Abstract
Detailed anatomical models can be produced with consumer‐level 3D scanning and printing systems. 3D replication techniques are significant advances for anatomical education as they allow practitioners to more easily introduce diverse or numerous specimens into classrooms. Here we present a methodology for producing anatomical models in‐house, with the chondrocranium cartilage from a spiny dogfish (Squalus acanthias) and the skeleton of a cane toad (Rhinella marina) as case studies. 3D digital replicas were produced using two consumer‐level scanners and specimens were 3D‐printed with selective laser sintering. The fidelity of the two case study models was determined with respect to key anatomical features. Larger‐scale features of the dogfish chondrocranium and frog skeleton were all well‐resolved and distinct in the 3D digital models, and many finer‐scale features were also well‐resolved, but some more subtle features were absent from the digital models (e.g. endolymphatic foramina in chondrocranium). All characters identified in the digital chondrocranium could be identified in the subsequent 3D print; however, three characters in the 3D‐printed frog skeleton could not be clearly delimited (palatines, parasphenoid and pubis). Characters that were absent in the digital models or 3D prints had low‐relief in the original scanned specimen and represent a minor loss of fidelity. Our method description and case studies show that minimal equipment and training is needed to produce durable skeletal specimens. These technologies support the tailored production of models for specific classes or research aims.
Keywords: cartilage, dogfish, frog, laser scanning, selective laser sintering, skeleton, Squalidae, structured light scanning
Introduction
Animal dissections teach structure, function and evolution, and are the basis for advanced skills in specimen preparation, comparative research and veterinary practice (e.g. Mooney et al. 1982; Thomas & Fordyce, 2012; Crowther & Baillie, 2016). Dissections prepare students for specialist training in comparative anatomy, which can open career paths into zooarchaeology and systematic taxonomy (including paleontology) (Grayson, 1984; Fisher et al. 2008; Martinón‐Torres et al. 2013; Meijer et al. 2015). Anatomy courses are also required for some graduate programs and are in high demand among biology, zoology and human biology students. However, access to the animal tissues underpinning these opportunities could be a limiting factor for some education programs. Supplying large classes with animal tissues could be impractical, and ethical and conservation considerations may instead encourage the use of high‐quality alternatives to dissection (e.g. Russell & Burch, 1959; Balcombe, 2001; Villiers & Monk, 2005). Also, local anatomy collections may be dominated by regional faunas and be of limited use for taxonomically broad comparisons. High‐fidelity synthetic specimens have been the traditional solution to these issues (e.g. Khot et al. 2013; Preece et al. 2013; Lombardi et al. 2014; Yammine & Violato, 2015), and there is a growing trend for educators and researchers to produce their own anatomy replicas (Niven et al. 2009; Kuzminsky & Gardiner, 2012; Preece et al. 2013; McMenamin et al. 2014; D'Souza et al. 2015).
We describe how to produce anatomical models with minimal training and equipment using 3D scanning and printing methods. We propose that the resulting 3D digital and printed models are sufficiently detailed for both research and teaching purposes (veterinary, medical or other biology courses). The educational value of anatomical models was recently emphasised in a meta‐analysis (Yammine & Violato, 2015). Eight studies representing 820 learners met the inclusion criteria for the meta‐analysis which had two key conclusions ‘…physical models appear to offer an effective educational value to understanding spatial arrangements of anatomical organs…’ and ‘…the easy affordability and educational effectiveness of 3D physical models could offer a practical tool to bring up the learners’ level of gross anatomy knowledge at low cost…’ An educational study based around a 3D‐printed horse foot clearly demonstrated both of these points (Preece et al. 2013).
A 3D‐printed horse foot was produced using structural information from magnetic resonance images (Preece et al. 2013). Students studied the 3D‐printed foot (Model group; 21 students), veterinary textbooks (Textbook group; 19 students) or a 3D computer simulation of horse anatomy (Glasshorse group; 24 students), then undertook a quiz on horse foot anatomy. Preece et al. (2013) reported that ‘…the percentage of correct answers overall was significantly higher in the Model group than that of both the Textbook group (P < 0.001) and the Glasshorse group (P < 0.001); 86.4% compared with 62.6% and 63.7%, respectively…There was no significant difference in the overall test scores between the Textbook and the Glasshorse group (P > 0.685)’. The authors concluded that the 3D‐printed model was a useful tool for teaching equine foot anatomy.
