Abstract
Electricity generation by Geobacter sulfurreducens biofilms grown on electrodes involves matrix-associated electron carriers, such as c-type cytochromes. Yet, the contribution of the biofilm's conductive pili remains uncertain, largely because pili-defective mutants also have cytochrome defects. Here we report that a pili-deficient mutant carrying an inactivating mutation in the pilus assembly motor PilB has no measurable defects in cytochrome expression, yet forms anode biofilms with reduced electroactivity and is unable to grow beyond a threshold distance (∼10 μm) from the underlying electrode. The defects are similar to those of a Tyr3 mutant, which produces poorly conductive pili. The results support a model in which the conductive pili permeate the biofilms to wire the cells to the conductive biofilm matrix and the underlying electrode, operating coordinately with cytochromes until the biofilm reaches a threshold thickness that limits the efficiency of the cytochrome pathway but not the functioning of the conductive pili network.
 The roles played by cytochromes and conductive filamentous appendages (pili) in the electrical conductivity of Geobacter bacterial biofilms are controversial. Here, Steidl et al. present evidence that both mechanisms cooperate in thin biofilms, while pili are important for conductivity across thicker biofilms.
The roles played by cytochromes and conductive filamentous appendages (pili) in the electrical conductivity of Geobacter bacterial biofilms are controversial. Here, Steidl et al. present evidence that both mechanisms cooperate in thin biofilms, while pili are important for conductivity across thicker biofilms.
The ability of Geobacter bacteria to completely oxidize organic compounds to CO2 with an electrode poised at a metabolically oxidizing potential shows promise for the conversion of renewable biomass into electricity, hydrogen and/or liquid fuels in bioelectrochemical systems1,2,3. Energy recoveries in these devices depend greatly on the electroactivity of the electrode-associated biofilms4, yet the mechanism of biofilm conductance is not fully understood. Genetic studies in the model representative Geobacter sulfurreducens have identified genes encoding the biofilm exopolysaccharide (EPS) that anchors c-type cytochromes to the biofilm matrix5. Although the genome of G. sulfurreducens contains over 100 genes annotated as c-type cytochromes, some of which are required for efficient extracellular electron transfer6,7,8, only one, OmcZ, is essential for biofilm electroactivity8,9. Other c-type cytochromes may be required, but their genetic identification is challenging because of compensatory effects observed when the encoding genes are mutated7,10.
In situ electrical conductivity measurements on living biofilms of G. sulfurreducens grown on an electrode demonstrated the thermal dependence of charge transport, which is consistent with the incoherent redox conductivity mediated by the haem groups of the matrix-associated c-type cytochromes11. The role of cytochromes as electron carriers in the biofilms is further supported by the studies showing their reversible oxidization or reduction as a function of the anode potential12,13. However, while it is possible to reduce all of the biofilm cytochromes of thin (<10 μm) biofilms with a positive voltage, only half are reduced in thicker (∼20 μm) biofilms12. Furthermore, redox potentials decrease sharply as the biofilms grow to or beyond a 20-μm thickness14 and a redox gradient is established in these spatial scales that results in the accumulation of reduced cytochrome species further away from the electrode15. Source–drain experiments of biofilms grown across 10-μm wide gaps between electrodes indicate that the redox potential already decreases significantly in biofilm regions 10 μm away from the oxidizing electrode16. Thus, a redox gradient is established in the biofilms whereby more cytochrome-associated electrons are concentrated in the upper, electron-acceptor-limited biofilm stratum and decrease progressively towards the electrode-attached layers. The redox gradient has been proposed to provide the driving force for electron transport across the biofilms16, in a manner analogous to how electrons diffuse through redox polymers hopping among immobilized redox cofactors17.
The progressive decline in the rates of cytochrome oxidation and reduction in biofilm layers 10–20 μm away from the electrode indicates that a rate-limiting step exists when biofilms grow to or beyond this thickness. Yet, despite this limitation, G. sulfurreducens can grow electroactive biofilms tens of micrometres from the electrode surface while generating current proportionally to the biofilm thickness18. Furthermore, cells in the upper biofilm layers are metabolically active and continue to oxidize acetate while contributing to current production19. Consistent with this, acetate kinase, which serves as a proxy for acetate oxidative metabolism, is uniformly abundant across the biofilm20. By contrast, the expression of the outer membrane c-type cytochrome OmcB increases fourfold in cells located in the upper stratum20, where the redox potential is lowest14. This has lead to the proposal that cells in these upper, electron-acceptor-limited zones compensate for the lower rates of cytochrome-mediated redox reactions and maintain optimal rates of respiration by expressing ‘extra electron-transfer machinery'20.
One obvious candidate for discharging respiratory electrons and alleviating the electron acceptor limitation of cells in the upper biofilm regions is the conductive pili that G. sulfurreducens produces to reduce Fe(III) oxides21 and uranium22. The pili are also required to grow multilayered biofilms on iron-coated and glass surfaces23, plastic surfaces24 and on electrodes poised at an oxidizing potential18. The need to express pili to form biofilms, and the immunodetection of the outer membrane c-type cytochrome OmcS on chemically fixed pili produced by planktonic cells25, suggested that the pili may serve as a scaffold for the matrix-associated cytochromes26. However, scanning tunnelling microscopy studies did not reveal spectroscopic haem signatures along cell-associated, untreated pili27. The significance of OmcS in electroactive biofilms is also questionable, because deletion of the omcS gene had no effect in biofilm growth and maximum catalytic current9. Furthermore, omcS transcripts are less abundant in current-harvesting biofilms than in biofilms grown on the same electrode but operated in open circuit and fed the soluble electron acceptor fumarate8. A mere structural role for the pili is also incongruent with in vitro conductivity measurements, which show that the pilus fibres are protein nanowires capable of transporting charges at rates several orders of magnitude higher than the rates of cellular respiration28. The conductivity measured along individual pilus fibres and thermal dependence of their differential conductance at biologically relevant voltages28 is also consistent with the incoherent redox conductivity of living biofilms11. Further, a structural model of the pilus fibre refined via molecular dynamics identified transversal and axial multistep charge hopping paths involving aromatic residues (phenylalanines and tyrosines) of the pili required for optimal biofilm electrochemical activity29. The pili thus have the attributes necessary to function as biofilm electron carriers and could promote the discharge of respiratory electrons from cells in the electron-acceptor-limited upper regions to the oxidized cytochromes below. This is in accordance with the prediction that electron transfer across electroactive biofilms requires the expression of both cytochromes and pili to promote short-distance, electron-transfer reactions30.
Much of the difficulty in assessing the contribution of the pili to biofilm electroactivity stems from the fact that pili-deficient mutants reported thus far also have defects in cytochromes required for extracellular electron transfer22,24,31. Here we investigate the effect of several pili-inactivating mutations in the expression of cytochromes and identify one, a deletion in the gene encoding the pilin polymerization motor PilB, that has no measurable effects in cytochrome expression. Using the pilB mutant, we demonstrate that pili expression is required for optimal biofilm electroactivity in thin (∼10 μm) biofilms and to grow the biofilms beyond this threshold distance, which otherwise limits the rates of redox reactions involving the biofilm cytochromes. We also show that the pilB defects are similar to Tyr3, a mutant that carries alanine replacements in the three tyrosines of the pilus multistep hopping pathway29 and produces pili with reduced conductivity. The results thus indicate that pili, like cytochromes, are electron carriers in anode biofilms, but their ability to discharge respiratory electrons becomes progressively more critical as redox reactions involving cytochromes become rate-limiting in the upper zones. This strategy confers on Geobacter cells a metabolic advantage over other bacteria relying solely on cytochromes or diffusible electron carriers, as it maximizes energy generation through extracellular electron transfer even in cells positioned at tens of micrometre distances from the electrode.
Results
Inactivation of PilB does not affect cytochrome expression
The assembly of type IVa pili on the bacterial inner membrane is mediated by a protein apparatus involving three interacting subcomplexes: the pilus subcomplex (major pilin and minor pilins, if present), the motor subcomplex (containing several proteins, including the PilB and PilT ATPases that power pilus polymerization and retraction, respectively) and the alignment subcomplex (which properly aligns the assembled pilus through the outer membrane PilQF secretin complex)32. As mutations that render the pilus and motor subcomplexes inoperative also prevent pili expression32,33, we constructed mutants of G. sulfurreducens carrying deletions in the genes encoding the PilA pilin subunit (pilA mutant) and the PilB ATPase (pilB mutant), respectively. In addition, we constructed a pili-deficient mutant (pilA-E5A) carrying an alanine substitution in the conserved glutamic E5 amino acid required for proper alignment and assembly of pilins32,34.
