Abstract
Aims
The high costs associated with treatment for atrial fibrillation (AF) are primarily due to hospital care, but there are limited data to understand the reasons for and predictors of hospitalization in patients with AF.
Methods and results
The ROCKET AF trial compared rivaroxaban with warfarin for stroke prophylaxis in AF. We described the frequency of and reasons for hospitalization during study follow-up and utilized Cox proportional hazards models to assess for baseline characteristics associated with all-cause hospitalization. Of 14 171 patients, 14% were hospitalized at least once. Of 2614 total hospitalizations, 41% were cardiovascular including 4% for AF; of the remaining, 12% were for bleeding. Compared with patients not hospitalized, hospitalized patients were older (74 vs. 72 years), and more frequently had diabetes (46 vs. 39%), prior MI (23 vs. 16%), and paroxysmal AF (19 vs. 17%), but less frequently had prior transient ischaemic attack/stroke (49 vs. 56%). After multivariable adjustment, lung disease [hazard ratio (HR) 1.46, 95% confidence interval (CI) 1.29–1.66], diabetes [1.22, (1.11–1.34)], prior MI [1.27, (1.13–1.42)], and renal dysfunction [HR 1.07 per 5 unit GFR < 65 mL/min, (1.04–1.10)] were associated with increased hospitalization risk. Treatment assignment was not associated with differential rates of hospitalization.
Conclusion
Nearly 1 in 7 of the moderate-to-high-risk patients with AF enrolled in this trial was hospitalized within 2 years, and both AF and bleeding were rare causes of hospitalization. Further research is needed to determine whether care pathways directed at comorbid conditions among AF patients could reduce the need for and costs associated with hospitalization.
Keywords: Hospitalization, Atrial fibrillation, Outcomes, Stroke, Rivaroxaban
What's new?
To our knowledge, this is the first study to describe and compare hospitalizations in patients with atrial fibrillation (AF) in a multinational dataset.
We found that nearly 1 of 7 patients enrolled in the ROCKET AF trial was hospitalized at least once within 2 years.
The reasons for hospitalization were roughly split between cardiovascular and non-cardiovascular causes, and both AF and bleeding were rare causes of hospitalization.
Patients in North America had the highest rate of hospitalization, and factors associated with hospitalization included age and comorbid conditions.
This study has important implications for providers and investigators considering interventions aimed at reducing AF hospitalizations.
Introduction
The prevalence of atrial fibrillation (AF) is high. In the USA alone, AF affects between 3 and 6 million people, and this number is expected to rise considerably by 2050.1–3 The economic burden associated with treating patients with AF is substantial. Estimated annual medical costs for patients with AF in the USA are 73% higher than medically matched controls, primarily due to inpatient care for conditions other than AF.4 In the UK, total expenditures for AF have nearly doubled over the past 5 years, and more than half of these costs are due to inpatient care.5 Given this growing economic burden, it is important to understand the reasons for hospitalization and factors associated with hospitalization in patients with AF.
We utilized data from ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation)6 to (1) assess the rates of and reasons for hospitalization of patients with AF and (2) determine patient factors associated with hospitalization.
Methods
The design of the ROCKET AF study has been previously described.7 Briefly, ROCKET AF was a multinational, randomized, double-blind, active-controlled trial of fixed-dose rivaroxaban vs. adjusted-dose warfarin for the prevention of stroke in patients with nonvalvular AF at moderate to high risk for stroke. The trial was designed to determine whether rivaroxaban was noninferior to warfarin for the primary endpoint of stroke or non-central nervous system (CNS) systemic embolism. The trial was conducted from December 2006 through May 2010 at 1178 participating sites in 45 countries and included 14 264 patients. Qualifying medical history included a history of stroke, transient ischaemic attack (TIA), or systemic embolism or at least two of the following risk factors: heart failure or a left ventricular ejection fraction of ≤35%, hypertension, age ≥75 years, or the presence of diabetes mellitus (i.e. a CHADS2 score of ≥2), and according to the protocol, patients with only 2 risk factors were capped at 10% of the overall trial population, with the remaining patients requiring ≥3 risk factors (CHADS2 ≥ 3) or a prior stroke, TIA, or systemic embolism.
