, experiential, and neural levels compared to neutral ones6,7 and there are also differences in the processing of positive and negative words8,9,10. The valence of the items that are used in the above-mentioned experiments is usually determined by large-sample rating studies. Databases like the Affective Norms for English Words (ANEW)2 or the Berlin Affective Word List - Reloaded (BAWL-R)9 are important tools for research on emotion and language7. On the other hand, such large-scale studies usually circumvent the issue of how the social construct ‘valence’ is neuronally represented and accessed: The evaluation process itself mainly is neglected and the ‘How’ of valence judgments still lacks proper (neuro)-cognitive modelling and explanation7.
Recent findings suggest that valence decisions may be supported by associations in semantic networks. For example, Hofmann and Jacobs11 found a correlation between the positive valence and the amount of associations of words. They showed that negative, but not positive valence induces false memory effects over and above those accounted for by the amount of associated words in the stimulus set. In a similar vein, Westbury et al.12 predicted the valence ratings of words by their association to a selected set of emotion labels taken from extant emotion theories. In general, semantic network models assume that the building and consolidation of semantic associations follows a Hebbian learning mechanism13. When two words systematically appear spatio-temporally close together their association is strengthened. These associations are estimated in a reversed approach by counting the events of co-occurrence within large text corpora, and correcting them for individual word frequencies. While computation of semantic associations can straightforwardly explain effects for words with a clear positive and negative valence, words also can have a mixed affective structure and thus the question arises how a mixed valence is represented and processed7.
When rated separately for positivity and negativity, some words score high on both scales14. Thus, like other non-verbal stimuli15 words can be perceived as being positive and negative at the same time. Here, we would like to propose that the evaluation of such bivalent words engages an affective-semantic integration process. In Panksepp’s hierarchical emotion theory16,17 valence is considered a cognitive emotion component of the tertiary process level that is supported by neocortical areas. Therefore, the evaluation of word valence engages activation in cortical areas such as the inferior frontal gyri18. In a recent test of Panksepp’s theory using both EEG and fMRI with human subjects, Briesemeister et al.19,20 showed that positive valence in comparison to happiness (as a discrete emotion attributed to the primary process level) recruits neocortical activity. These authors suggested a semantic foundation of this affective dimension. Following up on this work, Jacobs et al.7 recently proposed that valence and arousal are semantic superfeatures that result from a yet unknown integration of both experiential and distributional data, as assumed by the semantics theory of Andrews et al.21.
The present study
Inspired by the above mentioned research, we hypothesized that affective-semantic integration recruits the left Inferior Frontal Gyrus (LIFG), which is suggested to be a key region for integration processes, e.g. in the Memory, Unification, Control framework (MUC)22. Neurocognitive evidence for this assumption comes from a study by Forgács et al.23, who reported that newly combined noun-noun compounds (NNCs) engage semantic integration activity in the LIFG. More recently, Hofmann and Jacobs11 used theoretical word association strengths as simulated by the Associative Read-Out Model (AROM)24 to predict LIFG activation generated by the NNC stimuli of Forgács et al.23.They found that strong long-term associations between the constituent words of an NNC effectively inhibited the integrative function of the LIFG.
A particularly interesting group of words to probe affective semantic integration processes are affectively bivalent words in the form of novel (German) NNCs which combine two positive or negative constituents, a positive with a negative constituent, or vice versa7. In contrast to most English NNCs, German NNCs are assembled by writing two or more words together without a dividing space. With regard to studying integration processes, this is an advantage, because undivided compounds are processed faster than those divided by a space25. In German like in all Germanic languages compounds are left-branching and the rightmost constituent is the head which defines the meaning, while all other constituents are modifiers constraining the meaning26. In German compounds the use of interfixes, inserted letters that serve as a linking element, is fairly common. The most frequent is the letter ‘s’. This interfix also exists in English (e.g., salesman) and is a possessive inflection. Compounds contain the information of a minimal sentence of the structure ‘modifier has head’ (e.g., Fingerabdruck - fingerprint) or ‘head is for modifier’ (e. g., Regenmantel - raincoat). They are an important mechanism for the creation of new words (e.g., internet). The meaning of a compound may result from the combination of the meaning of the constituents (e.g., blueberry) thus being ‘transparent’. In the case of ‘opaque’ compounds the meaning of a constituent is unassociated with the meaning of the compound (e.g., ‘straw’ in strawberry). Compounds and similar word pairs thus engage semantic integration processes which is especially true for new compounds23,27,28.