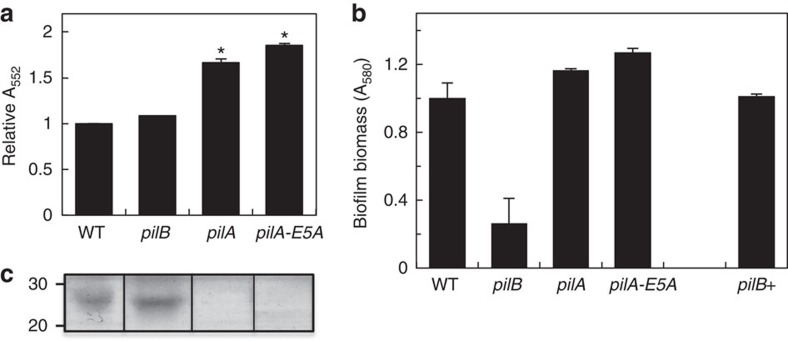
Two of the pili-inactivating mutations, pilA and pilA-E5A, had pleiotropic effects in cytochrome expression. Differential redox spectroscopy of reduced-minus-oxidized ultraviolet–visible spectra (Supplementary Fig. 1) revealed, for example, a greater cytochrome content in the pilA and pilA-E5A biofilms than in the wild type (WT; Fig. 1a). Similar increases were observed in planktonic cells of the two mutant strains (Supplementary Fig. 2), ruling out any influence of the physiological state of the cells (planktonic or biofilm) in the phenotype. The gene encoding the outer membrane c-type cytochrome OmcB was also upregulated (Supplementary Fig. 3), suggesting that some of the pleiotropic cytochrome effects observed in the pilA and pilA-E5A mutants were transcriptional in nature. By contrast, transcript levels for the gene encoding the outer membrane c-type cytochrome OmcZ were similar in the WT, and the pilA and pilA-E5A mutants (Supplementary Fig. 3). Yet, the processed form of this cytochrome (OmcZS, ∼30 kDa), which is released to the biofilm matrix5 and is required for optimal biofilm redox activity8,24, was absent in the matrix of the mutant biofilms (Fig. 1c). OmcZS was however detected in haem-stained preparations of planktonic cells (Supplementary Fig. 2), suggesting that the inability of the pilA and pilA-E5A mutant biofilms to secrete this cytochrome was related to the unique physiology of cells living within a surface-attached community.
Figure 1. Phenotypic characterization of pili-deficient mutant (pilB, pilA and pilA-E5A) biofilms.
(a) Relative cytochrome content in biofilm extracts of the pili-deficient mutants in reference to WT (average and s.e. of two biological replicates for each). Significant differences in cytochrome content (*P<0.01) relative to the WT were identified in t-test pairwise comparisons. (b) Biofilm biomass on plastic surfaces estimated from the OD580 of the biofilm-associated crystal violet solubilized with acetic acid (shown are averages and s.d. of six biofilm replicates of the WT, pili-deficient mutants and the genetic complemented pilB+ strain). (c) OmcZS band among haem-stained proteins isolated from the biofilm matrix of the WT and pili-deficient strains (full gels are shown in Supplementary Fig. 2c). Lanes were loaded with 20 μg of protein. Numbers at left are relative molecular masses of protein standards in kDa.
Pili-deficient mutants have been reported to build thin biofilms on glass surfaces23, presumably because the pili provide the structural support needed to stack cells on the surface. Consistent with this, 24-h biofilms of the pilA and pilA-E5A strains that formed on plastic surfaces were less dense than the WT (Supplementary Fig. 4); however, the mutant biofilms reached WT biofilm biomass levels after 48 h (Fig. 1b; Supplementary Fig. 4). The upregulation of the xapD gene, encoding the ATP-dependent exporter of the biofilm EPS5, in these mutants suggests that EPS synthesis could have compensated for the pili deficiency during the early stages of biofilm formation, as reported for other bacteria35. But once the mutant biofilms reached WT biomass levels at 48 h, the amount of EPS produced per cell was similar to the WT (Supplementary Fig. 5).
In contrast to the pleiotropic nature of the pilA and pilA-E5A mutations, preventing pilin assembly through the inactivation of the PilB motor (pilB mutant) had no measurable effects on the cytochrome content of the biofilms (Fig. 1a). Furthermore, the transcription of genes in the pilin operon (pilA and GSU1497) or those encoding the outer membrane c-type cytochromes OmcB and OmcZ was not affected either (Supplementary Fig. 3). Moreover, OmcZS was secreted in the pilB biofilm matrix (Fig. 1c), and xapD transcripts (Supplementary Fig. 3) and biofilm EPS produced per cell (Supplementary Fig. 5) were similar in the pilB and WT biofilms. As with other pili-deficient mutants23, the pilB biofilms that formed on plastic surfaces were less dense than the WT even after 48 h of incubation (Supplementary Fig. 4), as indicated by the reduced biofilm biomass stained with crystal violet (Fig. 1b) and the lower cell protein content extracted from the pilB biofilms (75±1% of the WT, average and s.e. of two independent experiments). Yet, the pilB biofilm defect was rescued in the genetically complemented pilB+ strain, which restored pili production through the expression of the pilB gene in trans from a medium-copy plasmid (Fig. 1b). Hence, the pilB strain provides the elusive genetic tool needed to assess the contribution of Geobacter conductive pili in the growth and electrochemical activity of anode biofilms.
Thin (∼10 μm) biofilms require both cytochromes and pili
We investigated the ability of the pili-deficient mutants to generate current in microbial electrolysis cells (MECs) fed an initial concentration of 1 mM acetate and operated in batch in reference to the WT strain. Under these conditions, all of the strains tested grew reproducibly to a thickness of ca. 10 μm, but the rates of current production and the maximum current reached were reduced in the three pili-deficient mutants (Fig. 2a). The pilA and pilA-E5A mutant biofilms, which in addition to the pili deficiency do not secrete OmcZS in the biofilm matrix (Fig. 1c), reached low levels of current (0.08±0.05 and 0.14±0.08 mA, respectively). As a result, prolonged incubations were required for the mutant biofilms to oxidize all the acetate in the anode chamber (Fig. 2a) and to reach WT thickness (∼ 13.5±3.5 and 11.6±1.9 μm, respectively, in duplicate MECs). Similar low levels of current (0.11±0.07 mA for duplicate MECs) and lengthy incubation times were recorded in control MECs driven by a null omcZ mutant (Fig. 2a), consistent with the need of the biofilms to concentrate OmcZS near and on the electrode for efficient electron transport to the anode surface36. The expression of OmcZS in the pilB biofilm matrix (Fig. 1c) improved MEC performance compared with the pilA and pilA-E5A mutants, but did not fully restore WT levels of current (Fig. 2a). Maximum current harnessed from the pilB anode biofilms in triplicate MECs averaged, for example, 0.52 (±0.06 mA), which is about half of the maximum current produced by WT biofilms (1.06±0.05 mA).
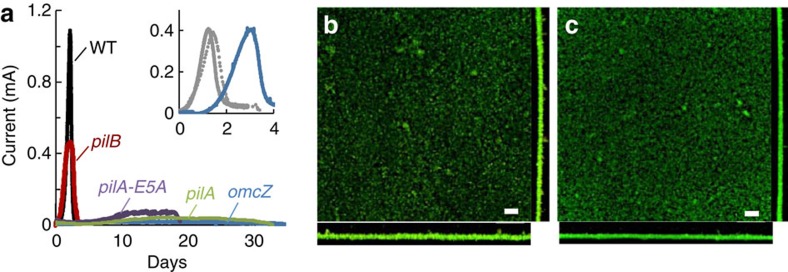
Figure 2. Current production by anode biofilms of WT and mutants in MECs fed with 1 mM acetate.