Patients were followed for the duration of the study to ascertain clinical events. An independent clinical endpoint committee adjudicated all suspected cases of stroke, systemic embolism, myocardial infarction, death, and bleeding events. Detailed definitions of these study endpoints were previously published.6 Patients were also evaluated at post-randomization study visits at 1, 2, and 4 weeks, and every month thereafter. As part of the study visits, a standardized questionnaire was used to query patients about emergency department visits and all-cause hospitalizations. Details regarding dates, location, and reasons for hospitalization were recorded. If a reason for hospitalization was not apparent from adjudication or recorded in the database, then adverse events occurring 0–3 days before the hospitalization date were reviewed to determine a cause of hospitalization. Patients were followed for a median of 707 days, and only 32 patients were lost to follow-up.
The present study is a post hoc analysis of all patients randomized in the trial (intention to treat). We assessed baseline characteristics and future unplanned hospitalizations. Reasons for hospitalization were broadly classified as bleeding, acute coronary syndrome, congestive heart failure, stroke/TIA, non-CNS embolism, other cardiovascular, and other.
The study conformed to the principles outlined in the Declaration of Helsinki and was approved by each participating centre's ethics committee or institutional review board. All patients provided written informed consent prior to randomization, and all study participants gave informed consent. The Duke Clinical Research Institute (Durham, NC) coordinated the trial and performed the statistical analyses for this study. The Institutional Review Board of the Duke University Health System approved this study.
Statistical methods
We summarized patient characteristics stratified by hospitalizations. Continuous variables were reported as medians and 25th and 75th percentiles; categorical variables were reported as counts and percentages. We also summarized the number of hospitalizations during study follow-up and stratified by region, broadly grouping into the following regions of interest: Latin America, Eastern Europe, East Asia, Western Europe, and North America. We tested for differences between the regions using χ2 tests for categorical variables and the Kruskal–Wallis tests for continuous variables.
We used Cox proportional hazards models to assess for baseline characteristics associated with risk of all-cause and cardiovascular hospitalizations. Baseline characteristics including demographic data, treatment assignment, and medical history were considered as candidate variables for the models with the exception of CHADS2 score, which was excluded due to concerns of colinearity. Continuous variables were evaluated for linear associations with outcomes and modifications (such as linear splines or truncations) were made when necessary. The outcomes were time to first all-cause hospitalization and time to first cardiovascular hospitalization. Forward stepwise selection was used to determine final variables utilized in the model. The final model included patients with complete data, and no missing data were imputed. Hazard ratios (HRs) [with 95% confidence interval (CI)] and P-values are presented. P-values <0.05 were considered statistically significant.
Results
Of 14 264 patients randomized in ROCKET AF, 93 (0.7%) were excluded due to violations in Good Clinical Practice guidelines at one site.
Patient characteristics
Of the 14 171 evaluable patients, 1925 (14%) were hospitalized at least once, and 474 of 1925 (25%) patients were hospitalized more than once. Characteristics of the patients stratified by hospitalizations during follow-up are shown in Table 1. Patients with no hospitalizations, compared with the other groups, were less likely to have a history of a prior myocardial infarction, diabetes, and chronic obstructive pulmonary disease (COPD). While history of heart failure was similar in all groups, baseline use of a diuretic was lower in patients with no hospitalizations compared with the other groups.
Table 1.