The educational benefits of studying plastic models have also been revealed in human anatomy training (Lombardi et al. 2014). Similar to the veterinary case study above, human anatomy and physiology students were divided into three groups: a plastic model group (eight students), a virtual dissection group (eight students), and an organ dissection group (13 students). The groups received a short lecture about heart anatomy before studying a life‐sized plastic model of a human heart (not 3D‐printed), a virtual heart in anatomy software or a sheep heart, respectively. Students were tested on their anatomy knowledge immediately after the quiz and 2 months later. ‘In general, students in the plastic model treatment group outperformed students in both virtual and organ dissection groups’ (Lombardi et al. 2014). The higher test scores of the plastic model group may have been due to the simplicity of the plastic models and Lombardi et al. (2014) caution that ‘…the plastic models may actually be an oversimplification for some higher education courses, and therefore incorporating more complex activities to augment plastic model use may be necessary’. A variety of hands‐on and model‐assisted activities were recommended.
Anatomical models do not account for biological variation and deprive students of dissecting experience (see Sholts et al. 2010 and references therein). However, a fundamental benefit of anatomical models is that they can provide educational opportunities to learners who may otherwise not have access to original specimens. Rare skeletal specimens that are not available through external model suppliers can be replicated in‐house. Here we describe the production of high‐fidelity anatomy specimens with 3D imaging and printing technologies, using a dogfish chondrocranium and a frog skeleton as case studies. Both case studies are common teaching specimens (e.g. De Iuliis & Pulerà, 2010) and each one is a different challenge for 3D scanning; the chondrocranium deforms as it desiccates and the frog skeleton contains many small bones.
Dogfish (Chondrichthyes: Squalidae) are small sharks with a cosmopolitan distribution and a long history in anatomical education (Griffin, 1922; Harrison, 1949; De Iuliis & Pulerà, 2010). The cartilaginous skull (chondrocranium) and gill supports (splanchnocranium) of sharks provide insight into the evolutionary precursors for bony skulls; by studying the shark chondrocranium, students discover that the orbit, occipital condyles and other anatomical features are homologous to structures in the skulls of tetrapods (De Iuliis & Pulerà, 2010). However, there are conservation considerations surrounding the use of dogfish in teaching, as the populations of some Squalidae species (e.g. Squalus acanthias) are in regional decline (Fordham et al. 2006). Moreover, cartilage harvested from dogfish may be fixed with an organic compound (e.g. formaldehyde) and represent a safe‐handling issue. 3D prints of dogfish cartilage could address both the conservation and handling issues.
Frog skeletons (Amphibia: Anura) are useful for teaching about tetrapod plesiomorphies (e.g. pelvic girdle) and anuran apomorphies (e.g. urostyle) (Griffiths, 1963; De Iuliis & Pulerà, 2010). Indeed, many physical models and virtual frog dissections are already available (e.g. digital frog 2.5, Digital Frog International, Ontario, Canada), reflecting the educational niche for dissection alternatives (e.g. Osenkowski et al. 2015). Synthetic alternatives to frog tissues may also be used in consideration of the global decline of amphibian species (Edwards et al. 2014; IUCN, 2015). 3D‐scanned and ‐printed frog skeletons may be a valuable supplement to existing learning resources.
We have produced a digital replica of a frog skeleton using an HD Desktop 3D laser scanner (Next Engine Inc., Santa Monica, CA, USA), and used an SLS‐2 structured light scanner (david Vision Systems GmbH, Koblenz, Germany) to produce a digital replica of a dogfish chondrocranium. The DAVID system was chosen because it produced good results from specimens that were partially submerged in water. The Next Engine system was used because it has an automated turntable. We show that key anatomical features can be resolved in the digital replicas and 3D prints of each case study specimen.
Material and methods
Specimens
Spiny dogfish Squalus acanthias were purchased from a commercial fishing company for use in undergraduate teaching at Massey University, New Zealand. The dogfish were caught between January and March 2014 (location unknown). The chondrocranium had been dissected out of one dogfish (sex unknown) and stored in 70% ethanol for transport to J.D.H. (Fig. 1). On arrival, the chondrocranium was decanted from ethanol and then partially submerged in water with the dorsal surface facing upwards for 3D scanning. Water covered approximately one‐third of the chondrocranium, which slowed desiccation and distortion (distortion would create scan alignment errors in later steps). Scanning was performed in a sunlit room and the chondrocranium was not coated with a contrast‐enhancing paint.
Figure 1.

3D‐printed dogfish chondrocranium (Squalus acanthias) and cane toad skeleton (Rhinella marina) alongside the specimens that were 3D‐scanned for printing. Original specimens are shown along the top row and 3D prints are shown underneath. Dried connective tissue between the ribs of the cane toad skeleton is represented in the 3D print but not present in the photograph, as it had been removed after 3D scanning. Images of the 3D prints and original specimens are at slightly different viewer angles, which results in different perceived proportions of some features (i.e. orbits in frog skeleton).
