Abstract
Carbon dots (CDs) as a new series of fluorescent nanomaterials have drawn great attention in recent years owning to their unique properties. In this paper, a simple carbonization approach to synthesize amino-functionalized CDs was developed by using chitosan as the carbon precursor. The as-prepared CDs possessed desirable amino function group on their surface and exhibited bright luminescence with absolute quantum yield (QY) of 4.34%, excitation-, pH-dependent and up-conversion fluorescence behaviors. Furthermore, we have investigated the cytotoxicity and biocompatibility of the as-prepared CDs, which demonstrated that the as-prepared CDs have the potential applications in biosensing, cellular imaging and drug delivery.
Carbon dots (CDs) as a new member of carbon nanomaterials have drawn a great deal of attention, owning to their chemical stability, excellent water solubility, tuneable fluorescence properties, low cost, low toxicity, good biocompatibility and environmental friendliness1,2,3,4. These outstanding merits make them show promising prospects as benign candidates for various potential applications, such as fluorescent markers, bioimaging agents, photocatalysts, sensors and optoelectronic devices5,6,7,8,9. Therefore, exploring alternative routes and starting materials for the synthesis of CDs are highly needed.
Up to now, a variety of methods have been developed to synthesize CDs, which can be roughly classified into “top-down” and “bottom-up” approaches10. The “top-down” approach includes chemical oxidation, electrochemical synthesis, arc-discharge and laser-ablation, where the CDs are formed from larger carbon materials11. The “bottom-up” approach refers to obtaining CDs from molecular precursors. Generally, “bottom-up” approach consists of microwave/ultrasonic preparation, plasma treatment, hydrothermal/acidic oxidation route and carbonizing organics route12,13,14. Nevertheless, many of these synthesis methods involve expensive or toxic starting materials, high temperature, long reaction time and further surface-passivation15,16.
In recent years, the preparation of fluorescent CDs without using organic chemicals in a simple, economical and environmentally friendly way is of great interest, which is so-called the green chemistry concept17. Some green synthetic routes have been developed for the preparation of CDs by using inexpensive and renewable resources as starting materials, like watermelon peels, orange juice, chicken eggs, gelatin, etc11,18,19. Remarkably, chitosan is the N-deacetylated derivative of chitin (a naturally abundant mucopolysaccharide), containing high amount of amino (-NH2) and hydroxyl (-OH) functional groups, also accepted as carbon source to synthesize CDs owing to its natural, nontoxic and biocompatible properties20,21.
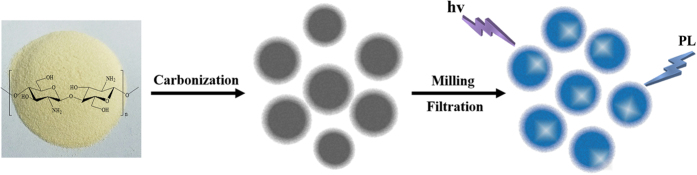
In this work, we presented a very simple approach to synthesize amino-functionalized CDs by one-step carbonization treatment of chitosan (Fig. 1), which was scarcely studied before. In this method, neither a strong acid solvent nor surface passivation reagent was used. Both the formation and the functionalization of CDs were accomplished simultaneously. The as-prepared amino-functionalized CDs exhibited excellent fluorescence together with excitation-, pH-dependent and up-conversion fluorescence behaviors. Besides, due to their low toxicity and fine aqueous dispersibility, the as-prepared CDs showed great potential in biosensing, cellular imaging and drug delivery applications.
Figure 1. Schematic illustration of the preparation of CDs from chitosan.
