Abstract
Aims
Implantable cardioverter-defibrillators (ICDs) have been shown to improve survival, although a considerable number of patients never receive therapy. Implantable cardioverter-defibrillators are routinely implanted regardless of sex. There is continuing controversy whether major outcomes differ between men and women.
Methods and results
In this retrospective single-centre study, 1151 consecutive patients (19% women) undergoing ICD implantation between 1998 and 2010 were followed for mortality and first appropriate ICD shock over 4.9 ± 2.7 years. Sex-related differences were investigated using multivariable Cox models adjusting for potential confounders. During follow-up, 318 patients died, a rate of 5.9% per year among men and 4.6% among women (uncorrected P = 0.08); 266 patients received a first appropriate ICD shock (6.3% per year among men vs. 3.6% among women, P = 0.002). After multivariate correction, independent predictors of all-cause mortality were age (hazard ratio, HR = 1.04 per year of age, 95% confidence interval (CI) [1.03–1.06], P < 0.001), left ventricular ejection fraction (HR = 0.98 per %, 95% CI [0.97–1.00], P = 0.025), renal function (HR = 0.99 per mL/min/1.73 m2, 95% CI [0.99–1.00], P = 0.009), use of diuretics (HR = 1.81, 95% CI [1.29–2.54], P = 0.0023), peripheral arterial disease (HR = 2.21, 95% CI [1.62–3.00], P < 0.001), and chronic obstructive pulmonary disease (HR = 1.48, 95% CI [1.13–1.94], P = 0.029), but not sex. Female sex (HR = 0.51, 95% CI [0.33–0.81], P = 0.013), older age (HR = 0.98, 95% CI [0.97–0.99], P < 0.001), and primary prophylactic ICD indication (HR = 0.69, 95% CI [0.52–0.93], P = 0.043) were independent predictors for less appropriate shocks.
Conclusion
Women receive 50% less appropriate shocks than men having similar mortality in this large single-centre population. These data may pertain to individually improved selection of defibrillator candidates using risk factors, e.g. sex as demonstrated in this study.
Keywords: Cardiovascular mortality, Risk factors, Implantable cardioverter-defibrillator, Sex difference, Sudden cardiac death
What's new?
This study shows for the first time that women receive 50% less appropriate shocks than men over long-term follow-up in a large single-centre ICD patient population, and after adjustment for possible confounders.
At the same time, they have a similar risk of all-cause mortality after adjustment.
Annual mortality in this study was 5.9% per year among men and 4.6% among women.
Annual rate of first appropriate ICD shock was 6.3% per year among men vs. 3.6% among women.
Female sex, older age, and primary prophylactic ICD indication were independent predictors for less appropriate shocks.
Introduction
Implantable cardioverter-defibrillators (ICDs) have been shown to improve survival1–4 and current guidelines recommend their use for primary and secondary preventions of sudden cardiac death (SCD). A large number of patients never receive appropriate therapy from their device, thus on an individual basis have not derived benefit.5 This may be explained by a lower all-cause and arrhythmic mortality, lower risk of life-threatening arrhythmias6 despite significant mortality, or with risk of death competing with risk of shock in patients with high mortality.7
Women are an important patient subgroup on whom controversial results have been reported regarding their ICD mortality benefit. Smaller treatment benefit for women was reported in the original SCD in Heart Failure Trial publication4—although not a specified subgroup—with a non-significant hazard ratio (HR) vs. placebo (HR = 0.96 [0.56–1.61], when compared with HR = 0.73 [0.57–0.93] for men). Subsequent analyses showed that similar sex-related differences of primary prevention mortality benefit were seen but could not be proved statistically as a factor interacting with ICD therapy.8–10 Recent registry data revealed that appropriate ICD shocks were ≈30% lower in women in the first year after ICD implantation.11 Van der Heijden et al.12 found lower mortality for women but no difference in appropriate ICD therapy. Therefore, the exact influence of sex on ICD therapy is yet unclear.
The aim of this retrospective study was to further investigate sex differences of all-cause mortality, death without prior shock, and appropriate ICD shock in a large single-centre population with long-term follow-up while correcting for a large number of possible confounders.
Methods
Patients
Consecutive patients undergoing ICD or cardiac resynchronization therapy with defibrillator (CRT-D) implantation between 1998 and 2010 at our institution for guideline recommended indications were recorded in a retrospective single-centre ICD registry.
Baseline characteristics
Baseline characteristics were retrieved from hospital records, among them, age, sex, body mass index, systolic blood pressure, ischaemic or non-ischaemic disease, primary or secondary prophylactic defibrillator indication, New York Heart Association (NYHA) functional class, echocardiographic left ventricular ejection fraction (LVEF), cardiovascular drug treatment, and co-morbidities. In addition, serum creatinine at implantation was retrieved to calculate the estimated glomerular filtration rate (eGFR) according to the Modification of Diet in Renal Disease Study.13 Underlying rhythm, heart rate, QRS, and uncorrected QT duration were taken from the admission electrocardiogram.