(a) Representative plots of current generation by WT, pili-deficient strains (pilB, pilA and pilA-E5A) and omcZ mutant in MECs operated in batch and fed with an initial concentration of 1 mM acetate. Inset shows current generation (Y axis, in mA) over time (X axis, in days) by double mutants pilB mshE (solid grey), pilB gspE (dashed grey) and an equal mixture of pilB and omcZ cells (blue). (b,c) Confocal micrographs showing top and side views of anode biofilms of the WT (b) and pilB (c) strains collected at the end of the MEC experiment, when current had decreased to <0.1 mA, and stained with the BacLight viability kit (green, live; red, dead). Scale bar, 20 μm.
The partial restoration of biofilm electroactivity in pilB compared with the other pili-deficient mutants did not result from functional complementation by the PilB homologues MshE and GspE (putative ATPase motors of type II secretion systems) because deleting the pilB and mshE or gspE genes generated double mutants that were phenotypically indistinguishable from the pilB single mutant (Fig. 2a, inset). In addition, the pilB mutant defect could not be complemented in MECs co-inoculated with the pilB and omcZ mutant cells (Fig. 2a, inset). We did observe a delay in current generation in the co-culture-driven MECs, which is the phase when cells colonize the electrode before growing on it while coupling the oxidation of acetate to the reduction of the electrode37. However, once current production was initiated, the performance of the co-culture MECs was similar to the pilB MECs. This suggests that biofilm cells need to express both pili and OmcZS for optimal biofilm growth and electroactivity.
The rates of current increase in the pilB MECs, which correlate with the exponential phase of biofilm growth37, were also reduced in half (0.61±0.15 mA per day) compared with the WT biofilms (1.45±0.06 mA per day). However, coulombic efficiencies in the pilB-driven MECs (96.6±3.1) were similar to the WT (94.6±4.1), indicating that, on average, the pilB biofilms converted the same amount of acetate to electricity as the WT biofilms, they just did so at slower rates. Confocal micrographs of WT and pilB biofilms collected at the end of the experiment, when all of the acetate had been depleted, and stained with fluorescent viability dyes showed predominantly live cells and similar biofilm structure (Fig. 2b,c, respectively). Furthermore, the biofilm biomass estimated from the fluorescence emitted by the biofilm cells was similar in both strains (10.05±1.00 in WT and 9.06±1.46 μm3 μm−2 in pilB). Thus, the pilB defects cannot be attributed to a reduction in the number of cells actively contributing to electron transfer or structural variations of the biofilms. Furthermore, the pilB phenotypes in MECs fed with 1 mM acetate (Fig. 2) are similar to those reported for Tyr3, a mutant that produces pili with alanine replacements in the pilin's three tyrosines of the pilus multistep hopping pathway that impair the cell's ability to transfer electrons to extracellular electron acceptors such as Fe(III) oxides29. Hence, the pilB phenotypes are consistent with reductions in the respiratory rate of individual cells resulting from the inability of the mutant cells to discharge electrons using the pili.
The conductivity of the pili is required to grow thick biofilms
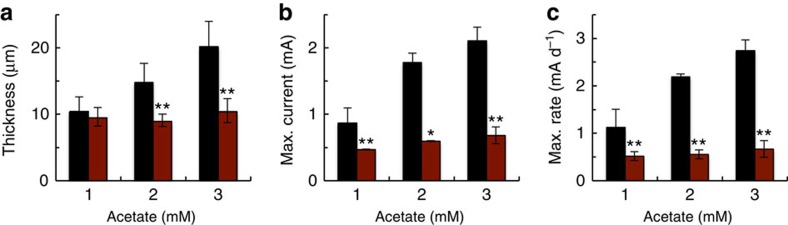
By increasing the initial amount of electron donor (acetate) added to the anode chamber of the MECs operated in batch, we were able to increase the thickness of the WT anode biofilms from ∼10 to 15 μm (2 mM acetate) and 20 μm (3 mM acetate; Fig. 3a). These are spatial scales that lead to the progressive accumulation of reduced cytochromes in the upper biofilm layers, thus limiting the ability of the cells to use cytochromes in these regions as electron acceptors for respiration12. Yet, cells in these electron-acceptor-limited layers are actively respiring and contributing to current production. To investigate a potential role for the conductive pili in alleviating the limitations imposed by the reductions of redox potential in the upper biofilm stratum, we grew the pilB mutant in MECs fed with 2 and 3 mM acetate. In contrast to the linear increases in WT biofilm thickness with initial acetate feeding, the pilB biofilms remained thin (∼10 μm) under all the conditions tested (Fig. 3a). Similarly, parameters that measure the biofilm electroactivity such as current maxima and rates of current production increased proportionally to acetate availability in the WT MECs, but were unaffected in the pilB MECs (Fig. 3b,c).
Figure 3. Thickness and electroactivity of WT and pilB biofilms.
Anode biofilm thickness (a), maximum current (b) and maximum rates of current production (c) in batch MECs fed with an initial concentration of 1, 2 and 3 mM acetate and driven by the WT (black) or the pilB mutant (maroon) strains. Shown are average and s.d.'s or errors of four (1 mM) or two (2 and 3 mM) independent MEC experiments, respectively. Significant changes between the WT and pilB values at each acetate concentration are indicated with stars (*P <0.05; **P<0.005). Representative plots of current generation used to estimate biofilm thickness and electroactivity at each acetate concentration are shown in Supplementary Fig. 6.
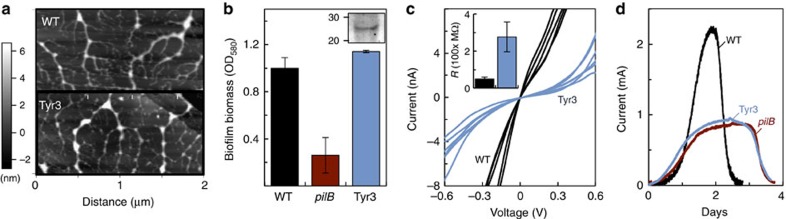
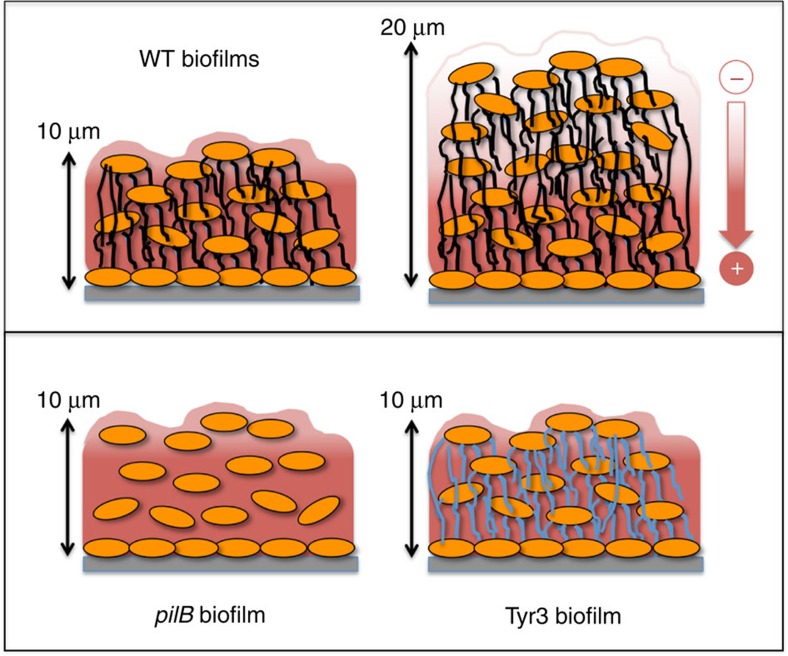
As a control, we also characterized the Tyr3 mutant strain phenotypically (Fig. 4). The Tyr3 mutant produced pili at WT levels (Fig. 4a), which provided the structural support needed to grow dense biofilms on plastic surfaces when fumarate was provided as the electron acceptor (Fig. 4b). The Tyr3 biofilms also expressed OmcZS in the biofilm matrix (Fig. 4b, inset). However, as predicted in molecular dynamics simulations29, the reduced number of aromatic contacts in the Tyr3 pili increased their electrical resistance (∼280 MΩ) fivefold over that measured in the WT pili (∼50 MΩ) (Fig. 4c). Furthermore, Tyr3 anode biofilms in MECs fed with 3 mM acetate remained thin (13.3±2.4 μm, for duplicate MECs) and reached current levels (0.81±0.10 mA, for duplicate MECs) comparable to those recorded in the pilB-driven MECs and fed the same amount of acetate (Fig. 4d). Thus, the ability of cells to grow thick (>10 μm) biofilms on the anode electrode while coupling the oxidation of acetate to current generation depends on the conductivity, rather than the structural support, provided by the pili.