When a compound made it into common usage, however, there is less need to derive the meaning gestalt by integrating the meanings of its constituents. The meaning of the compound is then hypothesized to be directly represented in semantic memory, much like a ‘dead’ metaphor. In contrast, the meaning of novel compounds can only be constructed by integrating the meaning of the constituents. Structure mapping theory29 suggests that the structure of one meaning must be mapped onto the other, and that comprehension is achieved by comparing the relations of the attributes of the words. Sopory28 suggested that valence can be considered such an attribute. Thus, because bivalent compounds consist of differential attributes, it should be more difficult to integrate their seemingly irreconcilable affective meanings.
Bivalent NNCs of the type we used in this study were already tested in behavioural pilot experiments partly reported in Jacobs et al.7. In a valence decision task (VDT) the responses to bivalent NNCs (i.e., positive-negative or vice versa) showed a negativity bias: far more often they were categorized as negative than as positive. Reaction times (RTs) were extremely slow and expectably slower than for control NNCs with equal valence constituents (e.g., positive-positive). These non-bivalent NNCs were usually evaluated in accordance with the valence of the constituents.
Here, we hypothesized that non-bivalent NNCs would not engage semantic integration processes, or to a much lesser degree, than the bivalent ones. To deal with the contradicting valence of the constituents, the latter should fully engage the LIFG, as can be expected from Hagoort’s22 MUC theory.
Methods and Materials
Participants
The 24 participants (7 male; aged 19–29; mean 23.38) who took part in our fMRI study were right handed, had normal or corrected-to-normal vision and were native speakers of German. They were recruited at the Free University Berlin and gave written informed consent. They either received course credit or were paid for participation. The study was carried out in accordance with the approved guidelines, and was also approved by the ethic committee of the Free University Berlin.
Stimuli
A set of 120 NNCs (see Supplementary Table S1) was generated by randomly pairing nouns that met our requirements for general positive or negative valence taken from the BAWL-R9. The mean valence ratings are given in Table 1. Valence ranged from −3 to −1.3 for negative, and from 1.3 to 3 for positive words (total scale is 7 points ranging from −3 for very negative through 0 for neutral to + 3 for very positive) and the absolute value was larger than 1.5 times the individual standard deviation. Word length was restricted to 5 to 8 letters per word, resulting in compounds with 10 to 16 letters length.
Table 1. Means and Standard Errors for lexical and semantic Variables for the constituents.
| Variable | Non-bivalent Compounds |
Bivalent Compounds |
||||||
|---|---|---|---|---|---|---|---|---|
| (m) = modifier (h) = head | Positive-Positive |
Negative-Negative |
Positive-Negative |
Negative-Positive |
||||
| M | SE | M | SE | M | SE | M | SE | |
| Valence (m) | 1.98 | 0.36 | −1.89 | 0.27 | 1.79 | 0.29 | −1.88 | 0.33 |
| Valence (h) | 1.86 | 0.31 | −1.84 | 0.34 | −1.89 | 0.3 | 1.73 | 0.27 |
| Imageability (m) | 4.42 | 1.33 | 4.08 | 1.06 | 4.06 | 1.25 | 4.31 | 1.1 |
| Imageability (h) | 4.24 | 1.36 | 4.20 | 0.95 | 4.22 | 1.21 | 4.55 | 1.19 |
| Word frequency (m) | 17.76 | 22 | 11.24 | 20.02 | 15.31 | 19.75 | 11.27 | 12.97 |
| Word frequency(h) | 13.82 | 18.59 | 8.09 | 10.15 | 9.11 | 14.08 | 11.49 | 16.71 |
| #Letters (m) | 6.87 | 1.04 | 6.47 | 1.14 | 6.4 | 1.04 | 6.63 | 1.07 |
| #Letters (h) | 6.4 | 1.19 | 6.7 | 1.09 | 6.8 | 1.03 | 6.4 | 1.07 |
| #Syllables (m) | 2.1 | 0.4 | 2.1 | 0.4 | 2.3 | 0.53 | 2.1 | 0.66 |
| #Syllables (h) | 2.17 | 0.53 | 2.13 | 0.63 | 2.03 | 0.49 | 2.13 | 0.43 |
The set was subdivided into four conditions defined by the valence of the constituents (positive-positive, negative-negative, positive-negative, negative-positive). The two conditions with incongruent constituents formed the bivalent compounds.