Device programming and interrogation
Implantable cardioverter-defibrillator devices from three manufacturers were implanted (Biotronik, Berlin, Germany; Boston Scientific, Natick, MA, USA, formerly CPI, Guidant, St Paul, MN, USA; and Medtronic Inc., Minneapolis, MN, USA). Implantable cardioverter-defibrillator programming varied over time according to available publications and recommendations. In general, a programming using two zones was used (ventricular tachycardia, VT zone and ventricular fibrillation, VF zone). Ventricular fibrillation was detected for heart rates >210–230 b.p.m. Detection counters were set to between 12 and 18 out of 16 and 24 beats, 20 of 30 or 30 of 40 settings were not used. Ventricular tachycardia was identified at heart rates >170 b.p.m. and treated by alternating burst and ramp trains involving a total of 6–12 antitachycardia pacing (ATP) cycles. If unsuccessful, shocks of maximal energy followed. For secondary prevention ICDs, programming was individually tiered to documented arrhythmias. Algorithms for improved detection of supraventricular arrhythmias (onset, stability, Biotronik SMART®, Medtronic Wavelet®, PRLogic®, and Boston Scientific RhythmID®) were programmed ON where available. Detection zones were reprogrammed in case of VT episodes.
Follow-up and endpoints
All-cause mortality and first appropriate ICD shock were predefined as endpoints before initiation of the registry. To reflect competing risks of deaths and shock, the rate of death without prior appropriate shock was determined. Mortality was assessed based on information from our institution, other hospitals, general practitioners, or local authorities. Outpatient ICD follow-up was done every 3–6 months, or when necessary. Episodes stored by the ICD were classified by the treating physician at first hand, these were re-evaluated by the investigators (J.S., M.D., and M.Z.). If sustained VT or VF had occurred, ICD treatment was considered appropriate and shocks, particularly first shocks were documented as endpoints. Appropriate ATP without shock or inappropriate ICD shocks/ATP were not considered as endpoints. The majority of patients was followed exclusively at our ICD clinic (71%). Additional information about ICD shocks was obtained from outside clinics, treating cardiologists, or patients by questionnaire or telephone. Retrieval of outside patient data and contacting patients was approved by the local ethics committee.
Statistical analysis
Baseline characteristics are presented as mean ± SD for continuous variables and as proportions for categorical variables. Continuous variables are compared by Student's t-test; categorical variables by Pearson's χ2 test.
Predictors of mortality and appropriate ICD shocks were analysed in univariate and multivariate Cox proportional hazards regression models. For appropriate ICD shocks, death was regarded as a competing risk.14 For all models, missing values were imputed by chained equations15,16 as recently applied in a heart failure study.17 Forward variable selection was used to obtain the final multivariable model using a P-value of <0.05 for inclusion of a given variable. Sex category was entered into the multivariate models regardless of P-value. To adjust for a non-proportional effect in the date of implantation, patients were stratified into three equally sized groups according to their dates of implantation, and stratified Cox regression was performed.18 Survival rates are illustrated by Kaplan–Meier curves.
Multivariable Cox models were built using the software R (version 3.0, www.r-project.org), competing risk models were implemented using the R-package ‘cmprsk’. Missing values imputation was done with the R-package ‘mice’ using the default setting. All other statistical analyses were performed using SPSS (version 21.0, IBM Corporation). For all tests, a P-value of <0.05 was required for statistical significance.
Results
Patient characteristics
The data set included 1151 patients, among them 216 (19%) females. Mean age was 64 ± 13 years. Implantable cardioverter-defibrillator therapy was prescribed for primary prophylaxis of SCD to 632 (55%) patients, and for secondary prophylaxis to 519 (45%) patients, respectively. Of the latter, 236 of 519 (45%) were successfully resuscitated from cardiac arrest due to VF, the remaining 283 (55%) had a history of sustained symptomatic VT. A single-chamber ICD was implanted in 375 (33%) patients, 375 (33%) received dual-chamber ICDs, and 401 (34%) a CRT-D. Other baseline characteristics stratified by sex are presented in Table 1.
Table 1.