Figure 4. Role of pilus conductivity in the growth and electroactivity of anode biofilms.
(a) AFM topographic images of pili purified from the WT and Tyr3 strains and deposited on a HOPG surface. (b) Crystal-violet-stained biomass of 48-h-old biofilms of the pilB and Tyr3 mutants relative to WT (shown are averages and s.d.'s of six biofilm replicates for each strain). Inset: OmcZS band in haem-stained proteins isolated from the biofilm matrix of the Tyr3 biofilms (20 μg of protein loaded per lane; full gel is shown in Supplementary Fig. 2d). Numbers at left are relative molecular masses of protein standards in kDa. (c) Representative current–voltage (I–V) plots obtained after probing the transversal conductivity of individual WT and Tyr3 pili (a). Inset shows the average and s.d. of the electrical resistance of four or more WT or Tyr3 pili, each probed in at least three positions. (d) Current production by WT, pilB and Tyr3 biofilms grown in MECs fed initially with 3 mM acetate.
Discussion
The studies described herein provide the elusive genetic evidence, in the form of the pilB mutant, needed to assess the contribution of the conductive pili of G. sulfurreducens to the electrochemical activity of electrode-associated biofilms. Indeed, the pilB mutant had no measurable changes in c-type cytochromes (Fig. 1) and was able to form biofilms of WT thickness (∼10 μm) in MECs operated in batch and fed an initial acetate concentration of 1 mM (Fig. 2). Yet, despite forming anode biofilms as thick as the WT under these conditions, the pili deficiency prevented the mutant from generating current at the same rates as the piliated WT strain and reduced current maxima (Fig. 3). Furthermore, the pilB mutant was unable to grow biofilms beyond this threshold thickness when the initial concentration of acetate fed to the MECs was increased from 1 to 2 or 3 mM, which are conditions that allow the WT biofilms to grow ∼15- and 20-μm thick, respectively (Fig. 3). We can rule out mass transport limitations because the biofilms investigated in this study were too thin (<50 μm) to restrict the diffusion of the electron donor, acetate38. Furthermore, the phenotypes of the pili-deficient pilB mutant in MECs were similar to those of a Tyr3 mutant, which produces pili with reduced conductivity (Fig. 4). This finding challenges the proposal that the pili's role is restricted to providing structural support needed for cytochrome organization26 and is instead consistent with the need of the biofilm cells to express conductive pili to transport electrons.
The genetic evidence therefore supports a model in which the conductive pili and the cytochromes work coordinately as electron carriers to maintain optimal rates of electron transfer in thin (∼10-μm thick) biofilms (Fig. 2). This is in accordance with the early models that suggested that electron transfer across electroactive biofilms may require the expression of both cytochromes and pili to promote short-distance, electron-transfer reactions30. However, as the biofilms grow further away from the electrode, the cytochromes become progressively more reduced and the ability of the pili to discharge respiratory electrons becomes more critical (Fig. 3). The redox potential decreases significantly already in regions of the biofilm only 10 μm away from an oxidizing electrode16. When compared with a 10-μm thick WT biofilm, the electrochemical activity of the pilB biofilms (rates of current production and maximum current) was reduced approximately in half (Figs 2 and 3). The pilB defects cannot be attributed to decreases in the number of biofilm cells contributing to electron transfer, because the biofilm thickness and the number of viable cells in the pilB biofilms were comparable to the WT. EPS production (Supplementary Fig. 5) and OmcZS expression in the biofilm matrix (Fig. 1c) were also similar in the pilB and WT biofilms. The reductions in electrochemical activity of 10-μm pilB biofilms are also within the ranges reported for Tyr3 biofilms grown to a similar thickness29. Tyr3 pili are expressed at WT levels, but carry alanine replacements in the three tyrosines of the pilus multistep hopping pathway29 that reduce the conductivity of the pili more than fivefold (Fig. 4). We measured, for example, an average electrical resistance of∼50 MΩ for WT pili deposited on highly oriented pyrolytic graphite (HOPG), which is within the ranges reported for pili deposited on gold electrodes28, and ∼280 MΩ in the Tyr3 pili. Thus, the reduced electrochemical activity of the pilB and Tyr3 biofilms results from the inability of the mutant cells to discharge respiratory electrons via the pili.
In vitro charge transport measurements indicate that individual pilus fibres can transport charges at micrometre distances and at rates (ca. 109 electrons per second for a 1-μm-long pilus at 100 mV) that greatly exceed the cellular respiratory rates28. Furthermore, the pili can reach micrometre lengths21 and physically interact with extracellular cytochromes25 of the biofilm matrix5. This could allow the biofilm pili to directly discharge electrons from the cells to matrix-associated cytochromes such as OmcZS. This multihaeme c-type cytochrome preferentially localizes closer to the electrode and on its surface36 and has the wide redox potential working range39 needed to function as an electrochemical gate between the biofilm and the electrode36. Indeed, pilin mutations that resulted in defects in OmcZS expression (pilA and pilA-E5A; Fig. 1) reduced the capacity of the biofilms to produce current to levels similar to a control omcZ mutant (Fig. 2). The pilA and pilA-E5A mutant cells also grew very slowly on the anode, taking several days to oxidize the acetate and reach WT biofilm thickness (∼10 μm). The expression of OmcZS in the pilB biofilm matrix (Fig. 1c) alleviated the defect but only partially (Fig. 2). Thus, the pili are required for optimal current production within spatial scales (∼10 μm) where biofilm cytochromes, including OmcZS, are available as electron carriers.
The coordinated interaction between pili and cytochrome electron carriers in the biofilm layers closer to the electrode is further supported by the cross-regulation of pili assembly and cytochrome export observed in this and one other study40. Earlier studies demonstrated that the PilA pilin protein of G. sulfurreducens is translated as both a short and a long prepilin isoform, which interact to promote the processing and assembly of the short pilin and the export of c-type cytochromes such as OmcZ to the outer membrane40. Indeed, we showed that mutations that prevented pilin expression or disrupted pilin–pilin interactions (pilA and pilA-E5A, respectively) interfered with the pilin's role in secretion and lead to defects in cytochrome expression and localization (Fig. 1). Similarly, pilA-deficient mutants used in earlier genetic studies18,21,41 also had defects in c-type cytochromes required for optimal extracellular electron transfer22,24. The dual role that Geobacter pilins play in pili synthesis and cytochrome export is in accordance with the multiple biological roles reported for type IV pilins from other bacteria. In Pseudomonas aeruginosa, for example, the structural subunit of the pilus fibre (the PilA pilin) interacts with components of the general secretion pathway to promote the export of proteins across the outer membrane42. As a result, mutations that inactivate PilA in this bacterium affect the secretion of numerous proteins and produce mutant strains with multiple phenotypes. By contrast, the function of PilB is restricted to pilus biogenesis. Thus, inactivating mutations in the pilB gene of P. aeruginosa prevent pili expression, but do not cause export defects43. Similarly, inactivating the PilB motor of G. sulfurreducens prevents the assembly of the pilins, but does not interfere with pilin interactions needed for cytochrome export. As a result, cytochrome expression in the pilB mutant was as in the WT strain (Fig. 1).