An articulated skeleton of a cane toad Rhinella marina was also 3D‐scanned (Fig. 1). The skeleton was from the Massey University zoology museum, with sex, age, provenance and preparation details unknown. The skeleton was mostly complete and articulated by dried remnants of muscle tissue; only the hyoid structure and some elements of the sternum were absent from the skeleton. Scanning was performed in a darkened room and the skeleton was not coated with a contrast‐enhancing paint. It was our experience that the laser scanner required a darkened room to generate high‐quality scans, whereas the structured light scanner could produce high quality data in a sunlit room. We suspect that this is because the perceived edge of the laser line becomes diffuse under ambient light.
Dogfish chondrocranium 3D scanning
The structured light 3D scanning system included a 1280 × 960 pixel CMOS monochrome camera (DAVID‐CAM‐3.1‐M; DAVID Vision Systems) and a K132 + DLP projector (Acer Incorporated, New Taipei City, Taiwan) mounted on a support bar, with the support bar attached to a tripod. Cables from the scanner and camera were attached to a laptop computer operating david 3 software (version 3.10.4.4657; david Vision Systems). The 3D scanning system was set up for small objects as outlined in the user manual. Specifically, the camera was positioned to the left of the projector on the support bar and rotated approximately 21° clockwise. The position and height of the tripod relative to the chondrocranium, as well as the distance between camera and projector, were adjusted until the specimen appeared in the centre of the camera view (determined from the live camera feed). The projector focus, camera focus, and camera exposure were each adjusted to produce the sharpest image of the chondrocranium (determined from the live camera feed). The dogfish specimen was moved aside for the scanner to be calibrated with the 60‐mm pattern on the supplied calibration panels. Calibration allows the absolute size of scanned objects to be recorded in the scan data. The dogfish specimen was returned to the centre of the camera view for 3D scanning, and positioned with a dorso‐lateral view to the camera.
The dorsal surface was imaged in eight scans by rotating the specimen approximately 45° clockwise between scans. The specimen was flipped for the ventral side to be imaged in eight scans. The specimen was then rotated onto a lateral edge for imaging, and a small dark object was positioned under the specimen to rest against. The object was repositioned for the anterior and posterior surfaces to be imaged. Additional scans were collected from foramina, sulci and other recessed structures to ensure that the features were completely imaged. Fifty‐three scans contributed to the final model. The collection, editing and fusing of 3D scan data was performed with david 3 software.
Scans were loaded into david 3 and vertices from the scanning backgrounds were removed. Individual scans were aligned using the inbuilt alignment tools and a composite model was formed. Some scans completely overlapped with other scans already in the composite model and were discarded. The composite model was fused using the inbuilt fusion tool at 500 resolution (filled model and high resolution options were selected). The final fused model had a resolution of 0.20 mm between vertices.
Frog skeleton 3D scanning
The cane toad (i.e. frog) skeleton was scanned with a laser‐based system instead of a structured light system. The laser 3D scanning system included a scanning unit with eight 650‐nm 10 mW lasers and two 3 megapixel CMOS cameras (Next Engine HD Desktop 3D laser scanner). The scanning unit was attached to a computer‐controlled tilting turntable (Multi Drive, Next Engine Inc., Santa Monica, CA, USA). A round plate and an adjustable arm for holding specimens were centered over the turntable (Partgripper, Next Engine Inc.). The laser 3D scanner was placed on a desk surface and connected to a PC workstation.
The skeleton was 3D‐scanned in four orientations on the turntable. First, the skeleton was positioned with the posterior end against the round plate and the anterior end pinned by the adjustable arm (i.e. antero‐posterior). The specimen was also scanned in postero‐anterior, dorso‐ventral and ventro‐dorsal positions. Scan data were collected using scanstudio hd 1.3.2 software (Next Engine Inc.). Prior to scanning, the scanner collected a series of size calibration measurements to allow absolute size to be recorded in the scan data. This is a feature of the Next Engine scanner and it differs from the calibration panel method used in the DAVID Vision Systems scanner. The skeleton was then scanned and subsequently rotated 22.5° in the horizontal plane (rotated 16 times under software control). The skeleton was then rotated 10° and a further set of 16 scans were collected (i.e. 32 scans for each of the four orientations). Scan resolution was set at maximum (160 k points per square inch), RGB textures were not collected, and target colour was set as neutral. In all, 93 scans contributed to the final model; scans without novel information were discarded. Just as with the dogfish scan, the frog skeleton was also edited with david 3 software. The final model was fused at 700 resolution to produce a water‐tight model with 0.20 mm between vertices.
Comment on file size
The relatively small file sizes of the digital dogfish chondrocranium and frog skeleton (around 30 MB; Supporting Information) are ideal for 3D printing and online display (i.e. www.sketchfab.com). However, 3D digital replicas with substantially larger file sizes (> 64 MB) may be unsuitable for current systems. Digital replicas will have large files sizes if they include many scans (i.e. large original specimens) or if the scan data are collected at fine‐scale resolution. We have printed and displayed large specimens (not presented in this study) by first reducing the file size with the ‘decimate’ tool in blender 2.74 (Blender online community, 2015). Note that decimation eliminates finer‐scale anatomical features, balancing file size against model detail.