Patient characteristics by sex
| Female | Male | P-value | |
|---|---|---|---|
| n | 216 | 935 | |
| Age (years) | 62 ± 15 | 65 ± 12 | 0.01 |
| LVEF (%) | 34 ± 13 | 29 ± 11 | <0.001 |
| Ischaemic cardiomyopathy | 111 (51%) | 668 (71%) | <0.001 |
| Primary prophylactic indication | 114 (53%) | 518 (55%) | 0.50 |
| Single-chamber device | 71 (33%) | 304 (33%) | 0.94 |
| CRT-D | 65 (30%) | 336 (36%) | 0.11 |
| QRS duration (ms) | 112 ± 30 | 123 ± 32 | <0.001 |
| β-Adrenergic blocker | 180 (87%) | 823 (91%) | 0.07 |
| Peripheral arterial disease | 15 (7.1%) | 85 (9.4%) | 0.35 |
| COPD | 20 (9%) | 157 (17%) | 0.0047 |
| Diabetes | 42 (19%) | 254 (27%) | 0.02 |
| NYHA functional class | 2.3 ± 0.9 | 2.4 ± 0.9 | 0.14 |
| eGFR (mL/min/1.73 m2) | 66 ± 24 | 67 ± 25 | 0.63 |
| FU for mortality (years) | 4.9 ± 3.0 | 4.9 ± 2.6 | 0.89 |
| Mortality | 49 (23%) | 269 (29%) | 0.08 |
| Mortality/year | 4.6% | 5.9% | |
| FU until appropriate shock | 4.2 ± 2.9 | 4.0 ± 2.7 | 0.21 |
| Appropriate shock | 32 (15%) | 234 (25%) | 0.0012 |
| First appropriate shock/year | 3.8% | 6.3% | |
| Mortality without prior appr. shock | 38 (18%) | 196 (21%) | 0.16 |
| Mortality without prior appr. shock/year | 3.6% | 4.3% |
appr., appropriate; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; LV, left ventricular; NYHA, New York Heart Association; FU, follow-up.
Long-term follow-up for mortality
All-cause mortality
Overall follow-up was 4.9 ± 2.7 years (maximum 14.0 years), 318 (27.6%) patients died corresponding to an annualized mortality rate of 5.6% in all patients, 5.9% among men, and 4.6% among women. Uncorrected all-cause mortality in women was slightly lower (uncorrected P = 0.08).
Mortality without appropriate shock
Eighty-four (26.4%) of 318 deceased patients received at least one appropriate ICD shock, 234 patients (73.6%) died without prior appropriate shock. Among these, 196 men and 38 women did not experience appropriate shock before death. Uncorrected mortality without experiencing prior shock was similar between men and women (P = 0.16).
Long-term follow-up for implantable cardioverter-defibrillator therapies
First appropriate shock was observed in 266 (23.1%) patients after a follow-up of 4.0 ± 2.7 years (annualized rate: 5.8%). It was documented in the VF zone in 49% of patients (median cycle length, 235 ms; interquartile range, 210–258 ms) and in 51% in the VT zone (300 ms; 279–352 ms). Uncorrected annualized appropriate shock rate was 6.3% among men vs. 3.6% among women (P = 0.002). Accordingly, there were 32 of 216 women (15%) that did receive appropriate ICD shocks during the long-term follow-up. If primary and secondary preventions of SCD were analysed separately, uncorrected annualized ICD shock rate was significantly lower in women in both groups of prevention (2.6 vs. 4.8% p.a., P = 0.033, and 4.9 vs. 7.4% p.a., P = 0.018, respectively).
At least one inappropriate shock was delivered in 106 (9.2%) patients, 44 of whom were also treated with appropriate shock. Inappropriate shocks were equally distributed between men and women (9.5 vs. 7.9%, P = 0.45). Appropriate ATP was noticed in 247 (21.5%) patients, more frequently in men when compared with women (23.3 vs. 13.4%, P = 0.001). Any appropriate ICD therapy (appropriate ATP or appropriate ICD shock) was observed in 380 (33%) patients, and again more frequently in men (35.4 vs. 22.7%, P = 0.0003).
Univariate and multivariate predictors of mortality
Univariate Cox regression revealed a considerable number of predictors for mortality, as presented in Table 2, left column. Sex was not a univariate predictor of mortality, but a statistically non-significant trend for better survival of women vs. men was observed, as presented in Table 2 and Figure 1. Age, LVEF, renal impairment (eGFR), medication with diuretics, aspirin treatment, peripheral arterial disease (PAD), and chronic obstructive pulmonary disease (COPD) were selected as independent predictors for all-cause mortality in the final Cox model, as presented in Table 2, right columns. Sex was not a predictor of mortality in the multivariable model (HR = 1.15, 95% confidence interval (CI) [0.84–1.58], P = 0.536). Unadjusted annualized mortality in women vs. men amounted to 4.6 vs. 5.9% per year (see Figure 1, HR = 0.768, 95% CI [0.57–1.04], P = 0.090).
Table 2.