As the biofilms grow further apart from the electrode (beyond a threshold distance of ∼10 μm), the reversible oxidization or reduction of biofilm cytochromes becomes rate-limiting12. Redox potentials decrease sharply beyond this threshold distance14,16 and reduced species progressively accumulate in biofilm layers further away from the electrode15. The redox gradient provides the driving force for electron transport across the biofilms16, but also limits the ability of cells in the upper layers to discharge respiratory electrons to nearby cytochromes. It has been proposed that cells in these outer regions of the biofilm overcome this limitation by upregulating the expression of electron transport proteins involved in extracellular electron transfer20. Consistent with this model, while it was possible to grow thicker (ca. 15 and 20 μm) WT biofilms in MECs, preventing the assembly of the pilins (pilB mutant) or reducing the conductivity of the assembled pili (Tyr3 mutant) effectively prevented the biofilms from growing >10 μm away from the electrode (Fig. 4). Hence, the expression and conductive properties of the pili are required to maintain optimal electrical connectivity as the biofilms grow in thickness and the cytochromes become progressively more reduced. These upper layers of the biofilm may be electron acceptor limited, but are also the regions where electron donor (acetate) availability is highest19. Studies show that G. sulfurreducens can continue to metabolize acetate in the absence of an electron acceptor by overexpressing its extracytoplasmic cytochromes and using them as a capacitor to store up to 106 electrons per cell44. Indeed, the outer membrane c-type cytochrome OmcB, which is transcriptionally upregulated under conditions of electron acceptor limitation45, is four times more abundant in cells from the 10–20-μm upper biofilm stratum than in the biofilm layers closer to the electrode20. This suggests that cells in these upper biofilm regions could continue to metabolize acetate and generate a proton motive force for energy generation, accumulating electrons in the cytochromes of the cell envelope. The pilus apparatus is anchored in the cell envelope of Gram-negative bacteria; hence, it could potentially accept electrons from the extracytoplasmic cytochromes, promoting their discharge from the cell to the biofilm matrix. By growing several micrometres in length21 and intertwining to form a complex web of nanowires27, the pili could electronically connect the upper and bottom strata of the biofilm, effectively bypassing the rate limitation imposed by the accumulation of reduced cytochromes in the electron-acceptor-limited regions.
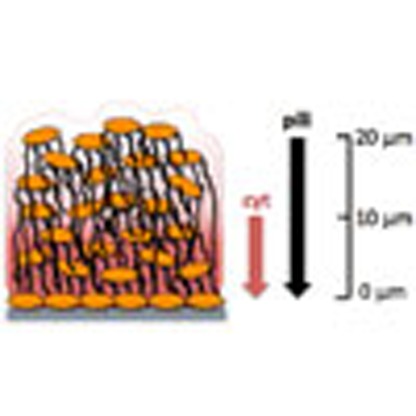
Taken together, the results support a model of mechanistic stratification in electroactive biofilms mediated by the pilus nanowires (Fig. 5). According to the model, the conductive pili network works coordinately with the matrix-associated cytochrome carriers to transfer electrons in the region (<10 μm) closer to the electrode. However, the pili become the primary mechanism for discharging respiratory electrons in the upper (>10 μm) region, where cytochromes are predominantly in a reduced state (Fig. 5). The pili can discharge electrons at micrometre distances and at rates that greatly exceed the cellular respiratory rates28. These attributes could allow the pili network to electronically connect cells in the upper layers of the biofilm to the oxidized cytochromes present in the regions closer to the electrode. As a result, mutants that do not assemble (pilB) or produce poorly conductive (Tyr3) pili cannot grow beyond this threshold distance and have reduced electroactivity compared with WT biofilms of comparable thickness (Fig. 5). The coordinated interactions between pili and cytochrome electron carriers revealed in our genetic studies challenge the proposal41 that metallic-like pilus conductivity fully accounts for electron flow across the biofilms and is in accordance with the prediction30 that electron transfer across electroactive biofilms involves short-distance, multistep hopping reactions between pili and cytochromes. Furthermore, living biofilms show the thermal dependence of incoherent conductivity predicted for electron-transfer reactions involving haems of c-type cytochromes11 and also demonstrated for charge transport via pili28. Phenylalanine and tyrosine residues cluster during pilin assembly, creating axial and transversal pathways with inter-aromatic distances and dimer geometries optimal for multistep hopping29. The tyrosine residues are in close proximity to acidic residues, which could be transiently protonated to promote proton-coupled electron transfer between tyrosine residues29. The simultaneous transfer of a proton and an electron lowers the oxidation potential of the tyrosine residue to the levels needed to enable fast rates of electron transfer in biological systems46,47. The transient protonation of acidic residues of the pili during charge transport also makes the rates of charge transport along the pili pH dependent. Thus, pH gradients that establish across anode biofilms14,48 likely tune the rates of charge transport via the pili and interactions with cytochromes, particularly in the biofilm regions closer to the electrode, where the pH is lower.
Figure 5. Model of mechanistic stratification of electroactive biofilms.
The pili permeate the biofilm matrix and promote the discharge of respiratory electrons to the oxidized cytochromes (in red, positive charge) in thin (∼10-μm thick) biofilms. As the biofilms grow in thickness, reduced cytochromes accumulate (in white, negative charge) and the pili provide the means for the cells to discharge respiratory electrons to the oxidized cytochromes below. In the absence of pili (pilB biofilms) or in biofilms expressing poorly conductive pili (Tyr3 biofilms), biofilm growth is limited to the thickness (∼10 μm), where oxidized cytochromes are available to serve as electron acceptor to the cells.
In addition to pH gradients, redox and electrical gradients form across the biofilms that can influence the physiology of the cells and could control the efficiency of charge transport along the pili and interactions with cytochromes to maintain optimal rates of electron transfer. Nutrient gradients also form in biofilms, which can influence the cell physiology and, therefore, the expression of biofilm electron carriers13,14. Microarray analyses of thin sections of anode biofilms of G. sulfurreducens did not detect changes in the expression of the pilA gene49. However, pilA is also transcribed under conditions that do not result in pili assembly50. Thus, the levels of cell piliation across the biofilms could be modulated by the local biofilm microenvironment to maintain optimal rates of respiration, as observed for other respiratory components20. The regulatory networks that control the expression and electrochemical activity of electron carriers such as the pili may be responsive to more than one biofilm parameter, such as pH, nutrient availability and redox potential. Biofilms of oxygen-respiring bacteria are, for example, responsive to redox potentials and use redox cues from their microenvironment to alter the biofilm structure, maximize oxygen diffusion and maintain redox homeostasis51. Similar mechanisms could control the expression of pili and cytochromes to facilitate their coordinated interactions and redox homeostasis in G. sulfurreducens biofilms. Thus, understanding the multiple functions that pili play in G. sulfurreducens and their regulation may prove instrumental to improve the performance of Geobacter-driven electrochemical systems for applications in bioenergy.
Methods
Bacterial strains and culture conditions
The bacterial strains used in this study are described in Supplementary Table 1. The WT strain, G. sulfurreducens strain PCA (ATCC 51573), was kindly provided by Daniel Bond (University of Minnesota) and was used to construct three knock-out mutants: pilB (carrying a deletion in the pilB gene, GSU1491), pilA (carrying a deletion in the pilin gene pilA, GSU1496) and omcZ (carrying a deletion in the omcZ gene, GSU2076). When indicated, the pilB mutation was complemented in trans by expressing a WT copy of pilB from the pRG5 plasmid (pilB+ strain). The WT strain was also used to construct the pilA-E5A mutant (carrying a single alanine replacement of the glutamic 5 residue in the mature pilin PilA protein) and the Tyr3 mutant (carrying alanine replacements in the pilin's three tyrosine residues Y27, Y32 and Y57). The WT and mutant strains were routinely cultured anaerobically in NB medium52 supplemented with 15 mM acetate as the electron donor and 40 mM fumarate as the electron acceptor (NBAF) and 0.1% yeast extract and 1 mM cysteine (NBAFYE). For biofilm assays, cells were cultured in modified freshwater FW medium22 with 30 mM acetate and 40 mM fumarate. Cells used to inoculate the anode chamber of MECs were grown in mineral DB medium53 supplemented with 20 mM acetate and 40 mM fumarate (DBAF).