All compounds were manually inspected and where appropriate, a binding letter was inserted in accordance with German morphosyntactic rules of compound construction. We cross-checked each compound with the data base of a standard German dictionary (Duden) to ensure that they were actually novel. In a pilot rating study (n = 36) we checked the comprehensibility and imageability of the new compounds on 7-point Likert scales (0 = uncomprehensible/non-imageable to 7 = comprehensible/imageable). Every single compound had an average comprehensibility score of above 3 (m = 4.33, sd = 0.58) indicating no uncomprehensible items. Comprehensibility and imageability of compounds did not differ significantly across conditions (ANOVAs, all F(3, 116) < 1; ns). In addition, we checked that imageability, word frequency (CELEX)30, letter, and syllable count of constituents did not significantly differ across conditions (ANOVAs, all F(3, 116) < 1; ns).
Procedure
The participants first received general information about the study and magnetic resonance imaging. They were then placed in the scanner by trained personal. The participant’s right hand was placed on the response box and with their left hand they had the possibility to press an emergency button at any time to signal abortion of the measurement. The screen, on which the stimuli were presented, was visible via a mirror system.
In a VDT the participants had to categorize each presented item as either positive or negative. Responses were given via the right hand’s index and middle finger on the buttons of a response box. The response mapping was balanced across participants. The participants were instructed to respond within the time window of presentation (3000 ms).
The instructions of the experiment were presented in written form on the screen. Each task began with 10 practice trials. Before and after the practice block the participants were asked if any questions remained open.
The design was event-related and the items were presented in Optseq231 calculated random order in two 60-trial blocks. Between the two blocks the participants could take a break. A trial began with the presentation of a fixation cross for between 1500 ms and 6500 ms, jittered in steps of 500 ms, in the centre of the screen. These durations were calculated with Optseq2 to ensure a maximal signal-to-noise ratio. The fixation cross was replaced by the item, which was presented for 3000 ms. All blocks were set to a fixed length of 200 volumes. Because of inhomogeneous timings due to the jittered intervals, after the last trial of a block a fix cross was presented till the end of the block.
Image acquisition
Scanning was done with a Siemens Tim Trio 3 tesla scanner (Siemens, Erlangen, Germany) with a standard 32 channel head coil at the Dahlem Institute for Neuroimaging of Emotion (D.I.N.E.) in Berlin. The parameters of the standard EPI sequence were TR = 2000 ms, TE = 30 ms, flip angle = 70°, 37 axial slices oriented along the AC-PC plane, interleaved from bottom to top, 3 mm slices, no gap, 3 mm × 3 mm, in-plane. A high resolution T1-weighted image for anatomical localization was collected and fMRI localized shimming reduced susceptibility artefacts.