Baseline characteristics of patients stratified by hospitalizations
| All patients (N = 14 171) | No hospitalizations (N = 12 246) | 1 Hospitalization (N = 1451) | ≥1 Hospitalization (N = 474) | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 73 (65, 78) | 72 (65, 78) | 74 (67, 79) | 75 (67, 79) |
| Female | 40% (5605) | 40% (4904) | 37% (532) | 36% (169) |
| Medical history | ||||
| AF | ||||
| New onset | 1% (196) | 1% (170) | 1% (15) | 2% (11) |
| Paroxysmal | 18% (2490) | 17% (2128) | 18% (259) | 22% (103) |
| Persistent | 81% (11 485) | 81% (9948) | 81% (1177) | 76% (360) |
| CHADS2 score, mean (SD) | 3.5 (0.9) | 3.5 (0.9) | 3.5 (1.0) | 3.5 (1.0) |
| 1 | <1% (3) | <1% (3) | 0 | 0 |
| 2 | 13% (1857) | 13% (1540) | 16% (234) | 18% (83) |
| 3 | 44% (6169) | 44% (5401) | 41% (591) | 37% (177) |
| 4 | 29% (4067) | 29% (3547) | 26% (383) | 29% (137) |
| 5 | 13% (1797) | 13% (1532) | 14% (203) | 13% (62) |
| 6 | 2% (278) | 2% (223) | 3% (40) | 3% (15) |
| Prior stroke, TIA, or non-CNS embolism | 55% (7767) | 56% (6826) | 50% (723) | 46% (218) |
| Carotid artery disease | 4% (589) | 4% (469) | 6% (88) | 7% (32) |
| Congestive heart failure | 62% (8851) | 62% (7645) | 62% (899) | 65% (307) |
| Prior myocardial infarction | 17% (2446) | 16% (2003) | 22% (316) | 27% (127) |
| Peripheral arterial disease | 6% (832) | 6% (687) | 7% (104) | 9% (41) |
| Diabetes | 40% (5647) | 39% (4768) | 46% (879) | 46% (879) |
| Hypertension | 90% (12 824) | 90% (11 077) | 91% (1317) | 91% (430) |
| COPD | 10% (1481) | 10% (1172) | 15% (214) | 20% (95) |
| Baseline medications | ||||
| Prior aspirin | 37% (5184) | 36% (4456) | 38% (545) | 39% (183) |
| Prior vitamin K antagonist | 62% (8853) | 61% (7510) | 69% (1007) | 71% (336) |
| ACE-I/ARB | 74% (10 528) | 74% (9071) | 75% (1091) | 77% (366) |
| β-Blocker | 65% (9184) | 64% (7877) | 68% (993) | 66% (314) |
| Digitalis | 39% (5460) | 38% (4685) | 40% (576) | 42% (199) |
| Diuretic | 60% (8441) | 58% (7121) | 68% (980) | 72% (340) |
| Baseline evaluation | ||||
| Body mass index, kg/m2 | 28 (25, 32) | 28 (25, 32) | 28 (25, 32) | 28 (25, 33) |
| Heart rate, beats per minute | 76 (67, 86) | 76 (68, 86) | 75 (66, 85) | 75 (66, 86) |
| Systolic blood pressure, mm Hg | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) |
| Creatinine clearance,a mL/min | 67 (52, 87) | 68 (53, 87) | 64 (49, 85) | 63 (48, 87) |
| Left ventricular EF < 40% | 23% (2497) | 22% (2083) | 26% (304) | 28% (110) |
| Treatment assignment | ||||
| Rivaroxaban | 50% (7081) | 50% (6105) | 50% (732) | 51% (244) |
Continuous variables are shown as median (25th, 75th percentiles).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; SD, standard deviation; TIA, transient ischaemic attack.
aCreatinine clearance was calculated with the use of the Cockcroft–Gault formula.
Summary and classification of hospitalizations
A summary of hospitalizations during study follow-up are shown in Table 2, and reasons for hospitalization are displayed in Figure 1. Over a median follow-up of 22 months (25th, 75th percentile 16, 28), there were a total of 2614 hospitalizations. The median number was 1 (25th, 75th percentile 1, 1), and the overall range was from 1 to 9 hospitalizations per patient. There were a total of 2614 hospitalizations or 10.2 hospitalizations per 100 patient-years.
Table 2.