DNA manipulations and mutant construction
Deletions of the pilB, pilA and omcZ genes were constructed using the cre–lox system54 with the primers listed in Supplementary Table 2. The general procedure included the PCR amplification of the gentamycin (Gm) resistance cassette (aaaC1) flanked by loxP sites (Gm-loxP) from plasmid pCM351 (ref. 54) using primer set RS21–RS22 and of the upstream/downstream target chromosomal regions using primer sets RS1–RS2/RS3–RS4 (pilB), RS5–RS6/RS7–RS8 (pilA) and RS9–RS10/RS11–RS12 (omcZ). The upstream region of the target gene, the Gm-loxP cassette and the downstream region of each gene were then fused in that order by overlap extension PCR using the corresponding external forward and reverse primers, and the Herculase II Fusion DNA Polymerase (Agilent Technologies). PCR conditions for this last amplification step were: 2 min of denaturation at 95 °C; 35 cycles of 20 s at 95 °C, 25 s at 54 °C and 2 min at 72 °C; and a final 3-min extension at 72 °C. The PCR products were then separated on an agarose gel, purified using the Zymoclean Gel DNA Recovery kit (Zymo Research), and cloned into the pCR2.1 plasmid (Supplementary Table 2) using the TOPO TA Cloning kit (Life Technologies) for sequence confirmation. After cloning, the constructs were PCR-amplified with the external primers, purified from an agarose gel and electroporated into electrocompetent cells of G. sulfurreducens following a previously published procedure52. Selection of recombinant strains was performed on NBAFYE plates supplemented with 5 μg ml−1 of gentamycin. When indicated, the Gm cassette carried by the pilB mutant was excised from its chromosomal location by expressing the Cre recombinase from plasmid pCM158 (ref. 54) and selecting for transformants on NBAFYE plates supplemented with 200 μg ml−1 of kanamycin. The excision of the marker was confirmed by PCR and the resulting mutant (pilB) was transferred twice in NBAFYE medium without kanamycin and then plated on NBAFYE with and without kanamycin to confirm the loss of the plasmid. The pilB strain was used to construct the double pilB gspE and pilB mshE mutants (Supplementary Table 1) using the general PCR procedure described above but with primer sets RS9–RS12, RS13–RS16 and RS17–RS20 (Supplementary Table 2), respectively.
We constructed the pilA-E5A and Tyr3 mutants by introducing one (E5A) or three (Y27A, Y32A and Y57A) alanine replacements, respectively, in targeted codons in the pilA gene (GSU1496). The primers used to construct these mutants are listed in Supplementary Table 2. The general procedure was to PCR amplify the pilA gene with its upstream chromosomal region (primers RS23–RS24), the downstream region of pilA (primers RS25–RS26) and a spectinomycin cassette (Sp; aadA gene) from plasmid pRG5 (ref. 10; primers RS27–RS28). The three fragments were then fused by overlap extension PCR (primers RS23 and RS26) to generate a 1684-bp DNA construct (pilA-Sp) containing the pilA gene and its upstream region, the antibiotic cassette (aadA), and the pilA downstream region. The construct was gel-purified and cloned into plasmid pCR2.1-TOPO TA vector (Invitrogen). Targeted nucleotide substitutions in the pilA gene were introduced using the QuikChange Lightning Site-Directed Mutagenesis kit (Agilent Technologies) using primers RS29 and RS30 (E5A substitution). Tyr3 was generated by sequentially introducing Y27A (RS31–RS32), Y32A (RS33–RS34) and, last, Y57A (RS35–RS36), as previously described29. The mutated fragments were confirmed by sequencing before PCR amplification using the external primers, gel purification and electroporation into electrocompetent cells of G. sulfurreducens. Selection of recombinant strains was performed on NBAFYE plates supplemented with 75 μg ml−1 of spectinomycin.
Reverse Transcription-quantitative–PCR
Transcripts for key components of the biofilm matrix (pili, EPS matrix and matrix-associated c-type cytochromes) were quantified by reverse transcription-quantitative PCR (RT–qPCR). The gene targets were the pilin-encoding gene pilA (GSU1496) and its downstream gene (GSU1497) in the pilA operon21, the ATP-dependent EPS exporter xapD (GSU1501)5, the outer membrane c-type cytochrome omcB (GSU2737) (ref. 6) and omcZ (GSU2078), which encodes for the precursor of the matrix-associated c-type cytochrome OmcZS (ref. 39). The constitutive gene rpoD (GSU3089) was used as a control. WT, pilB, pilA and pilA-E5A biofilms were grown for 48 h in the wells of 48-well polystyrene plates containing 600 μl of FWAF before decanting the culture broth and resuspending the biofilm cells in 50% (v/v) of ice-cold methanol to stop transcription. The cell suspension was then centrifuged to isolate the cells as a pellet and trizol reagent (Invitrogen) was used to extract their RNA. RNA treatment with RNase-free DNase (Promega) and reverse transcription with random primers (Promega) using the Super Script III Reverse Transcriptase (Invitrogen) were carried out following manufacturers recommendations. RT–qPCR was performed using the rEVAluation qPCR Master Mix (Syzygy), as recommended by the manufacturer, using the primers listed in Supplementary Table 2. The comparative CT method55 was used to calculate the relative expression of each gene using rpoD constitutive expression as an internal control (ΔCT value or CT (target)−CT (rpoD)) for each strain, and the average of the difference between each mutant strain ΔCT and the WT ΔCT was used to calculate the ΔΔCT. The relative fold change of expression for each target gene versus the rpoD internal control for each mutant strain versus the WT was then calculated with the formula 2−ΔΔCT for four replicate samples. Statistically significant changes in gene expression relative to pilB were determined in pairwise comparisons using the t-test function of the Microsoft Excel software.
Microbial electrolysis cells
The growth and electrochemical activity of the WT and mutant biofilms were assayed in H-type MECs equipped with anode and cathode graphite rod electrodes (Alfa Aesar, 1.27-cm diameter, 99% metals basis, 12 cm2) and a 3 M Ag/AgCl reference electrode (Bioanalytical Systems, Inc.). The MECs were set up, inoculated with cell suspensions harvested from early stationary phase DBAF cultures, filled with DB medium and operated in batch with a poised anode electrode (0.24 V versus reference electrode) as described previously53, except that the electron donor in the anode chambers was acetate, provided in 1, 2 or 3 mM concentrations, as indicated. Supernatant samples were periodically removed from the anode medium broth, filtered (0.45 μm) and analysed by high-performance liquid chromatography (HPLC), as described elsewhere56, to monitor electron donor removal. Current production was recorded with a VSP potentiostat (BioLogic) and biofilm electroactivity was inferred from the rates of linear current production (mA per day) before reaching maximum current and initiation of the deceleration phase. The exponential phase of current production was also fitted statistically (R2>0.98) to an exponential curve and the exponent of the resulting formula was used to estimate the generation times of the biofilm cells growing exponentially on the anode electrode.
At the end of the MEC experiment, when current had decreased to <0.1 mA, the anode electrodes were removed and the live and dead biofilm cells were differentially stained in green and red, respectively, with the SYTO-9 and propidium iodide dyes of the BacLight viability kit (Invitrogen). The anode electrodes with the stained biofilms were then immersed gently in a Lab-Tek coverglass chamber (Nunc) filled with 3 ml of PBS and examined using a FluoView FV1000 inverted microscope system (Olympus, Center Valley, PA) equipped with an Olympus UPLFLN × 40 oil immersion objective (numerical aperture, 1.30). SYTO-9 was excited at 488 nm and propidium iodide was excited at 543 nm. Vertical two-dimensional images of the biofilms were collected every 1 μm from ∼10 random fields (1,024 by 1,024 pixels, 0.31 μm per pixel) per electrode, using a minimum of two biological MEC replicates. The biofilm thickness was then manually analysed by averaging thickness measurements of five representative areas per field. When indicated, the COMSTAT software57 was used to estimate other biofilm structural parameters, as described previously53.
Static biofilm assays on plastic surfaces
The WT and mutant strains were also grown on plastic surfaces using a soluble electron acceptor (fumarate), essentially as described elsewhere24. Briefly, the strains were grown in FWAF to mid-exponential phase and inoculated to a final OD600 of 0.02 in 150 μl of FWAF dispensed in each of six replicate wells of a 96-well, polystyrene, tissue culture-treated plate (Costar, Corning Life Sciences). Unless otherwise indicated, the plates were incubated for 48 h at 30 °C and the optical density of the culture at 600 nm (OD600) measured before discarding the culture broth and staining the biofilms for 30 min with 150 μl of a 0.01% (w/v) aqueous solution of crystal violet. After staining, the biofilms were washed with double-distilled H2O and dried overnight before resolubilizing the biofilm-associated crystal violet with 33% (v/v) acetic acid. The optical density of the solution at 580 nm (OD580) was used to estimate the biofilm biomass (cells and matrix) and the biofilm biomass for each strain was averaged for six replicate samples. Time-course biofilm assays were performed the same way and biofilm growth was expressed as the biofilm biomass that stained with crystal violet (OD580) relative to the OD600 of the planktonic culture.