Univariate and multivariate Cox proportional hazards regression (final model) for all-cause mortality
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Age (years) | 1.058 [1.046–1.071] | <0.001 | 1.04 [1.03–1.06] | <0.001 |
| Sex category (male) | 1.302 [0.960–1.766] | 0.090 | 1.15 [0.84–1.58] | 0.536 |
| Body mass index (kg/m2) | 0.977 [0.951–1.003] | 0.082 | ||
| NYHA functional class | 1.587 [1.385–1.819] | <0.001 | ||
| Primary prophylactic indication | 1.444 [1.150–1.815] | 0.002 | ||
| CRT-D | 1.515 [1.212–1.893] | <0.001 | ||
| Ischaemic cardiomyopathy | 1.501 [1.166–1.932] | 0.002 | ||
| LVEF (%) | 0.972 [0.962–0.983] | <0.001 | 0.98 [0.97–1.00] | 0.025 |
| Heart rate (b.p.m.) | 1.002 [0.998–1.005] | 0.336 | ||
| QRS duration (ms) | 1.007 [1.004–1.011] | <0.001 | ||
| QT interval (ms) | 1.003 [1.000–1.005] | 0.027 | ||
| eGFR (mL/min/1.73 m2) | 0.979 [0.974–0.984] | <0.001 | 0.99 [0.99–1.00] | 0.009 |
| Medication | ||||
| ACE inhibitors/ARB | 0.997 [0.716–1.388] | 0.986 | ||
| β-Blocker | 0.923 [0.651–1.309] | 0.654 | ||
| Digitalis | 1.507 [1.197–1.898] | <0.001 | ||
| Diuretics | 2.872 [2.058–4.008] | <0.001 | 1.81 [1.29–2.54] | 0.002 |
| MRA | 1.012 [0.805–1.273] | 0.918 | ||
| Aspirin | 0.996 [0.792–1.251] | 0.971 | 0.73 [0.58–0.93] | 0.029 |
| Coumadin | 1.305 [1.025–1.661] | 0.031 | ||
| Amiodarone | 1.308 [1.016–1.683] | 0.037 | ||
| Hypertension | 1.268 [0.940–1.712] | 0.120 | ||
| Diabetes | 1.587 [1.259–2.001] | <0.001 | ||
| History of atrial fibrillation | 1.546 [1.240–1.928] | <0.001 | ||
| Peripheral arterial disease | 2.874 [2.149–3.843] | <0.001 | 2.21 [1.62–3.00] | <0.001 |
| COPD | 1.982 [1.520–2.584] | <0.001 | 1.48 [1.13–1.94] | 0.029 |
AAD, antiarrhythmic drug; AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; aspirin, acetylsalicylic acid; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Figure 1.
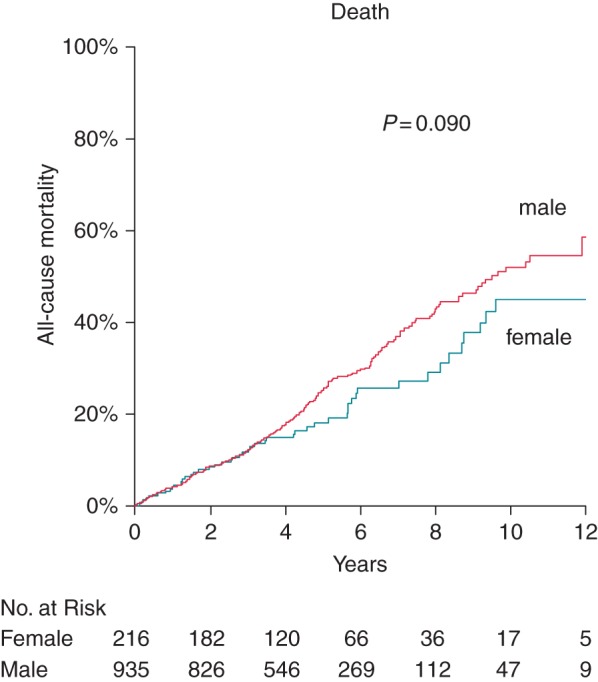
Cumulative Kaplan–Meier event probability of death according to sex: male (red line) and female (blue line) patients. Unadjusted P-value is shown in the figure.
Death without appropriate shock
Upon univariate Cox regression, a similar number of predictors were revealed as predictors of death without prior appropriate shock, as presented in Table 3, left column. The final Cox model identified higher age, renal impairment (eGFR), use of digitalis glycosides or diuretics, PAD, and COPD as independent predictors of death without appropriate shock, as presented in Table 3, right columns. Sex category was neither a univariate (P = 0.295) nor a multivariate (P = 0.708) predictor of death without appropriate shock. The incidence of death without prior appropriate shock in women vs. men amounted to 3.7 vs. 4.3% per year (see Figure 2, HR = 0.8, 95% CI [0.6–1.2], P = 0.295).
Table 3.
