Abstract
Aims
SYNARR-Flash study (Monitoring of SYNcopes and/or sustained palpitations of suspected ARRhythmic origin) is an international, multicentre, observational, prospective trial designed to evaluate the role of external 4-week electrocardiogram (ECG) monitoring in clinical work-up of unexplained syncope and/or sustained palpitations of suspected arrhythmic origin.
Methods and results
Consecutive patients were enrolled within 1 month after unexplained syncope or palpitations (index event) after being discharged from emergency room or hospitalization without a conclusive diagnosis. A 4-week ECG monitoring was obtained by external high-capacity loop recorder (SpiderFlash-T®, Sorin) storing patient-activated and auto-triggered tracings. Diagnostic monitorings included (i) conclusive events with reoccurrence of syncope or palpitation with concomitant ECG recording (with/without arrhythmias) and (ii) events with asymptomatic predefined significant arrhythmias (sustained supraventricular or ventricular tachycardia, advanced atrio-ventricular block, sinus bradycardia <30 b.p.m., pauses >6 s). SYNARR-Flash study enrolled 395 patients (57.7% females, 56.9 ± 18.7 years, 28.1% with syncope, and 71.9% with palpitations) from 10 European centres. For syncope, the 4-week diagnostic yield was 24.5%, and predictors of diagnostic events were early start of recording (0–15 vs. >15 days after index event) (OR 6.2, 95% CI 1.3–29.6, P = 0.021) and previous history of supraventricular arrhythmias (OR 3.6, 95% CI 1.4–9.7, P = 0.018). For palpitations, the 4-week diagnostic yield was 71.6% and predictors of diagnostic events were history of recurrent palpitations (P < 0.001) and early start of recording (P = 0.001).
Conclusion
The 4-week external ECG monitoring can be considered as first-line tool in the diagnostic work-up of syncope and palpitation. Early recorder use, history of supraventricular arrhythmia, and frequent previous events increased the likelihood of diagnostic events during the 4-week external ECG monitoring.
Keywords: External loop recorder, Syncope, Palpitation, Arrhythmia diagnosis, Ambulatory ECG monitoring
What's new?
The early use of external 4-week electrocardiogram (ECG) recorders in unexplained syncope of suspected arrhythmic origin proved to be a feasible ‘stepwise’ strategy, starting the recording as soon as possible after an event in order to maximize the diagnostic yield of external ECG monitoring.
In patients with unexplained syncope of suspected arrhythmic origin, in whom prolonged ECG monitoring is considered appropriate according to current guidelines, the 4-week external ECG monitoring should be utilized as first step, while more expensive and minimally invasive implantable loop recorder (ILR) should be reserved to those cases who remained undiagnosed after the 4-week external monitoring.
In patients with unexplained palpitation, the 4-week external ECG monitoring can be considered as first-line diagnostic tool, providing a conclusive diagnosis in most cases, avoiding useless repetition of standard 24 h Holter monitoring, with longer monitoring by ILR would be required only in few selected cases.
Introduction
The diagnosis of unexplained syncopal episodes and sustained palpitations remains a difficult task in clinical cardiology.1–6 Standard 24 h Holter monitoring has a low diagnostic yield, while implantable loop recorder (ILR) has a higher diagnostic yield, but it is expensive and mildly invasive, making its role in diagnostic work-up of unexplained syncope and palpitations questionable.7–10
The utility of external prolonged electrocardiogram (ECG) monitoring in work-up of syncope is still undefined. The few available studies, generally single-centre and retrospective, provided conflicting results, mainly due to heterogeneity of patient populations and devices capabilities. In sustained palpitations, the external loop recorders (ELRs) utility was more established.5 Earlier ELRs with relatively low-storage capacity and brief patient-activated ECG tracings had limited usefulness in syncope or asymptomatic arrhythmias.1,7,11–14 Recently, newer ELRs with auto-trigger capabilities showed 30% diagnostic yield for syncope and 75% for palpitations.15 External ECG recorders were also utilized to evaluate the burden of asymptomatic atrial fibrillation (AF), as in cryptogenic transient ischaemic attacks or after transcatheter ablation.16,17
Syncope and palpitations tend to occur in clusters, with higher reoccurrence rate early after an event, thus an early use of long-term ECG monitoring may increase the likelihood of clinical diagnosis.1,10
The SYNARR-Flash study (Monitoring of SYNcopes and/or sustained palpitations of suspected ARRhythmic origin; clinicaltrial.gov NCT02253134) is the first international, multicentre, prospective trial designed to evaluate the feasibility and usefulness of external prolonged ECG monitoring in the early clinical work-up of unexplained syncope and/or sustained palpitations of suspected arrhythmic origin.