3D printing
The digital models were 3D‐printed using a selective laser sintering system in the School of Engineering and Advanced Technology, Massey University, New Zealand. Practitioners who do not have direct access to a selective laser sintering system may instead use an online printing service (e.g. www.shapeways.com). The distinct advantage of selective laser sintering for anatomy replicas is that elevated features (i.e., vertebrae in the frog skeleton) are supported by the underlying powder and do not require additional scaffolding structures to be printed. Massey University has a TPM Elite P3200 printer (Trumpsystem Precision Machinery, Zhongshan City, China). Here, the dogfish chondrocrania and frog skeletons were constructed from successive layers of nylon powder. For each layer, powder was spread into the print chamber and heated and a 60 W CO2 laser traced out a cross‐section of the printed part (i.e. the layer was sintered). The print chamber was then lowered by 0.15 mm, a fresh layer of powder was spread, and the next layer was laser‐sintered. After all layers were printed, the powder was cooled and raised out of the printer. The models were extracted from the block of powder and brushed before being ‘sand‐blasted’ with glass beads to remove unsintered powder.
Results
Details in 3D digital models
The default size of the 3D digital models is the same as the physical size of the scanned specimens. The 1 : 1 size correlation between the original object and the 3D digital object (and 3D print) is the default output after calibrating the 3D scanner. The chondrocranium has an anteroposterior length of 98 mm, a dorsoventral depth of 27 mm, and is 46 mm wide. The smallest resolvable features in the chondrocranium are 0.2 mm long. Anatomical features in the 3D digital model compared well with a shark chondrocranium described in a vertebrate dissection laboratory manual (De Iuliis & Pulerà, 2010). The rostrum, orbit, occiptal region and other larger‐scale features were all well‐resolved and distinct (Table 1; Fig. 2). Many finer‐scale features were also well‐resolved (e.g. perilymphatic foramina) but some features were not distinguishable in the digital model (e.g. endolymphatic foramina) (Table 1; Fig. 2). Poorly resolved features in the 3D digital model tended to have low relief in the physical specimen.
Table 1.
Anatomical features of the shark chondrocranium, as listed in De Iuliis & Pulerà (2010), are generally well‐resolved in the 3D digital model and 3D print. Some features were absent from the digital model because they were not part of the scanned specimen (e.g. nasal capsules), and some low relief features were not well‐resolved (e.g. nerve foramina). Some features in the digital model were not well‐resolved in the 3D print. A, feature was absent from the physical specimen; NR, feature is not well‐resolved and is effectively absent; P, feature is present. See also Fig. 2
| Anatomical feature | Scan | Anatomical feature | Scan | ||
|---|---|---|---|---|---|
| Abducens foramina | P | P | Optic foramen | P | P |
| Antorbital processes | P | P | Orbit | P | P |
| Antorbital shelves | P | P | Otic capsules | P | P |
| Basal plate | P | P | Perilymphatic foramina | P | P |
| Basitrabecular processes | P | P | Postorbital processes | P | P |
| Carotid foramen | P | P | Precerebral cavity | P | P |
| Endolymphatic foramina | NR | A | Precerebral fenestra | A | A |
| Epiphyseal foramen | NR | A | Rostral carina | P | P |
| Foramen magnum | P | P | Rostral fenestrae | P | P |
| Glossopharyngeal foramina | NR | A | Rostrum | P | P |
| Hyomandibular foramina | NR | A | Superficial ophthalmic foramina | P | P |
| Nares | P | P | Supraorbital crest | P | P |
| Nasal capsules | A | A | Trigeminofacial foramen | NR | A |
| Occipital condyles | P | P | Trochlear foramina | NR | A |
| Oculomotor foramina | P | P | Vagus foramina | P | P |
Figure 2.