Summary of hospitalizations during study follow-up
| Frequency | |
|---|---|
| Patients with at least one hospitalization during follow-up | 14% (1925) |
| Days to first hospitalization, median (25th, 75th percentile) | 181 (70 391) |
| Hospitalizations among patients with any hospitalization (N = 1925) | |
| 1 | 75% (1451) |
| 2 | 18% (340) |
| 3 | 5% (88) |
| 4 | 2% (30) |
| 5 | 0.2% (4) |
| 6 | 0.4% (7) |
| ≥7 | 0.3% (5) |
| Median (25th, 75th percentile) | 1 (1, 1) |
| Total number of all-cause hospitalizations | 2614 |
| Total all-cause hospitalizations per 100 patient-years of follow-up | 10.2 |
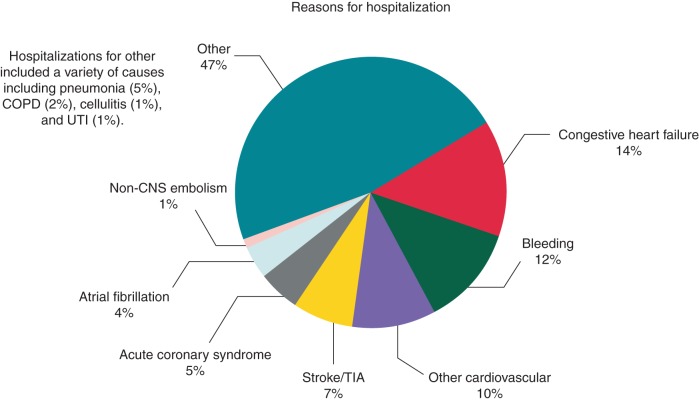
Figure 1.
Reasons for hospitalization during study follow-up. This figure displays the reasons for hospitalization among all hospitalizations (N = 2614). Atrial fibrillation accounted for 109 of 2614 (4%) total hospitalizations and is included under other cardiovascular causes. The reasons for other non-cardiovascular reasons are described in the Supplementary material online, Table. CNS, central nervous system; COPD, chronic obstructive pulmonary disease; TIA, transient ischaemic attack; UTI, urinary tract infection.
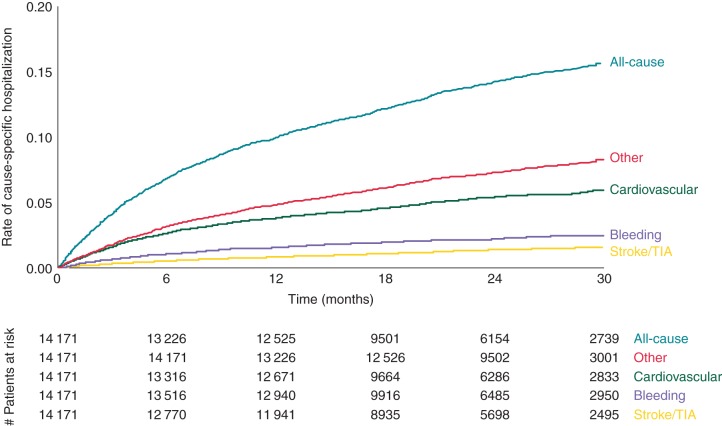
Cardiovascular causes (acute coronary syndrome, congestive heart failure, TIA, stroke, and other cardiovascular) were the primary reason for hospitalization in 41% of cases. Bleeding was the primary cause of hospitalization 12% of the time. Atrial fibrillation was the cause 4% of the time and is included under other cardiovascular causes. The largest reason was ‘other’ and including diagnoses such as pneumonia, COPD, and urinary tract infections. The complete list of other causes is listed in the Supplementary material online, Table. Time to first hospitalization by cause is displayed in Figure 2. The median time to first hospitalization was 181 days (25th, 75th percentile, 70, 391).
Figure 2.
Time to first hospitalization. This figure displays the time to first hospitalization stratified by type of hospitalization. TIA, transient ischaemic attack.
Hospitalizations by geographic region
The frequency and distribution of hospitalizations stratified by geographic region are shown in Table 3. Patients in Latin American and Eastern Europe were least likely to have a hospitalization, and patients in North America were most likely to have a hospitalization (P < 0.0001). In contrast, median hospital length of stay was longest in Latin American and Eastern Europe and shortest in North America (P < 0.0001).
Table 3.