Methods
Study design
At each enrolling centre, consecutive patients screened for enrolment had to meet both the following inclusion criteria: (i) recent (within 1 month) episode of syncope or sustained palpitations (index event), after being discharged from emergency room or hospitalization without a conclusive diagnosis, and (ii) suspected arrhythmic origin according to the clinical features defined in the 2009 Syncope Guidelines, including the presence of cardiovascular disease or channelopathy, family history of unexplained sudden death, or abnormal ECG findings.1
Syncope was defined as complete loss of consciousness with spontaneous recovery, while sustained palpitations as long-lasting sensation of irregular or fast heart rhythm. Abnormal ECG findings included atrio-ventricular conduction disorders [first-degree atrio-ventricular block (AVB) I, second-degree AVB II type Mobitz 1, left or right ventricular bundle branch block (LBBB/RBBB)], supraventricular rhythm disorders [sinus arrest or pauses <3 s, sinus bradycardia >40 b.p.m., sinus tachycardia >100 b.p.m., paroxysmal AF, frequent premature atrial contractions (PACs) (>10/min), chronic AF, paroxysmal atrial flutter/tachycardia], and ventricular rhythm disorders [frequent premature ventricular contractions (PVCs) (>10/min), couplets, non-sustained VT] (Table 1).
Table 1.
Demographic characteristics
| Parameters (number, %) | ITT population with syncope n = 110 |
ITT population with palpitation n = 282 |
P-value |
|---|---|---|---|
| Demographics | |||
| Age, mean (±SD) | 65.1 (±17.2) | 53.9 (±18.4) | <0.001 |
| Male gender | 64 (59.3%) | 101 (35.8%) | <0.001 |
| NYHA, n | 68 | 191 | |
| I/II | 66 (97.1%) | 190 (99.5%) | 0.170 |
| III/IV | 2 (2.9%) | 1 (0.5%) | |
| Previous diagnosis test, n | 106 | 262 | |
| 24 h Holter recording | 64 (60.4%) | 159 (60.7%) | 0.956 |
| HUT | 21 (19.8%) | 13 (5.0%) | <0.001 |
| CSM | 27 (25.5%) | 10 (3.8%) | <0.001 |
| Exercise stress test | 12 (11.3%) | 41 (15.6%) | 0.284 |
| History of previous rhythm disorders | |||
| Supraventricular rhythm disordersa | 40 (57.1%) | 103 (66.0%) | 0.200 |
| AV conduction disordersb | 32 (45.7%) | 63 (40.4%) | 0.453 |
| Ventricular rhythm disordersc | 29 (41.4%) | 65 (41.7%) | 0.973 |
| History of cardiovascular disease, n | 70 | 155 | |
| Systemic hypertension | 41 (58.6%) | 68 (43.9%) | 0.041 |
| Coronary artery disease | 8 (11.4%) | 12 (7.7%) | 0.368 |
| Cerebrovascular accident | 8 (11.4%) | 7 (4.5%) | 0.054 |
| Ischaemic cardiomyopathy | 5 (7.1%) | 9 (5.8%) | 0.701 |
| Previous myocardial infarction | 3 (4.3%) | 6 (3.9%) | 1.000 |
| Family history of sudden cardiac death | 9 (8.2%) | 7 (2.5%) | 0.006 |
| Cardiovascular medication at the time of the test | 82 (74.5%) | 190 (67.4%) | 0.166 |
SD, standard deviation; NYHA, New York Heart Association functional class; AV, atrio-ventricular.
aIncludes sinus arrest/pauses <3 s, sinus bradycardia >40 b.p.m., sinus tachycardia, paroxysmal AF, frequent PACs (>10/min), chronic AF, and paroxysmal atrial flutter/tachycardia.
bIncludes AVB I, AVB II Mobitz 1, LBBB, and RBBB.
cInclude frequent PVCS (>10/min), couplets, and non-sustained VT.
By study inclusion criteria, all patients were enrolled within 30 days after the index event, after being discharged from emergency room or hospitalization without a conclusive diagnosis. At enrolment, patient medical history was collected, including a detailed report of the index event (syncope or palpitation) and the history of events in the preceding year and of the previously performed diagnostic tests.
Patients were instructed by the study nurse on how to use the recorder and to report symptoms in the diary. Patients had to wear the recorder for 4 weeks, and a clinic visit was scheduled at the end of the recording period (M1). Patients had to contact the centre if any symptom occurred before M1 (in case an additional clinic visit was scheduled) or if any technical problem occurred or the recording stopped prematurely (in order to re-initiate the recording).
At M1, patients were interviewed, diaries were collected, and information was cross-checked. Electrocardiogram data were uploaded to the server. Clinical and device information, including events, device acceptance, device malfunction, adverse events, and medications, were reported in case report forms (CRFs).
The study was conducted in accordance with Guidelines for Good Clinical Practice and Declaration of Helsinki and approved by relevant local ethics committees. All enrolled patients provided written informed consent to study participation.
Modality of ECG recording
At enrolment, a prolonged high-capacity ELR (SpiderFlash-T®, Sorin Group SRL, Saluggia, Italy—sized 75 × 50 × 19 mm, powered by lithium battery) was provided to each patient. The three-wire recorder was connected to the chest using disposable adhesive electrodes, which the patient had to change daily. The recorder was carried in a disposable bag placed around the neck.