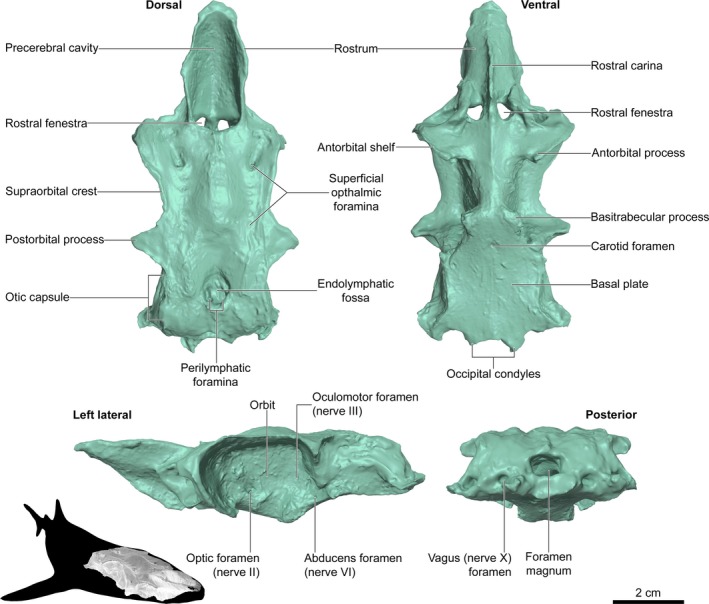
Anatomical features in a 3D digital model of a dogfish chondrocranium (Squalus acanthias). Features are identified in reference to a vertebrate zoology anatomy manual (De Iuliis & Pulera 2010). The chondrocranium was scanned with a DAVID Vision Systems SLS‐2 structured light scanner. See also Table 1.
The frog skeleton is 77 mm from the anterior skull to the posterior pelvic girdle. The dried muscle tissue that articulates the skeleton specimen is visible in the 3D digital model (Fig. 3). In particular, some remnant muscle extends between the transverse processes of the vertebrae (Fig. 3). As with the dogfish chondrocranium described above, key anatomical features of the frog skeleton are evident in the 3D digital model (i.e. De Iuliis & Pulerà, 2010). Larger scale structures (e.g. skull, vertebral column, limbs) are all well‐resolved and correctly proportioned (Fig. 3). Many finer‐scale structures (e.g. digits) are also clearly visible in the 3D digital model (Tables 2 and 3), although the sutures between fused long bones (e.g. radius and ulna) are not distinct.
Figure 3.
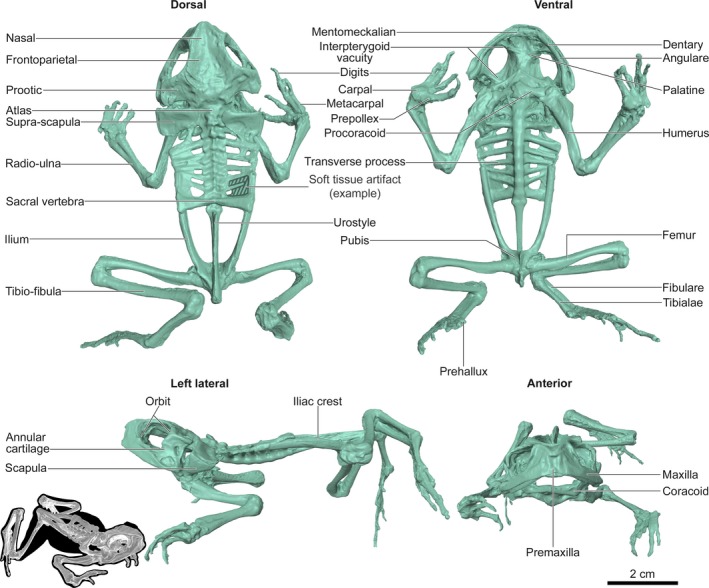
Anatomical features in a 3D digital model of a cane toad skeleton (Rhinella marina). Features are identified in reference to a vertebrate zoology anatomy manual (DeIuliis et al. 2007). The frog skeleton was scanned with a Next Engine HD Desktop 3D laser scanner. See also Tables 2 and 3.
Table 2.
Anatomical features in the skull of the frog skeleton, as listed in De Iuliis & Pulerà (2010), are generally well‐resolved in the 3D digital model and 3D print. The hyoid apparatus was not present in the scanned specimen and is therefore absent in the digital model and 3D print. Some features were not visible because the physical specimen was articulated and had remnant muscle tissue. A, feature was absent from the physical specimen; NR, feature is not well‐resolved and is effectively absent; O, feature is obscured by another structure; P, feature is present. See also Fig. 3
| Anatomical feature | Scan | Anatomical feature | Scan | ||
|---|---|---|---|---|---|
| Angulare | P | P | Meckel's cartilage | O | A |
| Annular cartilage | P | P | Mentomeckalian | P | P |
| Anterior cornu | A | A | Nasals | P | P |
| Columella | A | A | Occipital | O | A |
| Coronoid process | O | A | Occipital condyle | O | A |
| Dentary | P | P | Orbits | P | P |
| Foramen magnum | O | A | Palatines | P | NR |
| Foramen ovale | O | A | Parasphenoid | P | NR |
| Frontoparietals | P | P | Posterior cornu | A | A |
| Hyoid apparatus | A | A | Premaxilla | P | P |
| Interpterygoid vacuities | P | P | Premaxillary teeth | A | A |
| Mandible | P | P | Prootic | P | P |
| Maxilla | P | P | Sphenethmoids | P | NR |
| Maxillary teeth | A | A |
Table 3.