Results and Discussion
Physical structure of as-prepared CDs
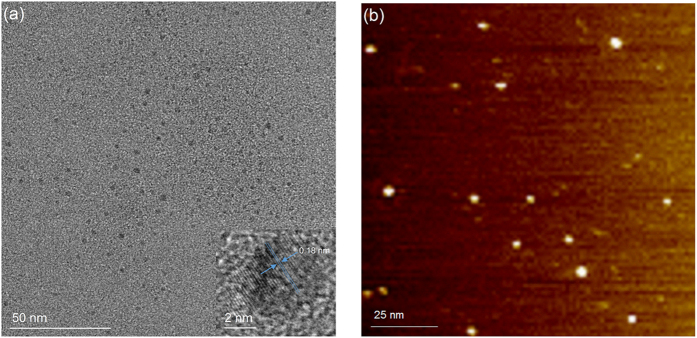
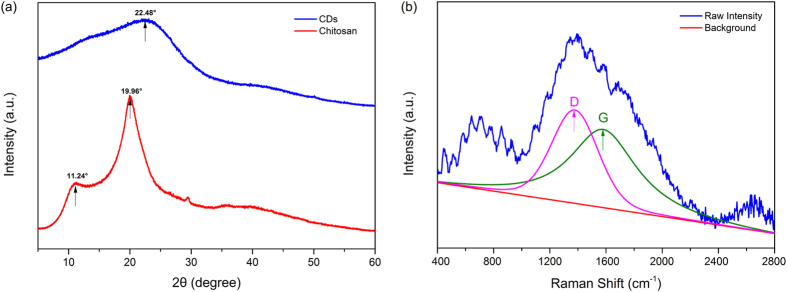
The transmission electron microscopy (TEM) and atomic-force microscopy (AFM) images (Fig. 2) illustrated that the as-prepared CDs were quasi-spherical and monodisperse with size distribution between 1–6 nm in diameter. Besides, the high resolution transmission electron microscopy (HRTEM) image (inset of Fig. 2(a)) clearly showed the lattice spacing of 0.18 nm, which might be assigned to the (102) facet of graphitic carbon15. The X-ray diffraction (XRD) pattern of chitosan and as-prepared CDs were displayed in Fig. 3(a). The spectrum of chitosan showed distinct diffraction peaks at 11.24 and 19.96°, corresponding to the amorphous peak and crystalline peak, separately22. After carbonization, the crystallinity of chitosan diminished and the spectrum of as-prepared CDs showed a broad peak at 2θ = 22.48°, similar to the graphite lattice spacing, which was attributed to highly disordered carbon atoms23,24. Additionally, the Raman spectrum of as-prepared CDs (Fig. 3(b)) revealed that although the fluorescence background signal was very strong, a relatively strong D band at 1378 cm−1 and G band at 1580 cm−1 could be obviously observed, and the ratio of intensities of ID/IG was calculated to be 1.15, indicating the presence of more defects or amorphous carbon as compared with sp2 hybridization in as-prepared CDs25,26,27, which coincided with the HRTEM and XRD results.
Figure 2.
TEM (a) and AFM (b) images of as-prepared CDs.
Figure 3.
(a) XRD patterns of chitosan and as-prepared CDs and (b) Raman spectrum of as-prepared CDs.
Chemical structure of as-prepared CDs
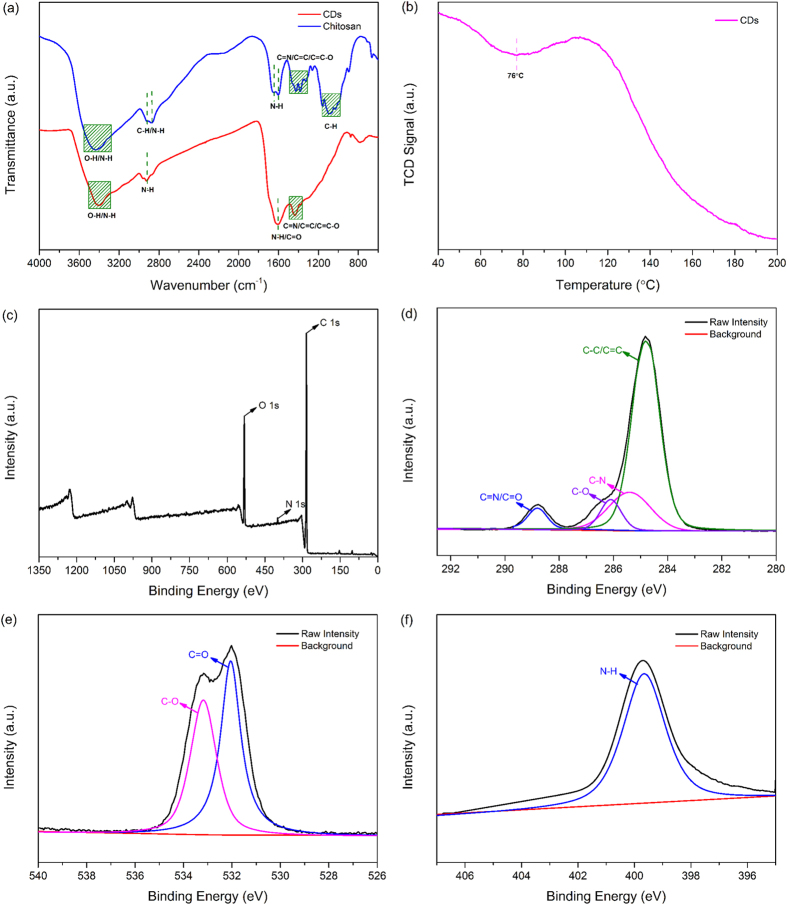
The Fourier transform infrared (FT-IR) spectra of chitosan and as-prepared CDs were presented in Fig. 4(a). The basic characteristic peaks of chitosan appeared at 3438 cm−1 (O-H and N–H stretching vibrations), 2920 and 2875 cm−1 (C-H stretching vibration), 1643 and 1602 cm−1 (N-H bending vibration), 1493–1245 cm−1 (C=C, C=N and C=C–O groups) and 1165–992 cm−1 (C-H bending vibrations of the pyranose ring)14. However, for the as-prepared CDs, the adsorption of O-H and N-H stretching vibration at 3401 cm−1 were decreased. Meanwhile, the C-H bending vibrations associated with pyranose ring in chitosan were completely disappeared. These differences between chitosan and as-prepared CDs might be ascribed to the decomposition of the chitosan chain and pyranose ring during the carbonization process. Notably, the bands at 2930 and 1615 cm−1 corresponded to N-H bending vibration of amino group increased28. The temperature programmed desorption of CO2 (CO2-TPD) profile of as-prepared CDs (Fig. 4(b)) suggested a distinct desorption of CO2 centered around 76 °C, similar to previous reports for amino-modified solids29, which might be due to the interaction between amino-functionalized CDs and CO2 via a chemisorption mechanism. The general formula of interaction between the functional amine and the CO2 molecules results in the formation of ammonium carbamates under anhydrous conditions can be seen in Equation (1) 30,31:
Figure 4.
(a) FT-IR spectra of chitosan and as-prepared CDs, (b)CO2-TPD profile, (c) Whole survey XPS spectrum, (d) C 1s XPS spectrum, (e) O 1s XPS spectrum and (f) N 1s XPS spectrum of as-prepared CDs.
 |
Also, the zeta-potential of as-prepared CDs was positive when the pH was below 6.67, indicating the existence of amine groups on the surface of as-prepared CDs together with the FT-IR and CO2-TPD results32.
X-ray photoelectron spectroscopy (XPS) measurement was carried out to further investigate functional groups presented on the surface of as-prepared CDs. The whole XPS survey spectrum of the as-prepared CDs (Fig. 4(c)) exhibited three obvious peaks at 284.81, 399.33, and 532.3 eV, which were attributed to C 1s, N 1s and O 1s, respectively. The C 1s XPS spectrum (Fig. 4(d)) can be further deconvoluted into four carbon states at 284.82, 285.40, 286.12 and 288.79 eV, which were attributed to C-C/C=C, C–N, C–O and C=N/C=O respectively and might result in a series of emissive traps between π and π* states of C=C15. The O 1s spectrum (Fig. 4(e)) showed two peaks at 532.01 and 533.17 eV, which were assigned to the C=O and C-O bands, respectively28,33. Furthermore, the N 1s spectrum (Fig. 4(f)) revealed an apparent peak at 399.83 eV, which was associated with N-H band14. The results from XPS spectra showed that the presence of hydrophilic functional groups on the surface of the as-prepared CDs without further modification. And these groups made as-prepared CDs aqueous dispersible and made a significant contribution to their optical properties34.
Optical properties of as-prepared CDs
Although the fluorescence emission mechanisms remain debatable, two classes of fluorescence emission mechanisms have been proposed. The first class mechanism is related to the bandgap transitions caused by conjugated π-domains, while the second class mechanism arises from the existence of multiple surface defects35. The Ultraviolet-visible (UV-Vis) absorption and photoluminescence (PL) spectra of as-prepared CDs were investigated as shown in Fig. 5. From UV-Vis absorption, it was obviously that a strong UV-Vis absorption peak located at 261 nm, which typically assigned to the π-π* transition of aromatic sp2 domains from the carbon core14,36. As shown in the PL spectra, when excited at the maximum excitation wavelength of 310 nm, the as-prepared CDs exhibited a strong PL peak at 390 nm. The aqueous solution of as-prepared CDs was nearly transparent under visible light, while it displayed strong blue fluorescence under UV light (365 nm) excitation. (inset, Fig. 5)
Figure 5. UV-Vis absorption, PL excitation and PL emission spectra of as-prepared CDs solution.