Recorders store two leads ECG tracings on a high-capacity removable digital card capable of up to 40 day monitoring. Three recording modalities are available: (i) manual patient activation in case of symptoms; (ii) automatic activation at predefined intervals; and (iii) auto-trigger activation at preselected rhythm disturbances, such as pauses or bradycardia or supraventricular tachycardia (SVT) and ventricular tachycardia (VT).
Event classification and computation of diagnostic yield
All ECG monitorings were reviewed by two separate investigators (E.T.L. and A.M.) and categorized based on both patient diary and interview and ECG data, as follows.
Diagnostic monitorings include the following: Non-diagnostic monitorings include the following:
Conclusive events, when syncope or palpitations were reported and a readable ECG recording was available at the time of symptom (independently of the presence or absence of arrhythmias);
Significant events, when the following predefined arrhythmias were detected by auto-trigger function in the absence of reported symptoms: (i) advanced AVB (third-degree AVB or second-degree AVB Mobitz type 2), sinus bradycardia (<30 b.p.m.), pauses ≥6 s; (ii) fast sustained supraventricular tachycardia (SVT), AF, or flutter (AFL) (rate >180 b.p.m., duration >3 min); and (iii) non-sustained ventricular tachycardia (NSVT, duration >10 s) or sustained ventricular tachycardia (>30 s). Those arrhythmias were considered diagnostic even in the absence of recurrent syncope according to the 2009 Syncope Guidelines.1
Suggestive events, when the following predefined arrhythmias were detected by auto-trigger function, in the absence of reported symptoms: (i) sinus bradycardia (30–40 b.p.m.), 3–6 s pauses; (ii) brief burst of SVT, AF, or AFL (15–180 s); and (iii) NSVT <10 s;
Negative monitorings: (i) monitorings without event recurrence reported in the patient diary and without recording of asymptomatic arrhythmia by auto-trigger function; (ii) monitorings with recurrence of symptoms different from the index event; and (iii) monitorings with recurrence of syncope or palpitations, but without available ECG recording.
Statistical analysis
All analyses were performed on the intention-to-treat (ITT) population, both overall and separately per index event (syncope or palpitations). Since the distribution of the data was normal, continuous data were expressed as mean ± standard deviation (SD), while categorical variables as frequencies and percentages and differences in categorical variables were tested by χ2 test or by Fisher exact test as appropriate.
The time-to-first occurrence of a diagnostic event during 4-week monitoring was analysed by Kaplan–Meier survival analysis, and survival curves of patients with syncope and palpitations were compared by log-rank test. Actuarial diagnostic yields for diagnostic events were retrieved from Kaplan–Meier statistics and compared by χ2 analysis when applicable.
Predictors of occurrence of diagnostic events during the 4-week monitoring were identified by multivariable Cox proportional-hazard model, after proportional-hazard assumptions were verified. A stepwise procedure was applied: first, univariable analyses determined statistically significant variables at 20%, and then significant variables were introduced in the multivariable model.
Two-sided P values of <0.05 were considered significant in all evaluations. Analyses were performed using SAS software release 9.2 (SAS Institute, Cary, NC, USA).
Results
Study population
The SYNARR-Flash study enrolled 395 patients (42.3% males, age 56.9 ± 18.7 years) from 10 centres in five countries between August 2010 and June 2013. As index event information was unavailable for three patients, the ITT population included 392 patients; 282 patients (71.9%) enrolled for palpitations and 110 (28.1%) for syncope.
Patients with syncope were older, more frequently males, with previous history of cardiovascular disease, family history of sudden death, hypertension, and use of cardiovascular medication (Table 1). Previous diagnostic tests, when available, were reported negative in all patients.
Duration of ECG monitoring
The mean duration of ECG monitoring was 23.0 ± 8.1 days, similar for syncope and palpitations. In 22 patients, the recording was stopped for the occurrence of a major event. In four patients (1%), the recording was interrupted due to intolerance to electrodes, while two patients (0.5%) withdrew from the study. Skin reactions to electrodes were reported in 12 patients (3%), but all were defined as non-serious and solved spontaneously.
Findings in patients with syncope
Twenty-seven patients with prior syncope (24.5%) had a diagnostic event within the 4-week monitoring. Conclusive events with ECG-documented syncope occurred in 11 patients (10%, Table 2): in 6 patients, supraventricular bradyarrhythmias were recorded during syncope, while in 5 cases, regular sinus rhythm or sinus tachycardia was recorded (Table 2). Auto-trigger function identified asymptomatic significant arrhythmias in 16 patients (14.5%, Table 2).
Table 2.