Univariate and multivariate Cox proportional hazards regression (final model) for death without appropriate shock
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR (95% CI) | P-value | |
| Age (years) | 1.063 [1.048–1.078] | <0.001 | 1.04 [1.03–1.06] | <0.001 |
| Sex category (male) | 1.204 [0.850–1.705] | 0.295 | 1.09 [0.77–1.55] | 0.708 |
| Body mass index (kg/m2) | 0.972 [0.942–1.003] | 0.076 | ||
| NYHA functional class | 1.711 [1.456–2.011] | <0.001 | ||
| Primary prophylactic indication | 1.658 [1.269–2.167] | <0.001 | ||
| CRT-D | 1.575 [1.216–2.039] | <0.001 | ||
| Ischaemic cardiomyopathy | 1.446 [1.080–1.936] | 0.013 | ||
| LVEF (%) | 0.974 [0.962–0.986] | <0.001 | ||
| Heart rate (b.p.m.) | 1.002 [0.998–1.006] | 0.298 | ||
| QRS duration (ms) | 1.007 [1.003–1.011] | <0.001 | ||
| QT interval (ms) | 1.001 [0.998–1.004] | 0.383 | ||
| eGFR (mL/min/1.73 m2) | 0.977 [0.971–0.983] | <0.001 | 0.99 [0.98–1.00] | 0.008 |
| Medication | ||||
| ACE inhibitors/ARB | 1.035 [0.696–1.541] | 0.864 | ||
| β-Blocker | 0.864 [0.577–1.293] | 0.477 | ||
| Digitalis | 1.558 [1.191–2.038] | 0.001 | 1.47 [1.11–1.94] | 0.021 |
| Diuretics | 2.925 [1.967–4.351] | <0.001 | 1.79 [1.19–2.69] | 0.016 |
| MRA | 1.014 [0.776–1.324] | 0.920 | ||
| Aspirin | 1.090 [0.832–1.428] | 0.530 | ||
| Coumadin | 1.268 [0.955–1.683] | 0.100 | ||
| Amiodarone | 1.071 [0.784–1.463] | 0.665 | ||
| Hypertension | 1.229 [0.868–1.741] | 0.244 | ||
| Diabetes | 1.509 [1.150–1.980] | 0.003 | ||
| History of atrial fibrillation | 1.594 [1.233–2.060] | <0.001 | ||
| Peripheral arterial disease | 3.474 [2.521–4.788] | <0.001 | 2.46 [1.76–3.44] | <0.001 |
| COPD | 2.003 [1.476–2.719] | <0.001 | 1.48 [1.08–2.02] | 0.040 |
AAD, antiarrhythmic drug; AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; aspirin, acetylsalicylic acid; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Figure 2.
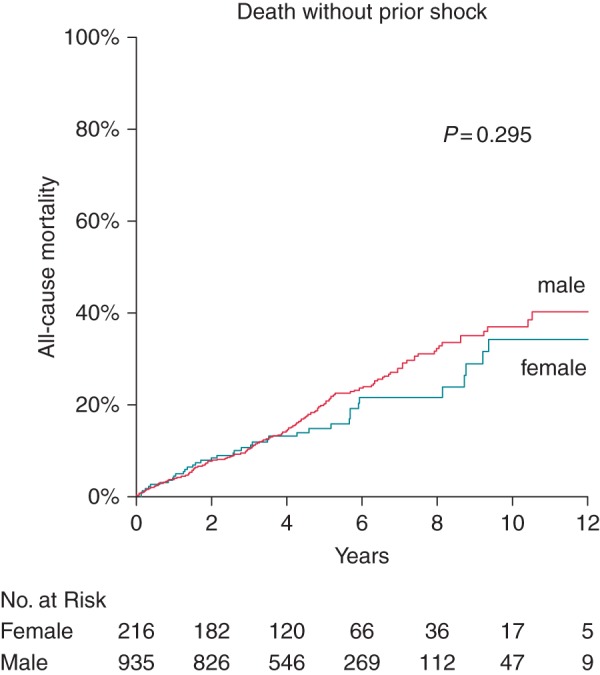
Cumulative Kaplan–Meier event probability of death without prior appropriate ICD shock: male (red line) and female (blue line) patients. Unadjusted P-value is shown in the figure.
Univariate and multivariate predictors of appropriate implantable cardioverter-defibrillator shock
Univariate Cox regression revealed a significant association of first appropriate shock with male sex, secondary prophylactic ICD indication, prolonged QT interval, oral anticoagulation, treatment with amiodarone, history of atrial fibrillation, and COPD (Table 4, left columns). Women were subject to significantly less appropriate ICD shocks (3.6 vs. 6.3% per year, P = 0.002, Figure 3). In the final multivariate Cox model, higher age, female sex, and primary prophylactic indication were identified as independent predictors of fewer appropriate ICD shocks (Table 4, right columns).
Table 4.