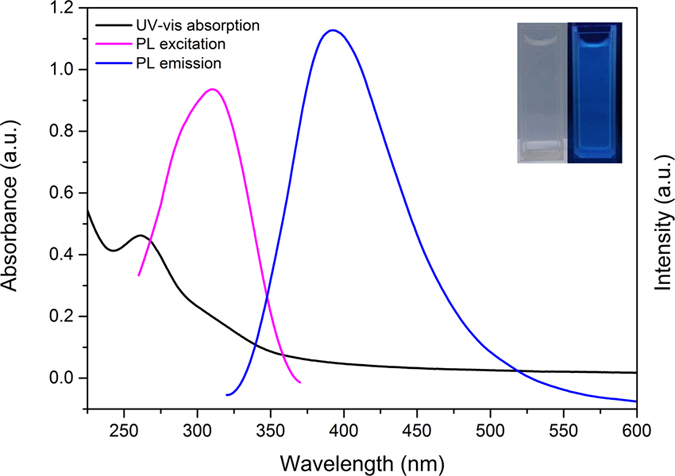
Inset are the photos of as-prepared CDs solution under sunlight (left) and 365 nm UV lamp (right) illumination.
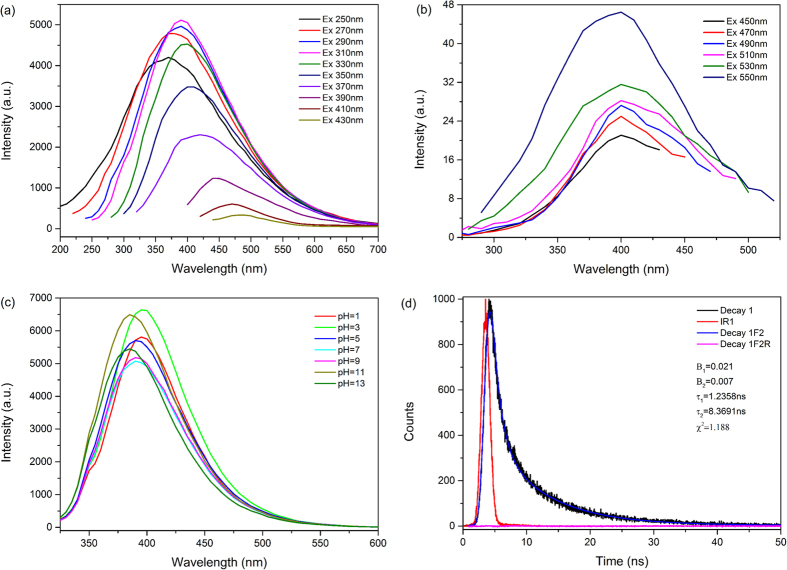
Furthermore, the phenomenon of excitation dependent was clearly observed in Fig. 6(a). The emission spectra were sensitive to the excitation wavelength and the emission peak varied from 370 nm to 490 nm as the excitation wavelength increased from 250 nm to 430 nm. When the excitation wavelength increased from 250 to 310 nm, the emission peak become stronger and redshift. When excited from 310 to 430 nm, the emission peak kept redshift with remarkably decreased intensities. The excitation-dependent and redshift optical properties were similar to previous studies37,38,39. As shown in Fig. 6(b), the up-conversion fluorescence emission phenomenon was observed that when as-prepared CDs were excited by longer wavelength (450–550 nm), shorter wavelength (400 nm) was emitted, which may be caused by the two or multiphoton active process40. Figure 6(c) illustrated that the optical responses of the as-prepared CDs with pH values ranging from 1 to 13. It was clearly that PL emission wavelength of as-prepared CDs were stable in the pH range of 5 to 9. In strong acidic condition, the emission wavelength evolved to the longer wavelength, while moved to shorter wavelength in strong basic solution, implying that the as-prepared CDs were very sensitive to acid/base solvent. These tunable fluorescence emission properties of as-prepared CDs were particular attractive for sensing, in vivo bioimaging and synthesizing novel photocatalysts7,11,40.
Figure 6.
(a) Excitation-dependent PL emission spectra and (b) Up-conversion PL emission spectra of as-prepared CDs. (c) PL spectra of as-prepared CDs dispersed in different pH solutions and (d) Time-resolved PL decay and fitting curves for as-prepared CDs.