Electrocardiogram findings during monitoring in patients with syncope and palpitation
| Patients | Syncope (n = 110) |
Palpitation (n = 282) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Diagnostic tests | 27 | 24.5 | 202 | 71.6 |
| Conclusive events (recurrence reported on patient diary) | 11 | 10.0 | 193 | 68.4 |
| Non-arrhythmic (sinus rhythm, sinus tachycardia) | 5 | 4.5 | 66 | 23.4 |
| Arrhythmic | 6 | 5.5 | 127 | 45.0 |
| Pauses (≥6 s), AVB 3 or AVB 2 Mobitz 2 | 4 | 3.6 | 1 | 0.4 |
| Pauses (3–6 s), sinus bradycardia | 2 | 1.8 | 7 | 2.5 |
| Paroxysmal AF, AFL (>3 min) | 0 | 0.0 | 22 | 7.8 |
| Paroxysmal SVT (>3 min) | 0 | 0.0 | 15 | 5.3 |
| Non-sustained SVT (15 s–3 min) | 0 | 0.0 | 40 | 14.2 |
| NSVT <10 s | 0 | 0.0 | 5 | 1.8 |
| NSVT >10 s | 0 | 0.0 | 1 | 0.4 |
| Frequent VPBs or APBs | 0 | 0.0 | 36 | 12.7 |
| Asymptomatic significant arrhythmias | 16 | 14.5 | 9 | 3.2 |
| Fast sustained AF/AFL/SVT >3 min >180 b.p.m. | 8 | 7.3 | 8 | 2.8 |
| Sinus bradycardia <30 b.p.m., Pauses ≥6 s, AVB 3 or AVB 2 Mobitz 2 | 5 | 4.5 | 1 | 0.4 |
| NSVT >10 s or sustained ventricular tachycardia >30 s | 3 | 2.7 | 0 | 0.0 |
| Non-diagnostic tests | 83 | 75.5 | 80 | 28.4 |
| Negative monitoring | 73 | 66.4 | 59 | 20.9 |
| Asymptomatic suggestive arrhythmias | 10 | 9.1 | 21 | 7.5 |
| Sinus bradycardia 30–40 b.p.m., sinus pauses 3–6 s | 5 | 4.5 | 11 | 3.9 |
| NSVT <10 s | 5 | 4.5 | 9 | 3.2 |
| AF/AFL/SVT 15 s–3 min | 0 | 0.0 | 1 | 0.4 |
AVB 2, AVB 3, atrio-ventricular block (Grade 2 or 3); AF, atrial fibrillation; AFL, atrial flutter; SVT, supraventricular tachycardia; NSVT, non-sustained ventricular tachycardia; VPBs, ventricular premature beats; APBs, atrial premature beats.
The actuarial diagnostic yield, calculated by the Kaplan–Meier cumulative occurrence of diagnostic events, was 13.2% at 1 week, 19.1% at 2 weeks, and 29.4% at 4 weeks (Figure 1).
Figure 1.
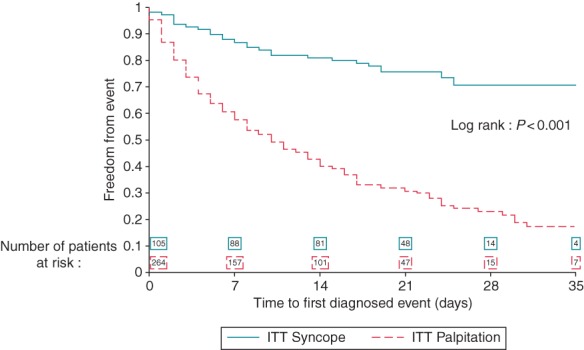
Kaplan–Meier analysis of recurrence-free rate in patients with syncope (continuous line) and palpitations (dashed line) of first diagnostic event during prolonged ECG monitoring (log rank: P< 0.001).
Auto-trigger function detected asymptomatic suggestive arrhythmias in 10 patients (9.1%, Table 2), although per definition were not included as diagnostic events. The cumulative yield including conclusive, significant, and suggestive events would be 33.6%.
Multivariable analyses to identify potential predictors of diagnostic events included age, gender, previous recording of supraventricular or ventricular arrhythmia, time from index event to enrolment (0–15 days vs. >15 days after index syncope), the number of events in preceding year, history of cardiovascular disease, and family history of sudden cardiac death. The only two significant predictors for diagnostic findings during the 4-week monitoring were (i) early start of monitoring after index event and (ii) previous history of supraventricular arrhythmias [sinus arrest or pauses <3 s, sinus bradycardia >40 b.p.m., sinus tachycardia >100 b.p.m., paroxysmal AF, frequent PACs (>10/min), chronic AF, paroxysmal atrial flutter/tachycardia—Table 3]. Monitoring was started within 15 days from index event in 74 patients: 4-week actuarial diagnostic yield was 33.0% in patients with early initiation compared with 15.6% in later initiation (P = 0.021, Figure 2A). History of supraventricular arrhythmias was present in 39 patients: 4-week actuarial diagnostic yield was 43.6% in patients with supraventricular arrhythmias compared with 21.3% in those without (P = 0.018, Figure 2B).
Table 3.