Anatomical features in the postcranial frog skeleton, as listed in De Iuliis & Pulerà (2010), are generally well‐resolved in the 3D digital model and 3D print. Some elements of the sternum were not present in the scanned specimen and are therefore absent in the digital model and 3D print. Also, sutures within the long bones (i.e. between radius and ulna) were not visible in the scanned specimen. A, feature was absent from the physical specimen; NR, feature is not well‐resolved and effectively absent; P, feature is present; O, feature is obscured by another structure. See also Fig. 3
| Anatomical feature | Scan | Anatomical feature | Scan | ||
|---|---|---|---|---|---|
| Acetabulum | O | A | Phalanges | P | P |
| Atlas | P | P | Prehallux | P | P |
| Carpals | P | P | Prepollex | P | P |
| Clavicle | P | P | Procoracoids | P | P |
| Digits | P | P | Pubis | P | NR |
| Episternum | A | A | Radio‐ulna | P | P |
| Femur | P | P | Radius | NR | A |
| Fibula | NR | A | Sacral vertebra | P | P |
| Fibulare (= calcaneum) | P | P | Scapula | P | P |
| Glenoid fossa | O | A | Sternum | A | A |
| Humerus | P | P | Suprascapula | P | P |
| Iliac crest | P | P | Tibia | NR | A |
| Ilium | P | P | Tibiale (= astragalus) | P | P |
| Manus | P | P | Tibio‐fibula | P | P |
| Metacarpals | P | P | Transverse process | P | P |
| Omosternum | A | A | Ulna | NR | A |
| Pectoral girdle | P | P | Urostyle | P | P |
| Pelvic girdle | P | P | Vertebrae | P | P |
| Pelvis | P | P | Vertebral column | P | P |
| Pes | P | P | Xiphisternum | A | A |
Details in 3D prints
The 3D‐printed chondrocranium and frog skeleton were good facsimiles of the digital models and, consequently, of the physical scanned objects (Fig. 1). Almost all of the key anatomical features in the chondrocranium translated from the digital model into the 3D print (Table 1). Features that were not well‐resolved in the 3D digital model (e.g. endolymphatic foramina) were absent from the 3D print. Trigeminofacial foramina were present in the digital model but were not well‐resolved in the 3D print. The cranial cavity was printed as a solid structure as the internal surface was not scanned. Likewise for the frog skeleton, anatomical features in the digital model were almost all distinguishable in the 3D print (Tables 2 and 3). Data loss in the 3D print occurred at some bone sutures (e.g. suture between palatines and parasphenoids was not distinguishable in the printed model; Fig. 4).
Figure 4.
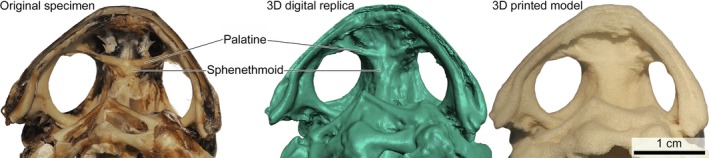
Fine‐scale anatomical features in the skull were poorly resolved in the 3D‐printed cane toad skeleton (Rhinella marina). Close up of the ventral surface of the cane toad skull showing the palatines and sphenethmoids. The palatines are distinct from the sphenethmoids in the original skeleton and 3D digital replica, but the suture between these bones is not distinguishable in the 3D‐printed model.
Discussion
Two skeletal models were produced using consumer‐level 3D scanning equipment, an HD Desktop 3D laser scanner and an SLS‐2 structured light scanner. A 3D digital replica of a dogfish chondrocranium retained 24 of 28 anatomical characters identified as key features in an undergraduate vertebrate zoology textbook (Table 1; De Iuliis & Pulerà, 2010). Two key features identified in the textbook were absent from the original specimen (30 characters in total). A 3D digital frog skeleton retained 44 of 48 characters, with 18 characters either absent from the original specimen or obscured by other anatomical features (Tables 2 and 3) (De Iuliis & Pulerà, 2010). The details that did not translate well from the original specimen to the 3D digital replicas tended to have low‐relief in the scanned objects (e.g. some nerve foramina). Models were 3D‐printed at 0.15 mm resolution (compared with 0.2 mm resolution of the 3D scans) using a selective laser sintering process. We have not printed bone structures that are less than 0.5 mm thick (i.e. frog toe bones), and there is no maximum size limit for printing or scanning, as bones that are larger than the printing chamber can be printed in multiple pieces. All characters in the digital chondrocranium could be identified in the subsequent 3D print; however, three characters in the 3D‐printed frog skeleton could not be clearly delimited (palatines, parasphenoid and pubis). These three characters are distinguished from adjacent bones by subtle sutures in the original specimen. Despite a minor loss of fidelity at fine scale, our results are consistent with favourable results reported elsewhere (Adams et al. 2010; Sholts et al. 2010; Belvedere et al. 2011; Tambusso et al. 2015).