Fluorescence decay curve and the exponential fitting curve of as-prepared CDs were showed in Fig. 6(d). The fitting formula is:
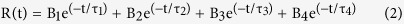 |
where t is time, B1, B2, B3and B4 are fractional intensities, τ1, τ2, τ3 and τ4 are fluorescence lifetime. The parameters generated from iterative reconvolution of the decay with the instrument response function (IRF) were listed in the inset of Fig. 6(d). And according to formula:
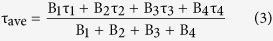 |
we calculated the amplitude-weighted average fluorescence lifetime (τave) of as-prepared CDs to be 6.0921 ns, which suggested that the as-prepared CDs are suitable for optoelectronic and biological application17,41. Additionally, the value of absolute quantum yield (QY) was measured to be 4.34%, which was comparable to the reported carbon dots2,42,43.
Cytotoxicity and cells imaging
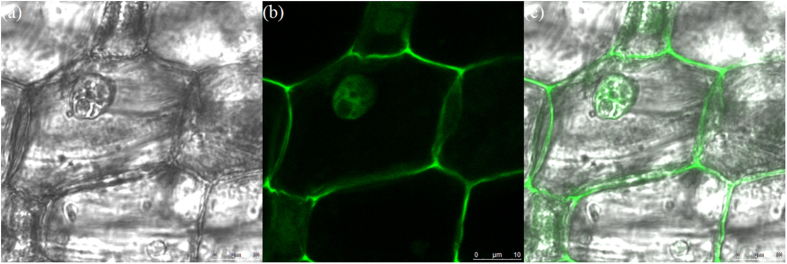
CCK-8 assay was used to evaluate the cytotoxicity of the CDs to living cells. As shown in Fig. 7, the cell viability were estimated to be greater than 80% after 24 h of incubation upon addition of the as-prepared CDs over concentration ranged from 0 to 200 μg/mL, which confirmed the low toxicity, excellent biocompatibility and safety in vitro and in vivo applications of the as-prepared CDs6,44. Furthermore, in order to investigate the imaging capability, we induced the as-prepared CDs into onion root tip cells. The confocal laser scanning microscopy (CLSM) images of onion root tip cells labeled with the as-prepared CDs for 12 h at 25 °C were shown in Fig. 8. Obviously, the as-prepared CDs have penetrated into the cells and labeled both the cell nuclei and membrane and shown significant green fluorescence emission under the 405 nm wavelength excitation. This result indicated that the as-prepared CDs possessed good biocompatibility and could serve in bioimaging and drug delivery applications45,46.
Figure 7. Cell viability of LLC cells in the presence of different concentration of as-prepared CDs.
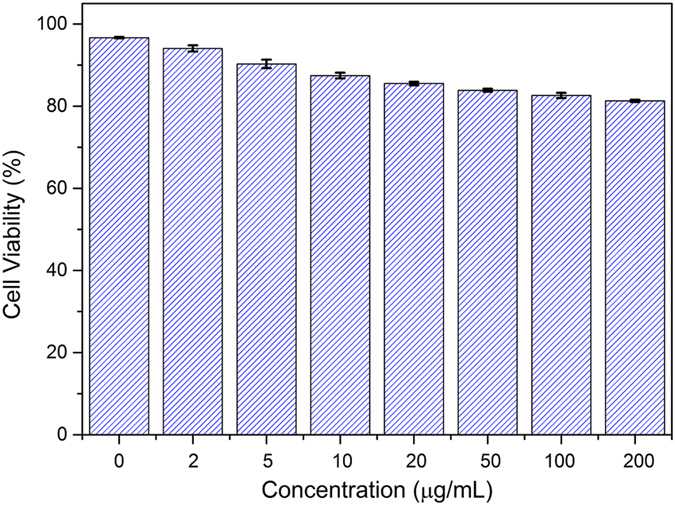
Figure 8.
CLSM images of onion root tip cells labeled with as-prepared CDs at 25 °C for 12 h (a) Bright field, (b) Fluorescent image excited with a 405 nm laser and (c) Overlap of the corresponding bright field image and the fluorescence image.
Conclusions
In summary, the amino-functionalized CDs have been successfully prepared by a simple carbonization approach. This green, cheap and convenient process represented a potential advance for large-scale production. The as-prepared CDs possessed desirable amino function group on the surface and exhibited bright luminescence with absolute quantum yield (QY) of 4.34%, excitation-, pH-dependent and up-conversion fluorescence behaviors. Furthermore, the as-prepared CDs showed good stability, less toxicity, biocompatibility and excellent fluorescence imaging ability. In the future, the as-prepared CDs could be utilized promisingly in optoelectronic materials, photocatalysts, cellular imaging and drug delivery applications.