Frequency and duration of hospitalizations stratified by region
| Latin America | Eastern Europe | East Asia | Western Europe | North America | P-value | |
|---|---|---|---|---|---|---|
| All patients | ||||||
| N | 1878 | 5407 | 2109 | 2096 | 2681 | |
| At least one hospitalization | 9% (164) | 10% (546) | 15% (318) | 17% (358) | 20% (539) | <.0001 |
| Days hospitalized per year, mean (SD) | 0.9 (10.9) | 1.0 (8.3) | 1.4 (8.9) | 1.6 (11.7) | 1.4 (7.5) | <0.0001 |
| Hospital length of stay, median (25th, 75th percentile) | 7 (4, 12) | 9 (6, 13) | 6 (4, 10) | 6 (4, 10) | 5 (3, 8) | <0.0001 |
SD, standard deviation.
Factors associated with all-cause and cardiovascular hospitalizations
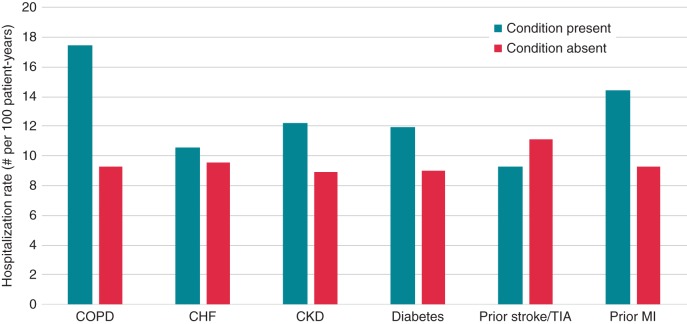
Patient factors associated with all-cause hospitalization are displayed in Table 4. Geographic region was independently associated with all-cause hospitalization with patients in Canada and USA having a higher hazard for hospitalization compared with Latin America (HR 0.53, 95% CI 0.44–0.64) and Eastern Europe (HR 0.64, 95% CI 0.56–0.74). Comorbid conditions were independently associated with increased risk of all-cause hospitalization including chronic lung disease (HR 1.46, 95% CI 1.29–1.66), diabetes (HR 1.22, 95% CI 1.11–1.34), prior myocardial infarction (HR 1.27, 95% 1.13–1.42), and impaired renal function (HR 1.07 per 5 unit decrease in creatinine clearance below 65 mL/min, 95% CI 1.04–1.10), and diuretic use (HR 1.37, 95% CI 1.24–1.52) was also independently associated with at least one hospitalization during follow-up. Randomized treatment (rivaroxaban vs. warfarin) was not associated with differential rates of hospitalization (P = 0.45). All-cause hospitalization rates by selected comorbid conditions are displayed in Figure 3. Patient factors associated with cardiovascular hospitalizations are displayed in Table 5.
Table 4.
Baseline patient characteristics associated with all-cause hospitalization
| χ2 | HR (95% CI) | P-value | |
|---|---|---|---|
| Geographic region (reference = Canada/US) | |||
| Latin America | 108.70 | 0.53 (0.44, 0.64) | <0.0001 |
| Eastern Europe | 0.64 (0.56, 0.74) | ||
| Western Europe | 1.05 (0.91, 1.20) | ||
| East Asia | 1.04 (0.89, 1.21) | ||
| COPD | 35.94 | 1.46 (1.29, 1.66) | <0.0001 |
| Baseline diuretic use | 35.91 | 1.36 (1.23, 1.51) | <0.0001 |
| Creatinine clearance, per 5-unit decrease below 65a | 25.90 | 1.07 (1.04, 1.10) | <0.0001 |
| SBP, per 5-mmHg decrease below 120b | 18.21 | 1.08 (1.04, 1.13) | <0.0001 |
| Diabetes | 17.38 | 1.22 (1.11, 1.34) | <0.0001 |
| Prior myocardial infarction | 16.91 | 1.27 (1.13, 1.42) | <0.0001 |
| Prior aspirin use | 12.39 | 1.21 (1.09, 1.34) | 0.0004 |
| BMI, per 1 unit increase above 31c | 9.79 | 1.02 (1.01, 1.03) | 0.0018 |
| Age, per 5 year increase | 8.78 | 1.05 (1.02, 1.08) | 0.0030 |
| Prior use of vitamin K antagonist | 7.43 | 1.18 (1.05, 1.32) | 0.0064 |
| Male | 6.14 | 1.13 (1.03, 1.25) | 0.013 |
| Carotid artery disease | 5.38 | 1.25 (1.04, 1.51) | 0.020 |
| Heart rate, per 5 bpm increase above 90d | 4.82 | 1.04 (1.00, 1.08) | 0.028 |
BMI, body mass index; SBP, systolic blood pressure; US, United States.