When indicated, 48-h WT and pilB biofilms grown in the wells of three 48-well plates were collected and treated with SDS, as described earlier24, to lyse the cells and solubilize the total biofilm protein. Protein in the biofilm lysates was estimated with the Pierce BCA protein assay (Thermo Fisher Scientific). We estimated that ∼90% of the protein solubilized with SDS was contributed by the biofilm cells, thus serving as a proxy of cell numbers in the biofilms.
Isolation and characterization of the biofilm matrix
The WT and mutant strains were each grown in the wells of two 48-well plates for 48 h to grow biofilms, as described above. The biofilm biomass from the two 48-well plates was then scraped and pooled together, and biofilm lysates were generated by SDS treatment, as described above for the isolation of cell protein. The biofilm matrix was isolated as previously described24, except that the biofilm lysate was generated by repeated passages of the biofilm biomass through an 18-G needle and included several steps of centrifugation and washes to isolate the insoluble biofilm matrix fraction. The sample was lyophilized and its EPS component was acid hydrolysed, as previously described1. EPS content was calculated as glucose equivalents using a published protocol5, except that the glucose content in the acid-hydrolysed sample was estimated by HPLC, using a Waters HPLC instrument (Milford, MA) equipped with the refractive index and ultraviolet detectors, and operated as described elsewhere58.
Assays for cytochrome content and profiling
The cytochrome content of 48-h biofilms and planktonic cells of the WT and mutant strains was estimated by redox difference spectroscopy. The biofilms were first grown for 48 h in 96-well plates, as described above for the biofilm assays on plastic surfaces, before discarding the culture broth and storing the plates at −80 °C for a minimum of 24 h to freeze the biofilms. Before redox difference spectroscopy, the plates with biofilms were thawed at room temperature for a minimum of 15 min before suspending the biofilms in FW medium. A total of 96 biofilm samples (that is, 96 wells) were pooled together for each strain and the OD600 of the biofilm cell suspension was adjusted to 0.2 with FW medium. SDS (final concentration of 0.1% w/v) was then added to the cell suspension to lyse the cells and fresh 1 mM dithionite was added to the cell extract as a reducing agent anaerobically inside a glove bag (Coy Laboratories). The ultraviolet–visible spectrum from 350 to 650 nm of the reduced cell extract was then collected before and after oxidation with 2 mM ferricyanide using a Shimadzu UV-2401 spectrophotometer. The difference between the reduced and oxidized spectra at 552 nm (the α-Soret band in the reduced state) of the biofilm cell extracts in the WT and mutants (pilB, pilA and pilA-E5A) was used to estimate the overall biofilm cytochrome content (cells and biofilm matrix)59. Spectra were also collected from cell extracts obtained from planktonic cells collected from exponential (OD600. 0.4–0.5) cultures grown in FWAF at 30 °C. The total haem content of the cells was analysed using the alkaline pyridine haemochrome method60. Statistically significant changes in cytochrome content relative to the WT were determined in pairwise comparisons using the t-test function of the Microsoft Excel software.
Cytochromes were also extracted with SDS from the biofilm matrix, isolated as described above and separated electrophoretically by SDS–polyacrylamide gel electrophoresis in 12% Mini-Protean TGX gels (Bio-Rad). Novex Sharp molecular-weight markers (Invitrogen) were used as standards. Haem-containing protein bands were stained with 3,3′,5,5′-tetramethylbenzidine, as previously described61. Replicate gels were run and stained with Coomassie blue to ensure that the total protein content per lane was comparable. When indicated, SDS–polyacrylamide gel electrophoresis and haem staining were also used to profile all haem-containing proteins within planktonic cells grown in FWAF to mid-exponential phase.
Pili purification and conductivity measurements by CP-AFM
Pili from WT or the Tyr3 mutant strain of G. sulfurreducens were purified as previously described22, except that all buffers contained 1 mM EDTA and all drying steps were carried out under a constant flow of filter-sterilized (0.22 μm) N2 gas. The pili were deposited on the surface of freshly cleaved HOPG for 30 min, then blotted dry, to probe the transversal conductivity of hydrated pili by conductive probe-atomic force microscopy (CP-AFM). The sample was scanned with the AFM tip in tapping mode to image the pili and to identify individual fibres. The CP-AFM tip was then positioned at different points along each pilus filament to measure its transversal conductivity while applying a bias voltage within the ±1 V range (3 nN force, 1 Hz rate). To ensure reproducibility, two to three current–voltage (I–V) curves were collected at each point, each pilus filament was probed in at least three positions, and four to five pilus fibres were probed for each strain. Furthermore, the tip was periodically moved to the HOPG surface adjacent to the pili to control tip quality. From the linear portion of each I–V curve, we estimated the electrical resistance (R), as described elsewhere28, and used the average and s.d. of all the resistance values to compare the conductivity of the WT and Tyr3 pili.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files, or from the corresponding author upon request.
Additional information
How to cite this article: Steidl, R. J. et al. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 7:12217 doi: 10.1038/ncomms12217 (2016).
Supplementary Material
Supplementary Figures 1-6, Supplementary Tables 1-2 and Supplementary References
Acknowledgments
This research was supported by grants MCB-1021948 (from the National Science Foundation) to G.R. We also acknowledge support from an MTRAC grant (supported by the State of Michigan 21st Century Jobs Funds received through the Michigan Strategic Fund and administered by the Michigan Economic Development Corporation) and the National Institute of Food and Agriculture of the US Department of Agriculture to GR and recruitment, dissertation completion and Hensley fellowships from Michigan State's College of Natural Sciences to R.J.S. We thank Michael Paxhia and Alexander Grohalski for assisting with the biofilm EPS extraction and characterization, Jihwan Hwang for designing the pilB deletion mutant, Melinda Frame at Michigan State's Center for Advanced Microscopy for microscopy support, Daniel Bond for providing the WT strain of G. sulfurreducens and Mary E. Lidstrom for providing the cre–lox antibiotic marker recycling system.