Behavioural analysis
RT data from the VDT were analysed with a mixed fixed and random effects model using the Statistical software JMP 11 (SAS Institute Inc.). The type of compound (positive-positive, negative-negative, positive-negative, negative-negative) was modelled as a fixed effect, participants, and items nested within conditions were modelled as random effects. We analysed with χ2-Tests if there were contingencies between the participants’ responses and the valence of the constituents.
fMRI analysis
All FMRI data analyses were conducted with the SPM8 (http://www.fil.ion.ucl.ac.uk/, downloaded: 11-14-2013) toolbox for Matlab (Mathworks, Inc.). Images were first slice time corrected, realigned to the first image of the volume and simultaneously unwarped. All interpolations were done with 4th degree B-spline. Images were coregistered with the image of the anatomical scan. A group anatomical template was created from the segmented white and grey mater images of the anatomical images of all participants using DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra)32. The template was used to normalize functional images to MNI-space (Montreal Neurological Institute). Thereby we achieved a homogeneous structural organization of brain anatomy for all participants.
Trials without response were excluded from the analysis. For each subject we specified and estimated a general linear model (GLM). Movement regressors from preprocessing were included in the computation and we build T-contrasts for each of the four factor combinations: positive-positive, negative-negative, positive-negative, negative-positive. Based on these T-contrasts a single GLM was computed for the whole sample. We calculated main effect T-contrasts for modifier and head of the compounds. For the interaction of modifier and head we calculated a contrast with all of the factor combinations and another contrasts that compares congruent (positive-positive and negative-negative) vs. incongruent (positive-negative and negative-positive) combinations.
Results
Behavioral data
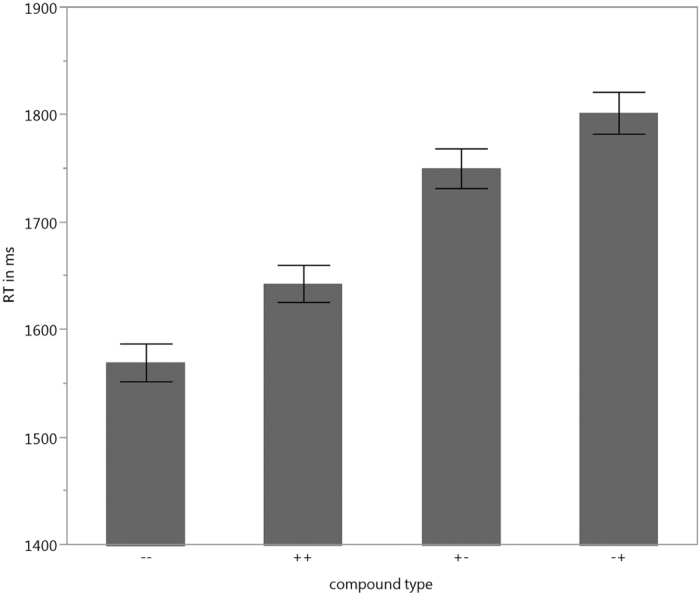
The linear mixed effects model showed a main effect of condition, F(3,115.9) = 13.4; p < 0.001. The effect was driven by faster responses to congruent conditions than to incongruent conditions (Fig. 1). Pair-wise comparisons with Bonferroni correction revealed a significant difference for the negative-negative-condition compared to both incongruent conditions, i.e. positive-negative, t(115.86) = 4.4; p < 0.001, and negative-positive compounds, t(115.86) = 5.74; p < 0.001. The other congruent condition, positive-positive, only differed significantly from the incongruent condition negative-positive, t(115.86) = 4.02; p < 0.001. The comparison with the other bivalent condition only approached significance, t(115.86) = 2.68; p = 0.051. The congruent conditions, positive-positive and negative-negative, did not differ significantly from each other, t(115.86) = 1.73; p < 0.52; neither did the incongruent conditions, positive-negative and negative-positive, t(115.86) = 1,34; p = 11.058. Responses to non-bivalent compounds were generally related to the valence of the constituents. For the bivalent compounds there was a strong tendency towards a negative response. In other words, when one of the constituents in the compound was negative the response was also likely to be negative, which could be confirmed with a significant χ2-Test, χ2 (1, N = 1866) = 546,59; p < .0001.
Figure 1. Reaction Times of the VDT (error bars represent standard error).