Predictors for diagnosis during the 4-week ECG monitoring
| Variables | P-value | Odds ratio | 95% Wald confidence limits |
||
|---|---|---|---|---|---|
| A—patients studied for syncope | |||||
| Time between index event and enrolment | 0–15 days vs. >15 days | 0.021 | 6.2 | 1.3 | 29.6 |
| History of supraventricular arrhythmiasa | Yes vs. No | 0.018 | 3.6 | 1.4 | 9.7 |
| B—patients studied for palpitations | |||||
| History of previous palpitations (in the previous 12 months) | 2–5 vs. ≤1 | 0.036 | 2.6 | 1.1 | 6.3 |
| 6–10 vs. ≤1 | 0.029 | 2.8 | 1.1 | 7.3 | |
| ≥11 vs. ≤1 | <0.001 | 4.3 | 2.0 | 9.4 | |
| Time between index event and enrolment | 0–7 days vs. 8–15 days | 0.004 | 3.0 | 1.4 | 6.4 |
| 0–7 days vs. >15 days | 0.031 | 2.3 | 1.1 | 4.9 | |
aSupraventricular arrhythmias include sinus arrest/pauses <3 s, sinus bradycardia >40 b.p.m., sinus tachycardia, paroxysmal AF, frequent PACs (>10/min), chronic AF, and paroxysmal atrial flutter/tachycardia.
Figure 2.
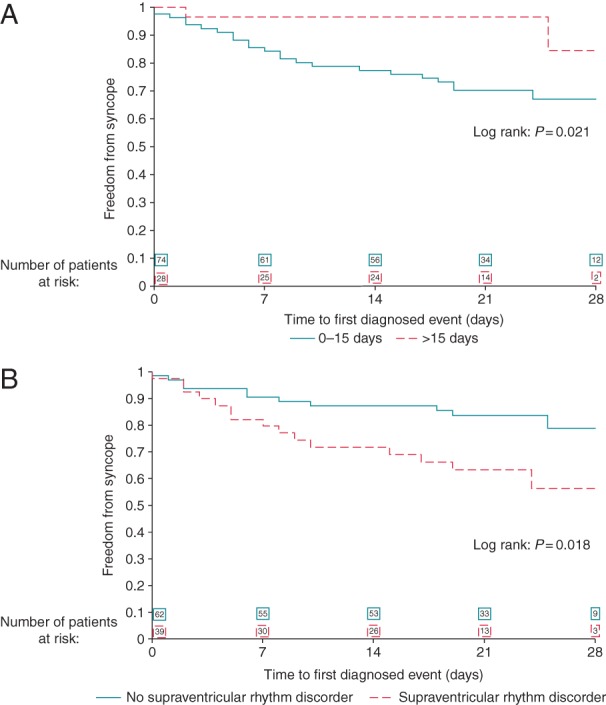
(A) Kaplan–Meier analysis of recurrence-free rate in patients with syncope enrolled at 0–15 days after the index syncope (continuous line) vs. >15 days after the index syncope (dashed line), OR = 6.222, 95% CI (1.309–29.573), P = 0.02 (see Table 3 for details). (B) Kaplan–Meier analysis of recurrence-free rate in patients with syncope without previous history of supraventricular rhythm disorder (continuous line) vs. patients with previous history of supraventricular rhythm disorder (dashed line), OR = 3.631, 95% CI (1.356–9.724), P = 0.01 (see Table 3 for details).
Findings in patients with palpitations
A total of 202 patients (71.6%) had a diagnostic event within the 4-week monitoring (Table 2). Conclusive events occurred in 193 patients (68.4%). Sinus rhythm or sinus tachycardia was present at patient-activated recording during palpitation in about two-thirds of the cases, while SVT, AF or AFL, bradycardia, pauses or NSVT were present in the remaining cases. Significant asymptomatic arrhythmias were detected by auto-trigger function in nine patients (3.2%).
Asymptomatic suggestive arrhythmias were detected by auto-trigger function in 21 patients (7.5%, Table 2), which per definition were not included as diagnostic events. The cumulative yield including conclusive, significant, and suggestive events would be 79.1%.
The Kaplan–Meier cumulative occurrence of diagnostic events was 42.4% at 1 week, 57.2% at 2 weeks, and 77.0% at 4 weeks (Figure 1). Previous frequent palpitations and early start of recording after the index event were significant predictors of diagnostic events (Table 3).
The diagnostic yield progressively increased with the number of palpitations during the year preceding the enrolment (Figure 3A). The diagnostic yield was 46.2% in patients with 1 or no palpitation, 68.1% in patients with 2–5 palpitations, 76.7% in patients with 6–10 palpitations, and 83.1% in patients with ≥11 palpitations (Figure 3A). The 4-week diagnostic yield was higher in patients starting the recording 0–7 days after the index event (Table 3, Figure 3B).
Figure 3.