Footnotes
Author contributions R.J.S., S.L.-P. and G.R. designed the experiments. R.J.S. performed all the experiments except for the WT and Tyr3 pili purification and CP-AFM conductivity measurements, which were performed by S.L.-P. R.J.S. and G.R. analysed the data and co-wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Speers A. M. & Reguera G. Consolidated bioprocessing of AFEX-pretreated corn stover to ethanol and hydrogen in a microbial electrolysis cell. Environ. Sci. Technol. 46, 7875–7881 (2012). [DOI] [PubMed] [Google Scholar]
- Speers A. M., Young J. M. & Reguera G. Fermentation of glycerol into ethanol in a microbial electrolysis cell driven by a customized consortium. Environ. Sci. Technol. 48, 6350–6358 (2014). [DOI] [PubMed] [Google Scholar]
- Sleutels T. H., Ter Heijne A., Buisman C. J. & Hamelers H. V. Bioelectrochemical systems: an outlook for practical applications. ChemSusChem 5, 1012–1019 (2012). [DOI] [PubMed] [Google Scholar]
- Borole A. P. et al. Electroactive biofilms: current status and future research needs. Energy Environ. Sci. 4, 4813–4834 (2011). [Google Scholar]
- Rollefson J. B., Stephen C. S., Tien M. & Bond D. R. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J. Bacteriol. 193, 1023–1033 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leang C., Coppi M. V. & Lovley D. R. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185, 2096–2103 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta T., Coppi M. V., Childers S. E. & Lovley D. R. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 71, 8634–8641 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin K. P. et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4, e5628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H. et al. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2, 506–516 (2009). [Google Scholar]
- Kim B. C. et al. OmcF, a putative c-type monoheme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J. Bacteriol. 187, 4505–4513 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. D. et al. Thermally activated long range electron transport in living biofilms. Phys. Chem. Chem. Phys. 17, 32564–32570 (2015). [DOI] [PubMed] [Google Scholar]
- Liu Y. & Bond D. R. Long-distance electron transfer by G. sulfurreducens biofilms results in accumulation of reduced c-type cytochromes. ChemSusChem 5, 1047–1053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kim H., Franklin R. R. & Bond D. R. Linking spectral and electrochemical analysis to monitor c-type cytochrome redox status in living Geobacter sulfurreducens biofilms. ChemPhysChem. 12, 2235–2241 (2011). [DOI] [PubMed] [Google Scholar]
- Babauta J. T., Nguyen H. D., Harrington T. D., Renslow R. & Beyenal H. pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer. Biotechnol. Bioeng. 109, 2651–2662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robuschi L. et al. Spectroscopic slicing to reveal internal redox gradients in electricity-producing biofilms. Angew Chem. Int. Ed. 52, 925–928 (2013). [DOI] [PubMed] [Google Scholar]
- Snider R. M., Strycharz-Glaven S. M., Tsoi S. D., Erickson J. S. & Tender L. M. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc. Natl Acad. Sci. USA 109, 15467–15472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton E. F. et al. Charge transport in electroactive polymers consisting of fixed molecular redox sites. Chem. Phys. 141, 143–157 (1990). [Google Scholar]
- Reguera G. et al. Biofilm and nanowire production lead to increased current in microbial fuel cells. Appl. Environ. Microbiol. 72, 7345–7348 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslow R. S. et al. Metabolic spatial variability in electrode-respiring Geobacter sulfurreducens biofilms. Energy Environ. Sci. 6, 1827–1836 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen C. S., LaBelle E. V., Brantley S. L. & Bond D. R. Abundance of the multiheme c-type cytochrome OmcB increases in outer biofilm layers of electrode-grown Geobacter sulfurreducens. PLoS ONE 9, e104336 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101 (2005). [DOI] [PubMed] [Google Scholar]
- Cologgi D. L., Lampa-Pastirk S., Speers A. M., Kelly S. D. & Reguera G. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc. Natl Acad. Sci. USA 108, 15248–15252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G., Pollina R. B., Nicoll J. S. & Lovley D. R. Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation. J. Bacteriol. 189, 2125–2127 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologgi D. L., Speers A. M., Bullard B. A., Kelly S. D. & Reguera G. Enhanced uranium immobilization and reduction by Geobacter sulfurreducens biofilms. Appl. Environ. Microbiol. 80, 6638–6646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leang C., Qian X., Mester T. & Lovley D. R. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 76, 4080–4084 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strycharz-Glaven S. M., Snider R. M., Guiseppi-Elie A. & Tender L. M. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 4, 4366–4379 (2011). [Google Scholar]
- Veazey J. P., Reguera G. & Tessmer S. H. Electronic properties of conductive pili of the metal-reducing bacterium Geobacter sulfurreducens probed by scanning tunneling microscopy. Phys. Rev. E 84, 060901 (2011). [DOI] [PubMed] [Google Scholar]
- Lampa-Pastirk S. et al. Thermally activated charge transport in microbial protein nanowires. Sci. Rep. 6, 23517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano G. T., Steidl R. J. & Reguera G. Structural and functional insights into the conductive pili of Geobacter sulfurreducens revealed in molecular dynamics simulations. Phys. Chem. Chem. Phys. 17, 22217–22226 (2015). [DOI] [PubMed] [Google Scholar]
- Bonanni P. S., Bradley D. F., Schrott G. D. & Busalmen J. P. Limitations for current production in Geobacter sulfurreducens biofilms. ChemSusChem 6, 711–720 (2013). [DOI] [PubMed] [Google Scholar]
- Juarez K. et al. PilR, a transcriptional regulator for pilin and other genes required for Fe(III) reduction in Geobacter sulfurreducens. J. Mol. Microbiol. Biotechnol. 16, 146–158 (2009). [DOI] [PubMed] [Google Scholar]
- Burrows L. L. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66, 493–520 (2012). [DOI] [PubMed] [Google Scholar]
- Pelicic V. Type IV pili: e pluribus unum?. Mol. Microbiol. 68, 827–837 (2008). [DOI] [PubMed] [Google Scholar]
- Craig L. et al. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23, 651–662 (2006). [DOI] [PubMed] [Google Scholar]
- Hentzer M. et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183, 5395–5401 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. et al. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ. Microbiol. Rep. 3, 211–217 (2011). [DOI] [PubMed] [Google Scholar]
- Marsili E., Sun J. & Bond D. R. Voltammetry and growth physiology of Geobacter sulfurreducens biofilms as a function of growth stage and imposed electrode potential. Electroanalysis 22, 865–874 (2010). [Google Scholar]
- Atci E., Babauta J. T., Sultana S. T. & Beyenal H. Microbiosensor for the detection of acetate in electrode-respiring biofilms. Biosens. Bioelectron. 81, 517–523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. et al. Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 76, 3999–4007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L. V., Sandler S. J. & Weis R. M. Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation. J. Bacteriol. 194, 2551–2563 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar N. S. et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6, 573–579 (2011). [DOI] [PubMed] [Google Scholar]
- Lu H. M., Motley S. T. & Lory S. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol. 25, 247–259 (1997). [DOI] [PubMed] [Google Scholar]
- Strom M. S., Nunn D. & Lory S. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from Pseudomonas aeruginosa. J. Bacteriol. 173, 1175–1180 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Nunez A., Sosnik J., Visconti P. & Lovley D. R. Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens. Environ. Microbiol. 10, 497–505 (2008). [DOI] [PubMed] [Google Scholar]
- Chin K. J., Esteve-Nunez A., Leang C. & Lovley D. R. Direct correlation between rates of anaerobic respiration and levels of mRNA for key respiratory genes in Geobacter sulfurreducens. Appl. Environ. Microbiol. 70, 5183–5189 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe J., Nocera D. G., Yee C. S. & Chang M. C. Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer? Chem. Rev. 103, 2167–2201 (2003). [DOI] [PubMed] [Google Scholar]
- Reece S. Y. & Nocera D. G. Proton-coupled electron transfer in biology: results from synergistic studies in natural and model systems. Annu. Rev. Biochem. 78, 673–699 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks A. E. et al. Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: monitoring the inhibitory effects of proton accumulation within the anode biofilm. En. Environ. Sci. 2, 113–119 (2009). [Google Scholar]
- Franks A. E., Nevin K. P., Glaven R. H. & Lovley D. R. Microtoming coupled to microarray analysis to evaluate the spatial metabolic status of Geobacter sulfurreducens biofilms. ISME J. 4, 509–519 (2010). [DOI] [PubMed] [Google Scholar]
- Childers S. E., Ciufo S. & Lovley D. R. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416, 767–769 (2002). [DOI] [PubMed] [Google Scholar]
- Okegbe C., Price-Whelan A. & Dietrich L. E. Redox-driven regulation of microbial community morphogenesis. Curr. Opin. Microbiol. 18, 39–45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi M. V., Leang C., Sandler S. J. & Lovley D. R. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67, 3180–3187 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers A. M. & Reguera G. Electron donors supporting growth and electroactivity of Geobacter sulfurreducens anode biofilms. Appl. Environ. Microbiol. 78, 437–444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx C. J. & Lidstrom M. E. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33, 1062–1067 (2002). [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D. & Livak K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
- McKinlay J. B., Zeikus J. G. & Vieille C. Insights into Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl. Environ. Microbiol. 71, 6651–6656 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A. et al. Experimental reproducibility in flow-chamber biofilms. Microbiology 146, (Pt 10): 2409–2415 (2000). [DOI] [PubMed] [Google Scholar]
- Sluiter A. et al. Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory, Technical Report NREL/TP-510-42618, 1–16 (2008).
- Senko J. M. & Stolz J. F. Evidence for iron-dependent nitrate respiration in the dissimilatory iron-reducing bacterium Geobacter metallireducens. Appl. Environ. Microbiol. 67, 3750–3752 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaichi S. & Morita S. Procedures and conditions for application of the pyridine hemochrome method to photosynthetically grown cells of Rhodopseudomonas sphaeroides. J. Biochem. 89, 1513–1519 (1981). [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D. & Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75, 168–176 (1976). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Tables 1-2 and Supplementary References
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files, or from the corresponding author upon request.