Neuroimaging results
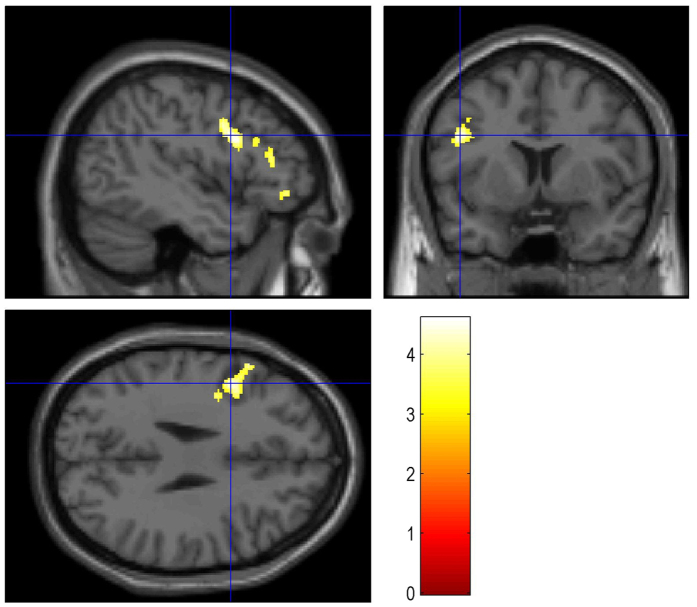
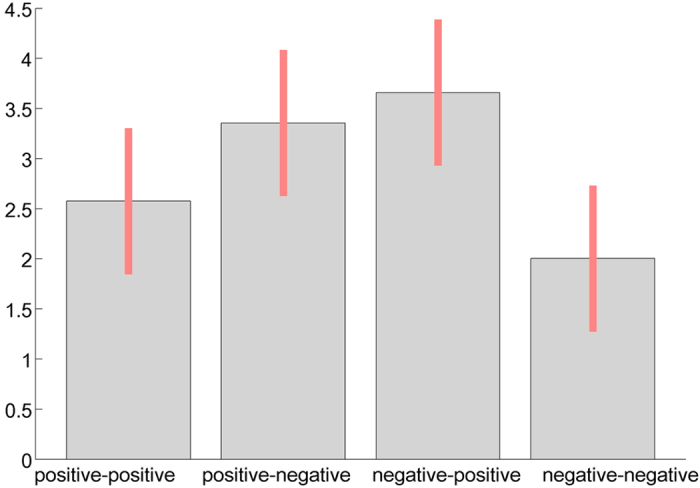
In line with our hypothesis that bivalent NNCs engage semantic integration processes we observed strong activation in the LIFG. All reported effects were significant at a cluster level of p < 0.05 corrected for family-wise error and surpassed a cluster size threshold of 10 voxels. There were no main effects for the valence of the compounds’ modifier or head. The contrast bivalent vs. non-bivalent compounds (Fig. 2) revealed a single large cluster of significant activity differences. This cluster was mainly located in the LIFG, and peaked there in activity (Table 2), but also partially extended into the left precentral gyrus (LPCG). An F-contrast of all factor combinations showed a gradual increase of BOLD-signal at the peak voxel of the LIFG cluster (Fig. 3). The increase in the signal was in the following order: negative-negative, positive-positive, positive-negative, and negative-positive compounds.
Figure 2. Activation in the LIFG from contrast: Bivalent > Non-bivalent (the cross-hair marks the voxel of maximal activation, the colour-bar represents t-values).
Table 2. Brain regions showing significantly greater BOLD signal (p FWE-corr < 0.001).
| Condition | Region | #Voxels | BA | x | y | z | t-value |
|---|---|---|---|---|---|---|---|
| Bivalent > Non-bivalent | L inferior frontal gyrus | 1213 | 6/44 | −45 | 8 | 28 | 4.60 |
| L precentral gyrus | 269 | 6 |
Figure 3. Percent signal change of BOLD-signal at LIFG peak voxel (−45, 8, 28) with red bars representing standard error.