Univariate and multivariate Cox proportional hazards regression (final model) for occurrence of the first appropriate shock
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Age (years) | 0.999 [0.990–1.008] | 0.838 | 0.98 [0.97–0.99] | <0.001 |
| Sex category (male) | 1.773 [1.225–2.567] | 0.002 | 1.94 [1.23–3.04] | 0.013 |
| Body mass index (kg/m2) | 1.001 [0.975–1.027] | 0.968 | ||
| NYHA functional class | 0.944 [0.823–1.083] | 0.411 | ||
| Primary prophylactic indication | 0.655 [0.512–0.838] | 0.001 | 0.69 [0.52–0.93] | 0.043 |
| CRT-D | 0.969 [0.751–1.249] | 0.806 | ||
| Ischemic cardiomyopathy | 1.102 [0.850–1.428] | 0.465 | ||
| LVEF (%) | 0.995 [0.985–1.006] | 0.358 | ||
| Heart rate (b.p.m.) | 0.992 [0.985–1.001] | 0.068 | ||
| QRS duration (ms) | 0.999 [0.995–1.003] | 0.734 | ||
| QT interval (ms) | 1.003 [1.001–1.006] | 0.013 | ||
| eGFR (mL/min/1.73 m2) | 0.998 [0.993–1.004] | 0.546 | ||
| Medication | ||||
| ACE inhibitors/ARB | 1.048 [0.722–1.519] | 0.807 | ||
| β-Blocker | 0.901 [0.609–1.334] | 0.603 | ||
| Digitalis | 1.019 [0.781–1.330] | 0.889 | ||
| Diuretics | 1.096 [0.831–1.445] | 0.515 | ||
| MRA | 0.959 [0.749–1.228] | 0.739 | ||
| Aspirin | 0.788 [0.616–1.007] | 0.057 | ||
| Coumadin | 1.391 [1.072–1.804] | 0.013 | ||
| Amiodarone | 1.900 [1.460–2.474] | <0.001 | ||
| Hypertension | 1.010 [0.741–1.377] | 0.950 | ||
| Diabetes | 1.008 [0.765–1.328] | 0.953 | ||
| History of atrial fibrillation | 1.335 [1.047–1.701] | 0.020 | ||
| Peripheral arterial disease | 0.901 [0.565–1.437] | 0.661 | ||
| COPD | 1.385 [1.010–1.900] | 0.043 | ||
AAD, antiarrhythmic drug; AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; aspirin, acetylsalicylic acid; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Figure 3.
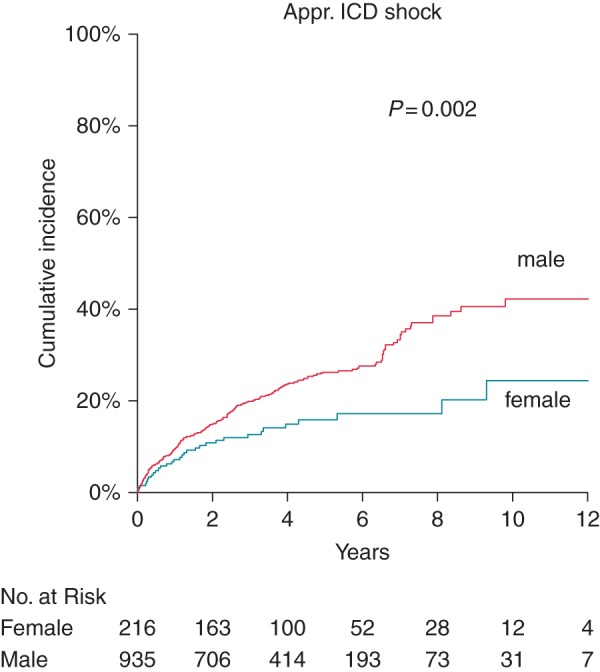
Cumulative Kaplan–Meier event probability of first appropriate ICD shock: male (red line) and female (blue line) patients. Unadjusted P-value is shown in the figure.
Discussion
In this large single-centre ICD population with extended follow-up, we identified seven independent predictors of all-cause mortality and three independent predictors of appropriate shock. These results highlight that risk factors for mortality do not correspond to those of malignant arrhythmias.
Lower appropriate shock rate in women
As one of the leading results, our data show that female sex was associated with a ≈50% reduced risk of appropriate shocks during the long-term follow-up, but did not influence mortality. For the long-term follow-up of ICD patients, this is a novel finding. One can conclude that women may derive a smaller benefit from their device if they exhibit less malignant ventricular arrhythmias and mortality is similar after multivariate correction. This would not obviate ICD therapy in women but provides new evidence for the hypothesis that individually a higher threshold for ICD indication may be useful in women. For instance, this could pertain to the level of left ventricular dysfunction in primary prophylactic indications or the presence of additional risk factors. Increased risk for ICD associated complications may further decrease benefit from the device among women11,19 but was not assessed in this study.
How sex differences translate into clinical arrhythmias and possibly appropriate ICD shocks is not fully studied. Arrhythmia susceptibility is influenced by hormonal differences between women and men. Among others, there are sex differences in electrophysiological properties such as repolarization, calcium handling, autonomic modulation, and ion channels.20,21 Furthermore, the pathologic substrate is different: gender-based differences in response to myocardial infarction, and with relevance for arrhythmogenesis, have been shown on a molecular level22 and in patients, by mechanism of different vagal tone.23 In addition, the distribution of ischaemic vs. non-ischaemic cardiomyopathy is not equal between women and men as also observed in our registry (51 vs. 71%).