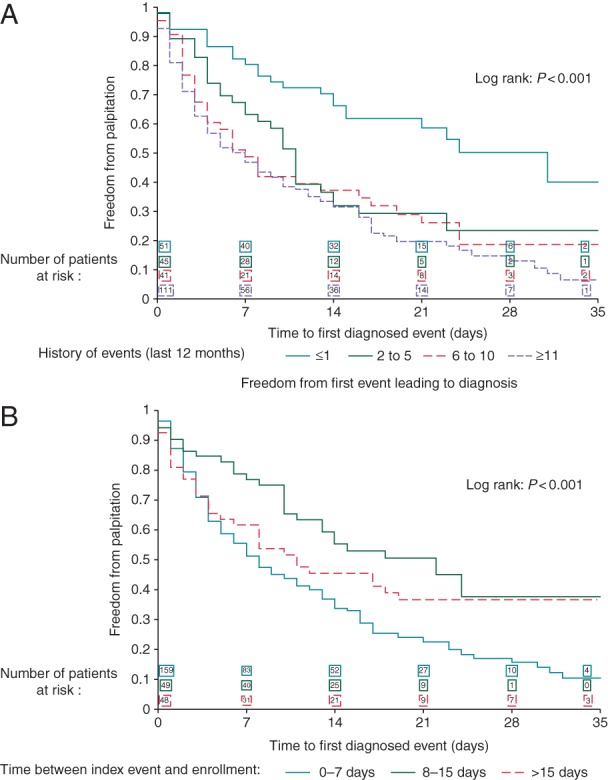
(A) Kaplan–Meier analysis of recurrence-free rate in patients with palpitations according to the previous number of events in the last 12 months (see Table 3 for details). (B) Kaplan–Meier analysis of recurrence-free rate in patients with palpitations according to the time between index event and enrolment (see Table 3 for details).
Discussion
The SYNARR-Flash study is the first international, multicentre, observational, prospective trial designed to prove the feasibility and usefulness of external prolonged ECG monitoring in early clinical work-up of unexplained syncope and/or sustained palpitations of suspected arrhythmic origin. In unexplained syncope, prolonged external ECG monitoring achieved a diagnosis in about one-third of the patients, and most diagnoses occurred in those who started the monitoring within 15 days from index syncope (Figure 2A) and in those with history of SVT (Figure 2B). These findings suggest two new clinical criteria in order to increase the diagnostic likelihood in unexplained syncope, namely early initiation of monitoring after an event and previous history of supraventricular arrhythmias. In unexplained palpitations, the 4-week actuarial diagnostic yield was 77.0%, increasing up to 81.3% in patients with previous frequent palpitations (Figure 3A), supporting its utility as first-line diagnostic tool in palpitation work-up.
Prolonged external ECG monitoring in unexplained syncope
All enrolled patients had unexplained syncope of suspected arrhythmic origin according to the criteria outlined in 2009 Guidelines for diagnosis and management of syncope.1 In this study, the actuarial diagnostic yield of the 4-week ECG monitoring was 29.4%, higher than the 1–10% diagnostic yield generally reported for 24 h Holter recording, and consistent with previous studies utilizing external long-term ECG monitoring, reporting diagnostic yields of 15–20% at 1 month.11–15,19
In our study, the 4-week diagnostic yield for syncope was similar or higher than in studies using ILR, when considering a similar monitoring period. In the PICTURE registry,9 the 3-month diagnostic yield was 19%, and in most studies, the diagnostic yield of ILR ranged from 30 up to 50%,7–10 indicating that syncope often remained unexplained even after 3-year ILR monitoring. These data support the concept that diagnostic findings in most patients with unexplained syncope occur relatively early after an index event, making prolonged external ECG monitoring sufficient to obtain a clinical diagnosis in most cases, with only a few selected patients requiring a much longer monitoring period.
In our study, arrhythmias were documented during syncope in about half of the patients, typically bradycardia or pauses leading to pacemaker implant, while in the remaining cases, no arrhythmias were detected, excluding an arrhythmic origin of syncope. These patients should then be referred for further clinical evaluation, possibly including carotid sinus massage (CSM) or head-up tilt test (HUT) or neurological work-up, while further prolonged ECG monitoring is not necessary.
Asymptomatic arrhythmias predefined as significant were detected by auto-trigger function in 16 patients (14.5%, Table 2). Significant arrhythmias were considered diagnostic even in the absence of recurrent syncope following the 2009 Syncope Guidelines, with evidence Class 1 Level C.1 This classification was specifically proposed by the International Study on Syncope of Unknown Etiology (ISSUE) investigators, with the aim to group the observations from prolonged ECG monitoring into homogeneous patterns in order to define an acceptable standard useful for future studies and clinical practice.20 Of note, this classification has been utilized in most studies with ILR, where unsuspected asymptomatic arrhythmias in the absence of syncope were generally considered diagnostic findings.7–10
To avoid potential bias, for this study, we utilized more stringent criteria for definition of significant asymptomatic arrhythmias than those proposed by the 2009 Syncope Guidelines. Asymptomatic suggestive arrhythmias were detected in 9.1% of patients (Table 2), which per definition were not included in the diagnostic yield, although some of these arrhythmias, such as 3–6 s pauses, would have been considered diagnostic according to 2009 Syncope Guidelines.1,20 Patients with asymptomatic suggestive arrhythmias seem to be the ideal candidates to continue prolonged ECG monitoring by ILR, to confirm or exclude the presence of significant arrhythmias.
In syncope, early initiation of recording after an index event was a significant predictor of diagnosis during the 4-week monitoring (Figure 2A). This is consistent with some preliminary observations suggesting that syncopal events occur in clusters,10 making it crucial to initiate the monitoring as soon as possible after an event.