Discussion
When the valence of a novel compound has to be evaluated, the incongruent valences of its constituents can make this task difficult, as can be seen by longer RTs for bivalent NNCs7. In the present study we not only replicated this finding, but we also hypothesized that this effect results from the effort required for semantic integration. Hagoort22 suggested that the LIFG integrates different types of information and among others presented supportive neuroimaging findings from a study with semantic violations33. In a similar vein the constituents of bivalent NNCs are semantically more dissociated and we therefore expected greater LIFG activation to bivalent than to non-bivalent compounds. The present results confirmed this hypothesis. This result may be explained by structure mapping theory29 suggesting that comprehension is achieved by comparing relations among attributes. For bivalent NNCs that comparison will result in fewer affective similarities than for non-bivalent NNCs, whose constituents share the same valence. Therefore, meaning making is more difficult for bivalent NNCs. It is questionable, however, whether the process of affectively toned meaning making is hosted more generally in bilateral inferior frontal regions. This is suggested by a PET study showing activity in the right inferior frontal gyrus to occur only if an explicit affective judgment of bivalent stimuli was required34.
A more detailed inspection of our BOLD-signal change at the peak voxel of LIFG activity revealed that the positive-positive compounds also elicited considerable LIFG activity in a magnitude between the negative-negative and the bivalent compounds. Again, this conforms to our behavioural data that show an RT advantage for negative-negative NNCs vs. positive-positive NNCs. Increased LIFG activity for positive-positive NNCs as compared to negative-negative ones may be explained by the number of associations (NA): Positive words usually have a higher number of associations than negative words11. Therefore, they may be more prone to elicit semantic integration. However, the influence of general NA on the process of semantic integration seems to be smaller than the influence from the semantic dissociation of the constituents. Bivalent compounds elicited the strongest LIFG activity and longest RTs, but they have a negative constituent and therefore introduce less average NA than the positive non-bivalent NNCs. The NA account could also be tentatively framed within structure mapping theory29, because a greater NA value may trigger more comparison processes until all similarities are found and semantic integration is reached. The constituents of negative-negative NNCs, in contrast, have fewer associations with many similarities, which should render meaning making easiest for them.
It is worth noting that in order to evaluate the valence of a compound, the meaning of both target words must be integrated. This contrasts with semantic priming, in which the decision concerns only one target word35. Nevertheless, we think that also in priming the meaning of the prime and the target are incidentally integrated, thus eliciting IFG effects36. However, in contrast to semantic priming, compound evaluation requires the semantic integration process to be relatively finished before a response can be given. This time consuming processing explains why the present RTs are twice as large as typical RTs in a semantic priming experiment35. Since no evidence was found suggesting that one of the constituents, ‘modifier’ or ‘head’, has a specific influence on the evaluation of NNCs, we think it safe to say that the evaluative decision in our experiment is based on the integrated information.
In sum, our findings further support the notion that the valence of a word is also derived from distributional data7. In our experiment we used novel NNCs, which precludes direct affective experience with that stimulus as a source of information. Hence the evaluation is solely grounded in associated affective information derived from the constituents of the NNCs and integrated in accordance with theories of metaphoric meaning making28,29.
Limitations and outlook
Our study was conducted with novel German NNCs. We think that the German language is very well suited to study effects of semantic integration, because of its strong productivity concerning compounds. Although they are common to most languages, compounds are especially abundant in German, and have a simple orthography, as they are all left branching and usually written as one word without interrupting spaces. We expect that LIFG-based semantic integration of novel compounds takes place in other languages too, but if the rules for compounds are more diverse than in German, this may affect their processing. Some languages, like Italian for example, have right and left branching compounds with differences in their lexical processing37. In English there is a more diverse compound orthography, with closed compounds (e.g., girlfriend), hyphened compounds (e.g., “credit-rating”), and open compounds (e.g., “tennis racket”), and again lexical processing and semantic integration might be different38. Therefore, an intriguing sequel might ask how such nuances in form, or how a novel compound becoming lexicalized, affect semantic integration and meaning making. Finally, future research may tackle the question whether affective incongruence produces a more vivid memory of the compound, e.g. in recognition memory tasks known to produce false alarms24.