Sex differences in SCD were reported in the Framingham Heart study24 and for out of hospital SCD.25 Several defibrillator trials8–10 provided evidence to the fact, but were underpowered to prove significance. For instance, Zareba et al.8 found identical mortality between women and men, similar ICD benefit, and a 40% lower appropriate shock rate (P = 0.039). In contrast, Russo et al.9 found a 32% lower mortality for women vs. men (P = 0.001) but no difference in the incidence of shock and ICD benefit for women. Albert et al.10 analysed 458 patients from DEFINITE including 63 women randomized to an ICD. Featuring only primary prevention indications and non-ischaemic patients, a 54% reduction of appropriate shocks was found (P = 0.10), a very similar risk reduction as in the current study, although not significant due to the patient number and duration of follow-up. In the current study, 219 women were followed for almost 5 years, which is longer than in most prior studies, increasing the power and generalizability of our findings.
Gender-focused meta-analyses from the above-mentioned older trials confirmed lower shock rates in women26,27 but were equivocal on ICD benefit depending on study selection.28 The largest gender-focused ICD study so far is the Ontario registry providing results in more than 6000 patients.11 These authors found a 31% lower rate of first appropriate shock in women (P = 0.015) as well as an absent difference in overall survival which is in line with our study. The main difference is the short follow-up of only 1 year. Wijers et al.29 and Weeke et al.30 provided ICD registry data recently not focusing on sex differences. Wijers et al.29 reported a 47% reduction of adjusted mortality for women (P = 0.004), and a 57% reduction of adjusted appropriate shock rate (P = 0.001), whereas Weeke et al.30 reported a significant multivariate HR of 0.33 (95% CI [0.11–0.57] for appropriate shocks in women and an insignificant HR for mortality of 0.75 (95% CI [0.49–1.14]). Our study as well as MacFadden et al.11 and Wijers et al.29 combined primary and secondary prophylactic indications, whereas Weeke et al.30 analysed only primary prevention patients. Importantly, we found no difference in our main finding between primary and secondary prevention patients. Van der Hejden et al.12 published a gender-focused analysis from the Leiden ICD registry in more than 1900 primary prevention patients including 418 women (21%). Adjusted all-cause mortality was significantly lower for women (HR = 0.65, 95% CI [0.49–0.84], P < 0.01) while the rate of appropriate shock showed an adjusted HR of 0.80 (95% CI [0.66–1.13], P = 0.19). Compared with our study, there were somewhat different baseline characteristics such as more frequent CRT-D treatment which may favour women.31 We chose not to define ATP therapies as endpoints to reflect more malignant arrhythmias in the endpoint; however, results were very similar when adding them. Counting appropriate ICD shocks only as a surrogate of arrhythmic mortality in ICD patients may still overestimate the potential risk of arrhythmogenic mortality.32 However, their unadjusted annualized mortality rates for men (6.8%) and women (5.3%) were in a very similar range as in our study. Therefore, the results of van der Heijden et al.12 are not contradictory with our findings or other studies.
Predictors of mortality
Mortality risk factors identified in our population are confirmatory of the literature in ICD patients. Kramer et al.33 validated a mortality risk score in of 2717 ICD patients. In multivariate regression, peripheral arterial disease, decreased LVEF, elevated serum creatinine, and higher age remained as independent factors. Sex category—as in our study—was not influencing survival. Bilchick et al.34 investigated >35 000 primary prophylactic ICD recipients for mortality predictors and identified age, NYHA status, atrial fibrillation, COPD, kidney disease, LVEF, and diabetes as independent predictors. Of note, no shock data were available in the latter two studies. The confirmation of mortality factors and HRs in our study is very precise. Independent risk factors may be combined to define higher risk groups which was not the purpose of this analysis.
The rate of patients never experiencing an appropriate shock in our study was high (75% in men, 85% in women). Death without previous appropriate shock was not predicted by sex, but by higher age, decreased renal function, PAD, and COPD. These factors are also mortality factors, and coincide with increased morbidity.
Limitations
The retrospective design of data collection may confer unknown biases; however, the results for many known variables are confirming other studies proving data validity of this series. Implantable cardioverter-defibrillator programming was not uniform over a period of 12 years, and may have influenced the results; however, systematic differences in programming between male and female patients are unlikely. However, our ICD technician never changed during the study guaranteeing ICD programming to be as consistent as possible.
Conclusions
Our data show that after ICD or CRT-D implantation, women receive 50% less appropriate shocks than men but have similar mortality. These data may pertain to an individually improved selection of candidates for defibrillator implantation using risk factors for the occurrence of malignant arrhythmias, e.g. sex as demonstrated in this study.
Funding
The research leading to the results has received funding from the European Community's Seventh Framework Programme FP7/2007-2013 under grant agreement n° 602299, EU-CERT-ICD. D.C. was supported by a grant of the Swiss National Science Foundation (PP00P3_133681 and PP00P3_159322). Funding to pay the Open Access publication charges for this article was provided by the European Community's Seventh Framework Program FP7/2007–2013 under grant agreement n° 602299, EU-CERT-ICD.