aFor creatinine clearance, there was equivalent risk for all values ≥65 mL/min.
bFor SBP, there was equivalent risk for all values ≥120 mmHg.
cFor BMI, there was equivalent risk for all values ≤31 kg/m2.
dFor heart rate, there was equivalent risk for all values ≤90 beats per minute.
Figure 3.
Hospitalization rates by the presence or absence of selected comorbid conditions. This figure displays the all-cause hospitalization rate per 100 patient-years by selected comorbid conditions. Patients may have more than one comorbid condition and be included in this figure. COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CKD, chronic kidney disease as is defined by a creatinine clearance <60 mL/min calculated with the use of the Cockcroft–Gault formula; TIA, transient ischaemic attack; MI, myocardial infarction.
Table 5.
Baseline patient characteristics associated with cardiovascular hospitalization
| χ2 | HR (95% CI) | P-value | |
|---|---|---|---|
| Creatinine clearance, per 5 unit decrease below 60a | 44.11 | 1.13 (1.09, 1.18) | <0.0001 |
| Prior myocardial infarction | 34.21 | 1.58 (1.35, 1.84) | <0.0001 |
| Geographic region (reference = Canada/US) | |||
| Latin America | 31.62 | 0.58 (0.44, 0.76) | <0.0001 |
| Eastern Europe | 0.81 (0.67, 0.96) | ||
| Western Europe | 1.17 (0.96, 1.43) | ||
| East Asia | 0.86 (0.68, 1.08) | ||
| Diabetes | 19.66 | 1.36 (1.19, 1.55) | <0.0001 |
| SBP, per 5 mmHg decrease below 125b | 12.75 | 1.08 (1.04, 1.13) | 0.0004 |
| Baseline diuretic use | 10.57 | 1.28 (1.10, 1.48) | <0.0001 |
| Baseline β-blocker | 8.04 | 1.24 (1.07, 1.44) | 0.0046 |
| Prior aspirin use | 4.89 | 1.17 (1.02, 1.34) | 0.027 |
| COPD | 4.57 | 1.24 (1.02, 1.50) | 0.032 |
SBP, systolic blood pressure; US, United States.
aFor creatinine clearance, there was equivalent risk for all values ≥60 mL/min.
bFor SBP, there was equivalent risk for all values ≥125 mmHg.
Discussion
The economic burden associated with treating AF is substantial and primarily due to hospital care.4,8 In this study, we assessed reasons for hospitalization and factors associated with hospitalization in patients with AF. To our knowledge, this is the first study to describe and compare AF hospitalizations in a multinational dataset. We found nearly 1 of 7 patients enrolled in the trial was hospitalized at least once within 2 years. The reasons for hospitalization were roughly split between cardiovascular and non-cardiovascular causes, and both AF and bleeding were rare causes of hospitalization. Patients in North America had the highest rate of hospitalization and factors associated with hospitalization included age and comorbid conditions.
Our study builds upon previous work on hospitalization in patients with AF. In a previous analysis of registry data of exclusively US patients, the rate of hospitalization was 31% annually, and comorbid conditions (heart failure, COPD, and diabetes) were associated with increased hazards for all-cause hospitalization.9 Our data confirm these findings in a multinational dataset. In a separate analysis of Medicare patients aged ≥65 years, 34% of patients had a hospitalization within the first year of follow-up, with both cardiovascular and non-cardiovascular reasons listed as the primary diagnoses code.10 The higher rates of hospitalization seen in these two studies compared with ours likely reflect the increased use of hospital-based care for patients with AF in the USA.