Additional Information
How to cite this article: Kuhlmann, M. et al. Mixing positive and negative valence: Affective-semantic integration of bivalent words. Sci. Rep. 6, 30718; doi: 10.1038/srep30718 (2016).
Supplementary Material
Acknowledgments
We thank Judit Pekár and Teresa Sylvester for their help during data acquisition. This research was partly supported by grants from the Deutsche Forschungsgemeinschaft to Arthur Jacobs (JA823/4-2) and to Markus Hofmann (HO5139/2-1).
Footnotes
Author Contributions M.K. conducted the analyses and prepared figures and tables. M.K., M.J.H., B.B.B., and A.M.J. wrote the manuscript.
References
- Wundt W. Grundriss der Psychologie. Philosophical Review 5, 331 (1896). [Google Scholar]
- Bradley M. M. & Lang P. J. Affective norms for English words (ANEW): Instruction manual and affective ratings. Technical report C-1, Gainesvile, FL . (The center for research in psychophysiology, University of Florida, 1999). [Google Scholar]
- Feldman Barrett L. & Russell J. A. The Structure of Current Affect: Controversies and Emerging Consensus. Current Directions in Psychological Science (Wiley-Blackwell) 8, 10–14 (1999). [Google Scholar]
- Larsen R. J. & Diener E. In Emotion 25–59 (Sage Publications, Inc, 1992). [Google Scholar]
- Reisenzein R. Pleasure-arousal theory and the intensity of emotions. Journal of Personality and Social Psychology 67, 525–539 (1994). [Google Scholar]
- Citron F. M. M. Neural correlates of written emotion word processing: A review of recent electrophysiological and hemodynamic neuroimaging studies. Brain and Language 122, 211–226 (2012). [DOI] [PubMed] [Google Scholar]
- Jacobs A. M. et al. 10 years of BAWLing into affective and aesthetic processes in reading: what are the echoes? Front. Psychol . 6, 714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. J., Kuchinke L., Tamm S., Võ M. L. H. & Jacobs A. M. Affective processing within 1/10th of a second: High arousal is necessary for early facilitative processing of negative but not positive words. Cognitive, Affective, & Behavioral Neuroscience 9, 389–397 (2009). [DOI] [PubMed] [Google Scholar]
- Võ M. L. H. et al. The Berlin Affective Word List Reloaded (BAWL-R). Behavior Research Methods 41, 534–538 (2009). [DOI] [PubMed] [Google Scholar]
- Herbert C., Junghofer M. & Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology 45, 487–498 (2008). [DOI] [PubMed] [Google Scholar]
- Hofmann M. J. & Jacobs A. M. Interactive activation and competition models and semantic context: From behavioral to brain data. Neuroscience & Biobehavioral Reviews 46, Part 1, 85–104 (2014). [DOI] [PubMed] [Google Scholar]
- Westbury C., Keith J., Briesemeister B. B., Hofmann M. J. & Jacobs A. M. Avoid violence, rioting, and outrage; approach celebration, delight, and strength: Using large text corpora to compute valence, arousal, and the basic emotions. The Quarterly Journal of Experimental Psychology 68, 1599–1622, 10.1080/17470218.2014.970204 (2014). [DOI] [PubMed] [Google Scholar]
- Hebb D. The Organization of Behaviour: A Neuropsychological Theory. (Wiley, 1949).