Methods
Materials
All chemicals, including chitosan (Sinopharm Chemical Reagent Co., Ltd. China), sulfuric acid (H2SO4; Beijing Chemical Works, China), sodium hydroxide (NaOH; Beijing Chemical Works, China) and phosphate buffer saline (PBS; Leagene, China), were in analytical grade and used without further purification. Deionized water was used throughout the whole experiment.
Preparation of CDs
CDs were prepared via carbonization of chitosan at 300 °C for 2 h at a heating rate of 5 °C/min under a nitrogen atmosphere. After cooled down to room temperature, the dark brown products were mechanically ground to fine powders. After that, obtained powders were dispersed in water, and the CDs were collected by filtrating for further characterization and use.
Characterization
Transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) micrographs were recorded on JEM-2100F (JEOL, Japan) operating at 200 kV. Atomic-force microscopy (AFM) investigation was done by a scanning probe microscope (BRUKER MULTIMADE 8, Bruker, USA). The crystal structure of samples was analyzed by an X-ray diffractometer (SHIMADZU XRD-6000, Shimadzu corporation, Japan) with Cu radiation (λ = 0.154 nm) at 40 kV and 40 mA. The Raman spectrum was recorded on Perkin Elmer Spectrum GX (Perkin Elmer, UK) at room temperature. Fourier-transform infrared (FT-IR) spectroscopy data were acquired using a Perkin Elmer Spotlight 400 imaging system (Perkin Elmer, UK). The X-ray photoelectron spectroscopy (XPS) measurements were performed on Thermo Scientific Escalab 250Xi XPS system (Thermo Fisher Scientific Ltd., UK) using Al Kα source. The temperature programmed desorption of CO2 (CO2-TPD) measurement was carried out on an AutoChem II 2920 analyser (Micrometrics, USA) fitted with a thermal conductivity detector (TCD). Ultraviolet-visible (UV-Vis) absorption spectra were carried out with UV2310II spectrometer (Techcomp, China). The fluorescence spectra were measured with a fluorescence spectrometer (F-7000, Hitachi High Tech CO., Japan). Fluorescence decay curves and photoluminescence (PL) absolute quantum yield (QY) for as-prepared CDs were measured at room temperature using an Edinburgh FLS 980 spectrometer (Edinburgh Instruments, UK) equipped with an integrating sphere under 310 nm excitation from a 450 W xenon lamp. The absolute fluorescence quantum yield was calculated as follows:
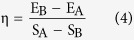 |
where η is the absolute fluorescence quantum yield, the indices “A” and “B” referred to the reference sample (deionized water) and carbon dots aqueous solution, separately, and SA, SB, EA and EB referred to the integral of the scans. The specific calculation was handled by using the Edinburgh FLS 980 software package (Quantum Yield Wizard)47,48.
Effect of pH on the PL of as-prepared CDs
The as-prepared CDs aqueous solutions were adjusted to the various target pH values by adding diluted H2SO4 or NaOH solution, then the corresponding fluorescence spectra were measured upon excitation at 310 nm separately.
Cytotoxicity evaluation
The cytotoxicity of as-prepared CDs was evaluated by a CCK-8 assay44. Lewis Lung Carcinoma (LLC) cells were seeded at a density of 4 × 103 cells per well in 180 mL culture medium and incubated for 24 h. Then, the cells were treated with different concentrations of as-prepared CDs solution at 37 °C in a humidified incubator with 5% CO2 for 24 h. Then, 20 mL of CCK-8 solution was added to each well and incubated for 1 h at 37 °C. The absorbance at 450 nm was measured using an infinite M200 microplate spectrophotometer (Tecan, Switzerland). The cell viability was expressed as percentage of absorbance relative to control, and the control was obtained in the absence of as-prepared CDs.
Cell imaging
Onion root tip cells were incubated with as-prepared CDs solution for 12 h at 25 °C, then washed with PBS for several times. The cellular localization was visualized using a confocal laser scanning microscope (SP-5, Leica, Germany) with laser excitation of 405 nm49,50.
Additional Information
How to cite this article: Liu, X. et al. Simple Approach to Synthesize Amino-Functionalized Carbon Dots by Carbonization of Chitosan. Sci. Rep. 6, 31100; doi: 10.1038/srep31100 (2016).
Acknowledgments
We are grateful for the financial support of this research from the Fundamental Research Funds for the Central Universities (No. 2016JX03), Natural Science Foundation of China (No. 31470606), China National Funds for Distinguished Young Scientists (31225005) and China Ministry of Education (No. 111).