A second significant predictor of diagnostic events was history of supraventricular arrhythmias, suggesting that patients with previous paroxysmal AF are reasonable candidates to prolonged ECG monitoring after an unexplained syncope. Of note, AF was not included among ECG criteria suggesting an arrhythmic origin for unexplained syncope in 2009 Guidelines.1
Prolonged external ECG monitoring in unexplained palpitations
In patients with unexplained sustained palpitations, the diagnostic yield of external monitoring was 42.4% at 1 week, 57.2% at 2 weeks, and 71.6% at 4 weeks. These data are consistent with previous studies,5,15,20,21 making the 4-week external ECG monitoring the first-choice tool in the diagnostic workflow of unexplained palpitations. Only patients with palpitations remaining unexplained after the 4-week monitoring may require further observation, either by ILR21 or by event recorders.22
The diagnostic yield was ∼50% at 1 week and 70% at 2 weeks in patients with previous frequent palpitations (Figure 3A) or in patients studied early after an index event (Figure 3B). These data support the utility of 1-week and 2-week external recordings, especially when utilized in high-risk patients and started early after an event.
Sinus rhythm or sinus tachycardia, or short episodes of SVT were observed during palpitations in most patients, while sustained and fast SVT, AF, or AFL were documented in 13% of the cases (Table 2). In fewer cases, sinus bradycardia and pauses were observed, often associated with dizziness or presyncope, besides palpitations. The clinical significance or the clinical management of such findings was beyond the scope of this study.
Although the main objective of prolonged external ECG monitoring was to correlate symptoms and arrhythmias, asymptomatic paroxysmal AF (either brief or sustained episodes) was detected in ∼10% of the patients with unexplained palpitations. These findings support the discrepancy between perceived symptoms and documented arrhythmias, and confirm that silent AF is relatively frequent even in patients with history of palpitations.4,5,16,17
Study limitations
One possible limitation was that not all centres contributed with the same amounts of patients, as three centres (Valld'Hebron, Milan, and Lavagna) enrolled ∼75% of the patients. However, no significant differences were observed among patients enrolled by the different centres.
The information provided in patients' diary was used to categorize for the absence or presence and kind of symptom (syncope or palpitation) at the time of arrhythmias. It is impossible to verify the accuracy of events reported in the diary by each patient, although the diary information was cross-checked at M1 clinic visits.
Predefined criteria were utilized to distinguish between ‘significant’ and ‘suggestive’ asymptomatic arrhythmias detected by auto-trigger function, based on recommendation from current guidelines1,20 and on our best clinical judgement. Due to lack of consensus on the minimum duration required to define an episode of paroxysmal AF,4,5 we utilized a restrictive criteria (duration >180 s) for significant events, while shorter events were only considered suggestive. Although the presence of fast and sustained supraventricular arrhythmias may suggest a syncope of arrhythmic origin, the precise mechanism provoking the syncope in the individual patient remains unknown, and the attribution of a specific aetiology for syncope in the individual patients is beyond the scope of this study. More stringent criteria were used also for pauses and NSVT, although some suggestive asymptomatic arrhythmias may represent meaningful findings in the diagnostic workflow and would have been considered diagnostic according to the 2009 Syncope Guidelines.1,20
Information about previous diagnostic tests, specifically CSM and HUT, was collected wherever available, but it was beyond the power of this study to verify why a specific diagnostic test was performed or not performed in the single patient. This study population was too small to draw any definitive conclusion on the possible correlation between ELR-negative and HUT-negative findings.
The cumulative diagnostic yield observed in this study both for syncope and palpitation may be an overestimation of the true clinical benefit. The capability of prolonged early monitoring to influence therapeutic decisions or to improve clinical outcomes was beyond of the scope of this study and remains to be demonstrated by an appropriately designed study.
Clinical implications and conclusions
The early use of external 4-week ECG recorders in unexplained syncope of suspected arrhythmic origin proved to be a feasible ‘stepwise’ strategy, starting the recording as soon as possible after an event in order to maximize the diagnostic yield of external ECG monitoring. In patients with unexplained syncope of suspected arrhythmic origin, in whom prolonged ECG monitoring is considered appropriate according to current guidelines, the 4-week external ECG monitoring should be utilized as first step, while more expensive and minimally invasive ILR should be reserved to those cases who remained undiagnosed after the 4-week external monitoring. In patients with unexplained palpitation, the 4-week external ECG monitoring can be considered as first-line diagnostic tool, providing a conclusive diagnosis in most cases, avoiding useless repetition of standard 24 h Holter monitoring, with longer monitoring by ILR would be required only in a few selected cases.22–25
The results of this study, which utilized external loop recording with auto-trigger function, may be extended to new systems of long-lasting external ECG recordings providing continuous ECG monitoring,21 that may have an even higher capability of detecting asymptomatic arrhythmias.
In patients with unexplained palpitation, the 4-week external ECG monitoring can be considered as first-line diagnostic tool, providing a conclusive diagnosis in most cases, avoiding useless repetition of standard 24 h Holter monitoring, with longer monitoring by ILR required only in few selected cases.
Funding
This study was supported by a research grant of SORIN GROUP, which supplied the SPIDERFLASH-T recorders free of charge to all enrolling centres and provided logistic support and assistance for database management and statistical analysis. R.W. was supported as a clinical researcher by the Fund for Scientific Research Flanders (FWO). Funding to pay the Open Access publication charges for this article was provided by SORIN GROUP.