Model production costs were relatively inexpensive after a moderate investment in scanning equipment. The HD Desktop 3D laser scanner was approximately £2,700 and the SLS‐2 structured light scanner approximately £2,100. Scanning time was between 1 and 2 h for each specimen: the SLS‐2 structured light scanner required the object to be manually repositioned between scans, whereas object positioning was automated with the HD Desktop 3D laser scanner. Processing of scan data into a 3D‐printable model carried an additional time burden of approximately 1 h. The dogfish chondrocranium cost approximately £13 to print and the frog skeleton approximately £10 to print. In contrast, a freeze‐dried dogfish chondrocranium costs approximately £40 and a grass frog skeleton approximately £150 from ward's science (https://www.wardsci.com/store/). Moreover, specimens printed in nylon using selective laser sintering are robust to undergraduate classrooms, assuming that the models are afforded the same respect as other lab specimens. The printed material has a small range of ductility which means that even small structures (e.g. frog toe bones) will bend a little bit before breaking.
Although both 3D scanning systems required minimal training and produced useful models, the SLS‐2 structured light scanner was more versatile and better suited for scanning cartilage. The cartilage needed to be partly submerged during scanning, which required the scanning system to be elevated above and angled down towards the cartilage. The camera and projector of the SLS‐2 system are easier to position than the HD Desktop 3D laser scanner and can tilted with reduced risk.
The skeletal models described here are valuable test cases for wider application of 3D printing in anatomy education. In particular, bones from other animals in the comparative anatomy sequence for vertebrate zoology are likely targets for reproduction and distribution (e.g. actinopterygiians, lepidosaurs, archosaurs and synapsids). 3D printing could also be a solution for replicating rare specimens, and for supplying anatomical models to large classes. 3D scanning and printing is thus a significant advance for anatomy education as it encourages user‐created content. Although purchasing skeletal specimens for teaching is a well‐established practice, 3D technologies allow practitioners to produce anatomy models suited for specific classes or research aims.
Authors’ contribution
D.B.T. and J.D.H. acquired the data and B.J.D. and J.P. printed the specimens. D.B.T. wrote the original manuscript and J.D.H., B.J.D. and J.P. critically revised and approved the paper.
Supporting information
3D Object S1. Dogfish chondrocranium. A 3D digital model of the chondrocranium of a spiny dogfish Squalus acanthias.
3D Object S2. Frog skeleton. A 3D digital model of the skeleton of a cane toad Rhinella marina.
Acknowledgement
We thank Mr C. Wallace (Institute of Agriculture & Environment, Massey University) for providing study materials. 3D scanning equipment was purchased with the Massey University INMS Minor Equipment Fund 2014 and Massey University Strategic Innovation Fund 2014. 3D printing costs were covered by the Massey University Innovation and Excellence Fund 2015.
References
- Adams TL, Strganac C, Polcyn MJ, et al. (2010) High resolution three‐dimensional laser‐scanning of the type specimen of Eubrontes (?) glenrosensis Shuler, 1935, from the Comanchean (Lower Cretaceous) of Texas: implications for digital archiving and preservation. Palaeontol Electron 13, Article‐Number 13.3.1T. [Google Scholar]
- Balcombe J (2001) Dissection: the scientific case for alternatives. J Appl Anim Welf Sci 4, 117–126. [Google Scholar]
- Belvedere M, Dyke G, Hadri M, et al. (2011) The oldest evidence for birds in Northern Gondwana? Small tridactyl footprints from the Middle Jurassic of Msemrir (Morocco). Gondwana Res 19, 542–549. [Google Scholar]
- Blender online community (2015) Blender 2.74 ‐ a 3D modelling and rendering package. http://www.blender.org.
- Crowther E, Baillie S (2016) A method of developing and introducing case‐based learning to a preclinical veterinary curriculum. Anat Sci Educ 9, 80–89. [DOI] [PubMed] [Google Scholar]
- De Iuliis G, Pulerà D (2010) The Dissection of Vertebrates: A Laboratory Manual, 2nd edn Burlington: Academic Press. [Google Scholar]
- D'Souza N, Mainprize J, Edwards G, et al. (2015) Teaching facial fracture repair: a novel method of surgical skills training using three‐dimensional biomodels. Plast Surg 23, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Jones SM, Bird FL, et al. (2014) Enhancing learning through the use of animals in undergraduate biology teaching: the student voice. Int J Innov Sci Math Educ 22, 35–54. [Google Scholar]
- Fisher RE, Adrian B, Elrod C, et al. (2008) The phylogeny of the red panda (Ailurus fulgens): evidence from the hindlimb. J Anat 213, 607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham S, Fowlerm SL, Coelhom R, et al. (2006) Squalus acanthias. The IUCN Red List of Threatened Species (accessed 23 September 2015). http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T39326A10201416.en.