The geographic variability for both hospitalization and lengths of stay observed in our study suggests that more work should be done to understand international differences in care and outcomes for patients with AF. To our knowledge, this has not been previously described for AF care, though geographic variability in lengths of stay and outcomes has been observed in other chronic diseases requiring hospital-based care, including heart failure.11 The reasons for geographic variability in our study are not apparent from our data but could be related to differences in patient characteristics or care delivery models. Further research should evaluate these geographic differences to determine if the value of healthcare could be improved in the USA with better outpatient care delivery models. These data also have implications for the design and interpretation of future multinational clinical trials of patients with AF that may include hospitalization as an endpoint.
Given the important overlap between AF and heart failure12 and the frequent use of hospital-based care for patients with heart failure,13 it was somewhat surprising that heart failure was not associated with hospitalizations in our study. However, chronic diuretic use was associated with hospitalizations in our study, and previous studies suggest that doses of diuretics are important markers of heart failure severity.14 Diuretic use in our study may be a marker of heart failure severity and concomitant renal dysfunction. We also found that randomized treatment assignment (rivaroxaban vs. warfarin) was not associated with differential rates of hospitalization. Whether these findings can be extended to patients outside of clinical trials or lower-risk populations remains unclear.
This study has important implications for providers and investigators considering interventions aimed at reducing AF hospitalizations. For example, a clinical trial of nurse-led care for outpatients with AF from the Netherlands demonstrated reduced cardiovascular hospitalizations and cardiovascular mortality compared with usual care,15 and a recent clinical trial of a nurse-led disease management programme for patients with AF recently discharged from the hospital in Australia demonstrated more days alive and out of the hospital compared with usual care.16 Both of these interventions focused on AF-specific care, such as providing and educating patients on guideline-directed medical therapy for AF17 or improving risk assessment to improve antithrombotic treatments.18 These interventions may be enhanced and treatment effects improved by incorporating elements of disease management for comorbid conditions that are associated with hospitalizations, such as chronic pulmonary disease.
This study also has significant policy implications for providers and payers considering bundled payments and/or reimbursement penalties for patients with AF. A large proportion of hospitalizations related to AF are attributable to non-cardiovascular causes and AF-specific hospitalizations and hospitalizations due to bleeding while on anticoagulation were relatively uncommon. Further research is needed to determine whether care pathways directed at coexisting pulmonary disease, diabetes, and impaired renal function among patients with AF could reduce the need for and the costs associated with hospitalization.
Limitations
The data for this study are derived from patients enrolled in the ROCKET AF trial with specific eligibility criteria and thus may not be generalizable to other patient populations. The reasons for hospitalization were not adjudicated for all medical conditions, but an independent clinical endpoint committee adjudicated all suspected cases of stroke, systemic embolism, myocardial infarction, death, and bleeding events. The treatments administered during the hospitalization or level of acuity was not recorded as part of study follow-up. Residual measured or unmeasured confounding may impact some of our findings.