Conflict of interest: none declared.
Acknowledgements
The authors thank all the doctors and register nurses from the 10 enrolling centres who contributed to patients' enrolment and clinic visits, and specially Dr J.M. Ormaetxe (Basurto Hospital, Bilbao), Dr M. Lopez-Gil (October 12 University Hospital, Madrid), Dr X. Sabate (Bellvitge University Hospital, Barcelona), and Dr N. Rivas (University Hospital Valld'Hebron and University Hospital Quiron Dexeus, Barcelona). The authors thank Mara Rolando, Andrea Pinciroli, Frederique Maneval, and Alessandra Pezzotta, from Sorin Group, for technical support and Franca Negrini, RN, for study assistance.
References
- 1. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB et al. . Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al. . ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 3. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann L, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am CollCardiol 2014;64:2246–80. 10.1016/j.jacc.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 5. Raviele A, Giada F, Bergfeldt L, Blanc JJ, Blomstrom-Lundqvist C, Mont L et al. . Management of patients with palpitation: a position paper from the European Heart Rhythm Association. Europace 2011;13:920–34. [DOI] [PubMed] [Google Scholar]
- 6. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J et al. . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 7. Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R et al. . Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009;11:671–87. [DOI] [PubMed] [Google Scholar]
- 8. Hong P, Sulke N. Implantable diagnostic monitors in the early assessment of syncope and collapse. Prog Cardiovasc Dis 2013;55:410–7. [DOI] [PubMed] [Google Scholar]
- 9. Edvardsson N, Frykman V, van Mechelen R, Mitro P, Mohii-Oskarsson A, Pasquié JL et al. . Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011;13:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Moya A, Wieling W et al. . Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006;27:1085–92. [DOI] [PubMed] [Google Scholar]
- 11. Schuchert A, Maas R, Kretzschmar C, Behrens G, Kratzmann I, Meinertz T. Diagnostic yield of external electrocardiographic loop recorders in patients with recurrent syncope and negative tilt table test. Pacing Clin Electrophysiol 2003;26:1837–40. [DOI] [PubMed] [Google Scholar]
- 12. Sivakumaran S, Krahn AD, Klein GJ, Finan J, Yee R, Renner S et al. . A prospective randomized comparison of loop recorders versus Holter monitors in patients with syncope or presyncope. Am J Med 2003;115:1–5. [DOI] [PubMed] [Google Scholar]
- 13. Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of ‘community acquired’ syncope. BMC Health Serv Res 2006;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations. A cost-effectiveness analysis. Ann Intern Med 1998;128:890–5. [DOI] [PubMed] [Google Scholar]
- 15. Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014;16:914–22. [DOI] [PubMed] [Google Scholar]
- 16. Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke 2012;43:2788–90. [DOI] [PubMed] [Google Scholar]
- 17. Müller A, Scharner W, Borchardt T, Och W, Korb H. Reliability of an external loop recorder for automatic recognition and trans-telephonic ECG transmission of atrial fibrillation. J Telemed Telecare 2009;15:391–6. [DOI] [PubMed] [Google Scholar]
- 18. Linzer M, Pritcett EL, Pontinen M, Mc Carthy E, Devine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990;66:214–9. [DOI] [PubMed] [Google Scholar]
- 19. Gula LJ, Krahn AD, Massel D, Skanes A, Yee R, Klein GJ. External loop recorders: determinants of diagnostic yield in patients with syncope. Am Heart J 2004;147:644–8. [DOI] [PubMed] [Google Scholar]
- 20. Brignole M, Moya A, Menozzi C, Garcia-Civera R, Sutton R. Proposed electrocardiographic classification of spontaneous syncope documented by an implantable loop recorder. Europace 2005;7:14–8. [DOI] [PubMed] [Google Scholar]
- 21. Krahn AD, Klein GJ, Yee R, Hoch JS, Skanes AC, Barrett PM et al. . Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127:95.e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giada F, Gulizia M, Francese M, Croci F, Santangelo L, Santomauro M et al. . Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007;49:1951–6. [DOI] [PubMed] [Google Scholar]
- 23. Park MH, de Asmundis C, Chierchia GB, Sarkozy A, Benatar A, Brugada P. First experience of monitoring with cardiac event recorder electrocardiography Omron system in childhood population for sporadic, potentially arrhythmia-related symptoms. Europace 2011;13:1335–9. [DOI] [PubMed] [Google Scholar]
- 24. Iglesias JF, Graf D, Forclaz A, Schlaepfer J, Fromer M, Pruvot E. Stepwise evaluation of unexplained syncope in a large ambulatory population. Pacing Clin Electrophysiol 2009;32:S202–6. [DOI] [PubMed] [Google Scholar]
- 25. Davis S, Westby M, Pitcher D, Petkar S. Implantable loop recorders are cost-effective when used to investigate transient loss of consciousness which is either suspected to be arrhythmic or remains unexplained. Europace 2012;14:402–9. [DOI] [PubMed] [Google Scholar]