Footnotes
Author Contributions X.Z. and X.L. conceived the project and designed the experiments; X.L. wrote the main manuscript text; X.L. and J.P. performed the experiments and analysed the data; X.Z. and F.X. supervised and directed the project; All authors reviewed the manuscript.
References
- Zhu S. et al. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed Engl. 52, 3953–3957 (2013). [DOI] [PubMed] [Google Scholar]
- Wang C., Sun D., Zhuo K., Zhang H. & Wang J. Simple and green synthesis of nitrogen-, sulfur-, and phosphorus-co-doped carbon dots with tunable luminescence properties and sensing application. RSC Adv. 4, 54060–54065 (2014). [Google Scholar]
- Sun Y. P. et al. Host-Guest Carbon Dots for Enhanced Optical Properties and Beyond. Sci Rep. 5, 12354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhao Y. & Zhang Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sensors Actua B-Chem. 196, 647–652 (2014). [Google Scholar]
- Jamieson T. et al. Biological applications of quantum dots. Biomaterials. 28, 4717–4732 (2007). [DOI] [PubMed] [Google Scholar]
- Luo P. G. et al. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B. 1, 2116–2127 (2013). [DOI] [PubMed] [Google Scholar]
- Li H., Kang Z., Liu Y. & Lee S.-T. Carbon nanodots: synthesis, properties and applications. J. Mater. Chem. 22, 24230–24253 (2012). [Google Scholar]
- Cao L. et al. Carbon dots for multiphoton bioimaging. J Am Chem Soc. 129, 11318–11319 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo T. C. et al. Hybrid heterostructures based on hematite and highly hydrophilic carbon dots with photocatalytic activity. Appl Catal B-Environ. 182, 204–212 (2016). [Google Scholar]
- Wang Y. & Hu A. Carbon quantum dots: synthesis, properties and applications. J. Mater. Chem. C. 2, 6921–6939 (2014). [Google Scholar]
- Lim S. Y., Shen W. & Gao Z. Carbon quantum dots and their applications. Chem Soc Rev. 44, 362–381 (2015). [DOI] [PubMed] [Google Scholar]
- Liu S.-S. et al. Hair-derived carbon dots toward versatile multidimensional fluorescent materials. J. Mater. Chem. C. 2, 6477–6483 (2014). [Google Scholar]
- Guo X., Wang C. F., Yu Z. Y., Chen L. & Chen S. Facile access to versatile fluorescent carbon dots toward light-emitting diodes. Chem. Commun. 48, 2692–2694 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. One-step synthesis of robust nitrogen-doped carbon dots: acid-evoked fluorescence enhancement and their application in Fe3 + detection. J. Mater. Chem. A 3, 17747–17754 (2015). [Google Scholar]
- Sahu S., Behera B., Maiti T. K. & Mohapatra S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun (Camb). 48, 8835–8837 (2012). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem Commun (Camb). 48, 380–382 (2012). [DOI] [PubMed] [Google Scholar]
- Xue M., Zou M., Zhao J., Zhan Z. & Zhao S. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B. 3, 6783–6789 (2015). [DOI] [PubMed] [Google Scholar]
- Zhu L., Yin Y., Wang C.-F. & Chen S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J. Mater. Chem. C. 1, 4925–4932 (2013). [Google Scholar]
- Song Y., Zhu S. & Yang B. Bioimaging based on fluorescent carbon dots. RSC Advances. 4, 27184–27200 (2014). [Google Scholar]
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 31, 603–632 (2006). [Google Scholar]
- Kumar M. N. V. R. A review of chitin and chitosan applications. React. Functi. Polym. 46, 1–27 (2000). [Google Scholar]
- Zhang Y., Xue C., Xue Y., Gao R. & Zhang X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr Res. 340, 1914–1917 (2005). [DOI] [PubMed] [Google Scholar]
- Wang F., Kreiter M., He B., Pang S. & Liu C. Y. Synthesis of direct white-light emitting carbogenic quantum dots. Chem Commun (Camb). 46, 3309–3311 (2010). [DOI] [PubMed] [Google Scholar]
- Wang W., Li Y., Cheng L., Cao Z. & Liu W. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B. 2, 46–48 (2014). [DOI] [PubMed] [Google Scholar]
- Qu S., Wang X., Lu Q., Liu X. & Wang L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew Chem Int Ed Engl. 51, 12215–12218 (2012). [DOI] [PubMed] [Google Scholar]