Conclusions
Nearly 1 of 7 of the moderate-to-high-risk ambulatory patients with AF in this trial was hospitalized within 2 years. The most common causes were cardiovascular related, but approximately half were for other medical conditions. Bleeding and AF were uncommon causes of hospitalization. Programmes and care pathways designed to reduce hospitalizations and healthcare expenditures in a similar moderate-to-high-risk population need to consider a variety of co-morbid medical conditions.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: A.D.D.: Consultant; Modest; Maquet; Research Grant: Modest; American Heart Association, Amgen, and Novartis Pharmaceuticals. R.C.B.: Consultant/Advisory Board; Modest; Bayer, Janssen, Daiichi Sankyo, Portola, Regado Biosciences, Boehringer Ingelheim. S.D.B.: Employment; Significant; Bayer. G.B.: Honoraria; Modest; Bayer HealthCare, BMS/Pfizer. Consultant/Advisory Board; Modest; Bayer HealthCare, BMS/Pfizer, Sanofi Aventis. W.H.: Research Grant; Significant; Boehringer Ingelheim. Consultant/Advisory Board; Modest; Sygnis Pharma Germany, Boehringer Ingelheim, Photothera USA, Codman USA, Bayer. J.L.H.: Consultant/Advisory Board; Modest; Bayer AG HealthCare, Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals, Pfizer, Sanofi Aventis, AstraZeneca, Boston Scientific, Janssen, Medtronic. Consultant/Advisory Board; Significant; Biotronik. G.J.H.: Honoraria; Modest; Bayer, Medscape (Heart.org). K.W.M.: Full disclosures prior to 1 August 2013 available at www.dcri.org. Disclosures after 1 August 2013 available at https://med.stanford.edu/profiles/47970?tab=research-and-scholarship. C.C.N.: Employment; Significant; Janssen Research & Development. D.E.S.: Research Grant; Significant; Johnson & Johnson, Bristol-Myers Squibb. Consultant/Advisory Board; Modest; Bayer HealthCare, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer. K.A.F.: Research Grant; Significant; Eli Lilly. Consultant/Advisory Board; Modest; Boehringer Ingelheim, Sanofi Aventis, AstraZeneca, Johnson & Johnson/Bayer. M.R.P.: Research Grant; Significant; Johnson & Johnson, AstraZeneca. Consultant/Advisory Board; Modest; Bayer, Janssen, AstraZeneca, Genzyme. J.P.P.: Research Grant; Significant; ARCA Biopharma, GE Healthcare, Johnson & Johnson, ResMed. Consultant/Advisory Board; Modest; Johnson & Johnson, Forest Laboratories, Spectranetics, Medtronic.
Funding
This work was supported by the Janssen Research & Development LLC, Raritan, NJ and Bayer HealthCare AG, Leverkusen, Germany. Funding to pay the Open Access publication charges for this article was provided by Janssen Research and Development.
Supplementary Material
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP et al. . Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 2. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009;104:1534–9. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ et al. . Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 5. Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace 2011;13:1375–85. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al. . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 7. Rocket AF study investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: Rationale and design of the rocket AF study. Am Heart J 2010;159:340–347 e341. [DOI] [PubMed] [Google Scholar]
- 8. Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the united states. Value Health 2006;9:348–56. [DOI] [PubMed] [Google Scholar]
- 9. Steinberg BA, Kim S, Fonarow GC, Thomas L, Ansell J, Kowey PR et al. . Drivers of hospitalization for patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J 2014;167:735–742.e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naccarelli GV, Johnston SS, Dalal M, Lin J, Patel PP. Rates and implications for hospitalization of patients ≥65 years of age with atrial fibrillation/flutter. Am J Cardiol 2012;109:543–9. [DOI] [PubMed] [Google Scholar]
- 11. Eapen ZJ, Reed SD, Li Y, Kociol RD, Armstrong PW, Starling RC et al. . Do countries or hospitals with longer hospital stays for acute heart failure have lower readmission rates?: Findings from ASCEND-HF. Circ Heart Fail 2013;6:727–32. [DOI] [PubMed] [Google Scholar]
- 12. Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014;64:710–21. [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol 2013;61:1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM et al. . Relation between dose of loop diuretics and outcomes in a heart failure population: results of the escape trial. Eur J Heart Fail 2007;9:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R et al. . Nurse-led care vs. Usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J 2012;33:2692–9. [DOI] [PubMed] [Google Scholar]
- 16. Stewart S, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C et al. . Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet 2015;385:775–84. [DOI] [PubMed] [Google Scholar]
- 17. Hendriks JM, de Wit R, Vrijhoef HJ, Tieleman RG, Crijns HJ. An integrated chronic care program for patients with atrial fibrillation: study protocol and methodology for an ongoing prospective randomised controlled trial. Int J Nurs Stud 2010;47:1310–6. [DOI] [PubMed] [Google Scholar]
- 18. Carrington MJ, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C et al. . Navigating the fine line between benefit and risk in chronic atrial fibrillation: rationale and design of the standard versus atrial fibrillation specific management study (SAFETY). Int J Cardiol 2013;166:359–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.