- Briesemeister B. B., Kuchinke L. & Jacobs A. M. Emotional Valence A Bipolar Continuum or Two Independent Dimensions? SAGE Open 2, 10.1177/2158244012466558 (2012). [DOI] [Google Scholar]
- Norris C. J., Gollan J., Berntson G. G. & Cacioppo J. T. The current status of research on the structure of evaluative space. Biological Psychology 84, 422–436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. A ffective Neuroscience: The Foundations of Human and Animal Emotions . (Oxford University Press, 1998). [Google Scholar]
- Panksepp J. The Philosophical Implications of Affective Neuroscience. Journal of Consciousness Studies 19, 6–48 (2012). [Google Scholar]
- Cunningham W. A., Raye C. L. & Johnson M. K. Implicit and Explicit Evaluation: fMRI Correlates of Valence, Emotional Intensity, and Control in the Processing of Attitudes. Journal of Cognitive Neuroscience 16, 1717–1729 (2004). [DOI] [PubMed] [Google Scholar]
- Briesemeister B. B., Kuchinke L., Jacobs A. M. & Braun M. Emotions in reading: Dissociation of happiness and positivity. Cogn Affect Behav Neurosci 15, 287–298, 10.3758/s13415-014-0327-2 (2014) [DOI] [PubMed] [Google Scholar]
- Briesemeister B. B., Kuchinke L. & Jacobs A. M. Emotion word recognition: Discrete information effects first, continuous later? Brain Research 1564, 62–71 (2014). [DOI] [PubMed] [Google Scholar]
- Andrews M., Vigliocco G. & Vinson D. Integrating experiential and distributional data to learn semantic representations. Psychological Review 116, 463–498 (2009). [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Sciences 9, 416–423 (2005). [DOI] [PubMed] [Google Scholar]
- Forgács B. et al. Neural correlates of combinatorial semantic processing of literal and figurative noun noun compound words. NeuroImage 63, 1432–1442 (2012). [DOI] [PubMed] [Google Scholar]
- Hofmann M. J., Kuchinke L., Biemann C., Tamm S. & Jacobs A. M. Remembering Words in Context as Predicted by an Associative Read-Out Model. Front Psychol 2, 10.3389/fpsyg.2011.00252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libben G., Gibson M., Yoon Y. B. & Sandra D. Compound fracture: The role of semantic transparency and morphological headedness. Brain and Language 84, 50–64 (2003). [DOI] [PubMed] [Google Scholar]
- Downing P. On the Creation and Use of English Compound Nouns. Language 53, 810–842 (1977). [Google Scholar]
- Graves W. W., Binder J. R., Desai R. H., Conant L. L. & Seidenberg M. S. Neural correlates of implicit and explicit combinatorial semantic processing. NeuroImage 53, 638–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopory P. Metaphor and Affect. Poetics Today 26, 433–458 (2005). [Google Scholar]
- Gentner D. Structure-Mapping: A Theoretical Framework for Analogy*. Cognitive Science 7, 155–170 (1983). [Google Scholar]
- Baayen R., Piepenbrock R. & Rijn H. The {CELEX} lexical data base on {CD-ROM} . (Linguistic Data Consortium, 1993). [Google Scholar]
- Dale A. M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 8, 109–114 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 38, 95–113 (2007). [DOI] [PubMed] [Google Scholar]
- Hagoort P., Hald L., Bastiaansen M. & Petersson K. M. Integration of Word Meaning and World Knowledge in Language Comprehension. Science 304, 438–441 (2004). [DOI] [PubMed] [Google Scholar]
- Jung Y. C. et al. Reciprocal activation of the orbitofrontal cortex and the ventrolateral prefrontal cortex in processing ambivalent stimuli. Brain research 1246, 136–143 (2008). [DOI] [PubMed] [Google Scholar]
- Neely J. H. Semantic priming and retrieval from lexical memory: Roles of inhibitionless spreading activation and limited-capacity attention. Journal of Experimental Psychology: General 106, 226–254 (1977). [Google Scholar]
- Kotz S. A., Cappa S. F., von Cramon D. Y. & Friederici A. D. Modulation of the Lexical–Semantic Network by Auditory Semantic Priming: An Event-Related Functional MRI Study. NeuroImage 17, 1761–1772 (2002). [DOI] [PubMed] [Google Scholar]
- Arcara G., Semenza C. & Bambini V. Word structure and decomposition effects in reading. Cognitive Neuropsychology 31, 184–218 (2014). [DOI] [PubMed] [Google Scholar]
- de Jong N. H., Feldman L. B., Schreuder R., Pastizzo M. & Baayen R. H. The Processing and Representation of Dutch and English Compounds: Peripheral Morphological and Central Orthographic Effects. Brain and Language 81, 555–567 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.