- Machado C. E. et al. Influence of Inert and Oxidizing Atmospheres on the Physical and Optical Properties of Luminescent Carbon Dots Prepared through Pyrolysis of a Model Molecule. Chem. Eur. J. 22, 4556–4563 (2016). [DOI] [PubMed] [Google Scholar]
- Amama P. B., Cola B. A., Sands T. D., Xu X. & Fisher T. S. Dendrimer-assisted controlled growth of carbon nanotubes for enhanced thermal interface conductance. Nanotechnology. 18, 385303 (2007). [Google Scholar]
- Liu S. et al. Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv Mater. 24, 2037–2041 (2012). [DOI] [PubMed] [Google Scholar]
- Madden D. & Curtin T. Carbon dioxide capture with amino-functionalised zeolite-β: A temperature programmed desorption study under dry and humid conditions. Microporous Mesoporous Mater. 228, 310–317 (2016). [Google Scholar]
- Knowles G. P., Graham J. V., Delaney S. W. & Chaffee A. L. Aminopropyl-functionalized mesoporous silicas as CO2 adsorbents. Fuel Process Technol. 86, 1435–1448 (2005). [Google Scholar]
- Amornvadee Veawab P. T. & Amit Chakma. Corrosion Behavior of Carbon Steel in the CO2 Absorption Process Using Aqueous Amine Solutions. Ind. Eng. Chem. Res. 38, 3917–3924 (1999). [Google Scholar]
- Xiao D., Yuan D., He H. & Lu J. Microwave-assisted one-step green synthesis of amino-functionalized fluorescent carbon nitride dots from chitosan. Luminescence. 28, 612–615 (2013). [DOI] [PubMed] [Google Scholar]
- Wu Q., Li W., Tan J., Wu Y. & Liu S. Hydrothermal carbonization of carboxymethylcellulose: One-pot preparation of conductive carbon microspheres and water-soluble fluorescent carbon nanodots. Chem. Eng. J. 266, 112–120 (2015). [Google Scholar]
- Li X., Rui M., Song J., Shen Z. & Zeng H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 25, 4929–4947 (2015). [Google Scholar]
- Luo P. G. et al. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 4, 10791–10807 (2014). [Google Scholar]
- Wang C. et al. A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon. 82, 87–95 (2015). [Google Scholar]
- Hola K. et al. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO− induced red-shift emission. Carbon. 70, 279–286 (2014). [Google Scholar]
- Pan D. et al. Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem Commun (Camb). 46, 3681–3683 (2010). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sensors Actua B-Chem. 190, 516–522 (2014). [Google Scholar]
- Zhu S. et al. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to Up-Conversion Bioimaging Applications. Adv. Funct. Mater. 22, 4732–4740 (2012). [Google Scholar]
- Peng J. et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 12, 844–849 (2012). [DOI] [PubMed] [Google Scholar]
- Liu H., Ye T. & Mao C. Fluorescent carbon nanoparticles derived from candle soot. Angew Chem Int Ed Engl. 46, 6473–6475 (2007). [DOI] [PubMed] [Google Scholar]
- Ling Y. et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv Mater. 28, 305–311 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Carbon-dots derived from nanodiamond: photoluminescence tunable nanoparticles for cell imaging. J. Colloid Interface Sci. 397, 39–44 (2013). [DOI] [PubMed] [Google Scholar]
- Ray Chowdhuri A., Tripathy S., Haldar C., Roy S. & Sahu S. K. Single step synthesis of carbon dot embedded chitosan nanoparticles for cell imaging and hydrophobic drug delivery. J. Mater. Chem. B. 3, 9122–9131 (2015). [DOI] [PubMed] [Google Scholar]
- Huang C.-L. et al. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B. 3, 651–664 (2015). [DOI] [PubMed] [Google Scholar]
- Tang M., Huang Y., Wang Y. & Fu L. An ytterbium complex with unique luminescence properties: detecting the temperature based on a luminescence spectrum without the interference of oxygen. Dalton Trans. 44, 7449–7457 (2015). [DOI] [PubMed] [Google Scholar]
- Dorneanu P. P. et al. Solvent effects on the photophysical properties of poly[1,4-dihydroxyanthraquinoneimine-1,3-bis(phenylene-ester-methylene)tetramethyldisiloxane]. Spectrochim. Acta, Part A. 134, 218–224 (2015). [DOI] [PubMed] [Google Scholar]
- Chen B. et al. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst. 138, 946–951 (2013). [DOI] [PubMed] [Google Scholar]
- Li F. et al. Mg/N double doping strategy to fabricate extremely high luminescent carbon dots for bioimaging. RSC Adv. 4, 3201–3205 (2014). [Google Scholar]