Conflict of interest: none declared.
References
- 1. The Antiarrhythmics AVID Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576–83. [DOI] [PubMed] [Google Scholar]
- 2. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999;341:1882–90. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al. . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al. . Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 5. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol 2008;52:1111–21. [DOI] [PubMed] [Google Scholar]
- 6. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H et al. . Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–96. [DOI] [PubMed] [Google Scholar]
- 7. Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation 2008;117:1918–26. [DOI] [PubMed] [Google Scholar]
- 8. Zareba W, Moss AJ, Jackson Hall W, Wilber DJ, Ruskin JN, McNitt S et al. . Clinical course and implantable cardioverter defibrillator therapy in postinfarction women with severe left ventricular dysfunction. J Cardiovasc Electrophysiol 2005;16:1265–70. [DOI] [PubMed] [Google Scholar]
- 9. Russo AM, Poole JE, Mark DB, Anderson J, Hellkamp AS, Lee KL et al. . Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol 2008;19:720–4. [DOI] [PubMed] [Google Scholar]
- 10. Albert CM, Quigg R, Saba S, Estes NA 3rd, Shaechter A, Subacius H et al. . Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am Heart J 2008;156:367–72. [DOI] [PubMed] [Google Scholar]
- 11. MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P et al. . Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 12. van der Heijden AC, Thijssen J, Borleffs CJ, van Rees JB, Hoke U, van der Velde ET et al. . Gender-specific differences in clinical outcome of primary prevention implantable cardioverter defibrillator recipients. Heart 2013;99:1244–9. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW et al. . Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- 14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 15. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–94. [DOI] [PubMed] [Google Scholar]
- 16. van Buuren S, Groothuis-Oudshoorn K. Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 17. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB et al. . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–13. [DOI] [PubMed] [Google Scholar]
- 18. Therneau TM, Grambsch PM. Stratified Cox models. In. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000, 44–7. [Google Scholar]
- 19. Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP et al. . Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circulation 2009;119:1078–84. [DOI] [PubMed] [Google Scholar]
- 20. Haigney MC, Zareba W, Nasir JM, McNitt S, McAdams D, Gentlesk PJ et al. . Gender differences and risk of ventricular tachycardia or ventricular fibrillation. Heart Rhythm 2009;6:180–6. [DOI] [PubMed] [Google Scholar]
- 21. Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 1996;94:1471–4. [DOI] [PubMed] [Google Scholar]
- 22. Korte T, Fuchs M, Arkudas A, Geertz S, Meyer R, Gardiwal A et al. . Female mice lacking estrogen receptor beta display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation 2005;111:2282–90. [DOI] [PubMed] [Google Scholar]
- 23. Airaksinen KE, Ikaheimo MJ, Linnaluoto M, Tahvanainen KU, Huikuri HV. Gender difference in autonomic and hemodynamic reactions to abrupt coronary occlusion. J Am Coll Cardiol 1998;31:301–6. [DOI] [PubMed] [Google Scholar]
- 24. Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J 1998;136:205–12. [DOI] [PubMed] [Google Scholar]
- 25. Wigginton JG, Pepe PE, Bedolla JP, DeTamble LA, Atkins JM. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: a multiyear, prospective, population-based study. Crit Care Med 2002;30:S131–6. [DOI] [PubMed] [Google Scholar]
- 26. Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S et al. . Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:1500–6. [DOI] [PubMed] [Google Scholar]
- 27. Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S et al. . Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm 2010;7:876–82. [DOI] [PubMed] [Google Scholar]
- 28. Bergau L, Seegers J, Zabel M. Sex differences in ICD benefit. J Electrocardiol 2014;47:869–73. [DOI] [PubMed] [Google Scholar]
- 29. Wijers SC, van der Kolk BY, Tuinenburg AE, Doevendans PA, Vos MA, Meine M. Implementation of guidelines for implantable cardioverter-defibrillator therapy in clinical practice: Which patients do benefit? Neth Heart J 2013;21:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weeke P, Johansen JB, Jorgensen OD, Nielsen JC, Moller M, Videbaek R et al. . Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter-defibrillators for primary prevention: data from the Danish ICD Register. Europace 2013;15:1150–7. [DOI] [PubMed] [Google Scholar]
- 31. Zabarovskaja S, Gadler F, Braunschweig F, Stahlberg M, Hornsten J, Linde C et al. . Women have better long-term prognosis than men after cardiac resynchronization therapy. Europace 2012;14:1148–55. [DOI] [PubMed] [Google Scholar]
- 32. Connolly SJ. Use and misuse of surrogate outcomes in arrhythmia trials. Circulation 2006;113:764–6. [DOI] [PubMed] [Google Scholar]
- 33. Kramer DB, Friedman PA, Kallinen LM, Morrison TB, Crusan DJ, Hodge DO et al. . Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter-defibrillators. Heart Rhythm 2012;9:42–6. [DOI] [PubMed] [Google Scholar]
- 34. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol 2012;60:1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