- Grayson DK (1984) Quantitative Zooarchaeology: Topics in the Analysis of Archaelogical Faunas. Orlando: Academic Press. [Google Scholar]
- Griffin LE (1922) A Guide for the Dissection of the Dogfish (Squalus acanthias), Portland: https://archive.org/details/guidefordissecti00grif. [Google Scholar]
- Griffiths I (1963) The phylogeny of the salientia. Biol Rev 38, 241–292. [DOI] [PubMed] [Google Scholar]
- Harrison BM (1949) The Dissection of the Shark (The Dogfish, Squalus acanthias): A Laboratory Manual. Minneapolis: Burgess Publishing Company. [Google Scholar]
- IUCN (2015) IUCN Red List Status for Amphibians (accessed 23 September 2015). http://www.iucnredlist.org/initiatives/amphibians/analysis/red-list-status.
- Khot Z, Quinlan K, Norman GR, et al. (2013) The relative effectiveness of computer‐based and traditional resources for education in anatomy. Anat Sci Educ 6,211–215. [DOI] [PubMed] [Google Scholar]
- Kuzminsky SC, Gardiner MS (2012) Three‐dimensional laser scanning: potential uses for museum conservation and scientific research. J Archaeol Sci 39, 2744–2751. [Google Scholar]
- Lombardi SA, Hicks RE, Thompson KV, et al. (2014) Are all hands‐on activities equally effective? Effect of using plastic models, organ dissections, and virtual dissections on student learning and perceptions. Adv Physiol Educ 38, 80–86. [DOI] [PubMed] [Google Scholar]
- Martinón‐Torres M, Spěváčková P, Gracia‐Téllez A, et al. (2013) Morphometric analysis of molars in a Middle Pleistocene population shows a mosaic of ‘modern’ and Neanderthal features. J Anat 223, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Quayle MR, McHenry CR, et al. (2014) The production of anatomical teaching resources using three‐dimensional (3D) printing technology. Anat Sci Educ 7, 479–486. [DOI] [PubMed] [Google Scholar]
- Meijer HJM, Kurniawan I, Setiabudi E, et al. (2015) Avian remains from the Early/Middle Pleistocene of the So'a Basin, central Flores, Indonesia, and their palaeoenvironmental significance. Palaeogeogr Palaeoclim Palaeoecol 440, 161–171. [Google Scholar]
- Mooney MP, Kraus EM, Bardach J, et al. (1982) Skull preparation using the enzyme‐active detergent technique. Anat Rec 202, 125–129. [DOI] [PubMed] [Google Scholar]
- Niven L, Steele TE, Finke H, et al. (2009) Virtual skeletons: using a structured light scanner to create a 3D faunal comparative collection. J Archaeol Sci 36, 2018–2023. [Google Scholar]
- Osenkowski P, Green C, Tjaden A, et al. (2015) Evaluation of educator & student use of & attitudes toward dissection & dissection alternatives. Am Biol Teach 77, 340–346. [Google Scholar]
- Preece D, Williams SB, Lam R, et al. (2013) ‘Let's Get Physical’: advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat Sci Educ 6, 216–224. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL (1959) The Principles of Humane Experimental Technique. London: Methuen and Co. [Google Scholar]
- Sholts SB, Wärmländer SKTS, Flores LM, et al. (2010) Variation in the measurement of cranial volume and surface area using 3D laser scanning technology. J Forensic Sci 55, 871–876. [DOI] [PubMed] [Google Scholar]
- Tambusso PS, McDonald HG, Fariña RA (2015) Description of the stylohyal bone of a giant sloth (Lestodon armatus). Palaeontol Electron 18, 1–10. [Google Scholar]
- Thomas DB, Fordyce RE (2012) Biological plasticity in penguin heat‐retention structures. Anat Rec 295, 249–256. [DOI] [PubMed] [Google Scholar]
- Villiers RD, Monk M (2005) The first cut is the deepest: reflections on the state of animal dissection in biology education. J Curriculum Stud 37, 583–600. [Google Scholar]
- Yammine K, Violato C (2015) The effectiveness of physical models in teaching anatomy: a meta‐analysis of comparative studies. Adv Health Sci Educ, 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D Object S1. Dogfish chondrocranium. A 3D digital model of the chondrocranium of a spiny dogfish Squalus acanthias.
3D Object S2. Frog skeleton. A 3D digital model of the skeleton of a cane toad Rhinella marina.


