Abstract
Background
Medicinal plants have proven their importance as a valuable source of molecules with therapeutic potential. Monotheca buxifolia (Falc.) A. DC. (family: Sapotaceae) is traditionally used as a hematinic, laxative, digestive, anthelmintic, antipyretic, and in the treatment of gastro-urinary disorders. To provide scientific evidence for its folkloric use, the present study investigated Monotheca buxifolia fruit hydro-ethanolic extract (MBHE) for its prospective antinociceptive, anti-inflammatory and antipyretic activities. MBHE was eluted through column chromatography to isolate the bioactive secondary metabolites which may probably involve in its beneficial properties.
Methods
The phytochemical constituents in MBHE was elucidated using UV, IR, 1H-NMR, 13C NMR, 2D-NMR spectra in combination with EIMS and FAB-MS spectrometric techniques and comparison with literature data of related compounds. The antinociceptive activity of MBHE was evaluated in the acetic acid induced abdominal constriction assay; the anti-inflammatory potential was assessed in the carrageenan induced paw edema, while the antipyretic effect was tested against brewer’s yeast induced pyrexia in BALB/c mice at doses of 50, 100 and 150 mg/kg.
Results
Elution of MBHE along with various characterization techniques led to the isolation of oleanolic acid and isoquercetin. Significant attenuation of chemical induced nociception was observed with MBHE at tested doses of 50 mg/kg (P < 0.01, 68.87 %), 100 mg/kg (P < 0.01, 68.87 %) and 150 mg/kg (P < 0.001, 83.02 %). During a duration of 1–5 h in the carrageenan induced paw edema assay, significant ameliorative effect (P < 0.01, P < 0.001) was demonstrated by MBHE at 50 mg/kg (22.94–20 %), 100 mg/kg (33.23–21.13 %) and 150 mg/kg (38.23–25 %). MBHE also significantly alleviated the brewer’s yeast induced pyrexic response when tested at doses of 50 mg/kg (P < 0.05 in 2nd h), 100 mg/kg (P < 0.05, P < 0.01 and P < 0.001 in 1–5 h) and 150 mg/kg (P < 0.01 and P < 0.001 in 1–5 h).
Conclusion
These findings suggest that Monotheca buxifolia possess pain, inflammation and pyrexia ameliorating properties, probably mediated by the presence of oleanolic acid and isoquercetin contents, though the involvement of other important phytochemicals constituents cannot be ignored.
Electronic supplementary material
The online version of this article (doi:10.1186/s12906-016-1257-z) contains supplementary material, which is available to authorized users.
Keywords: Sapotaceae, Oleanolic acid, Isoquercetin, Pain, Inflammation, Pyrexia
Background
Discovering excellent remedies for diseases that are efficacious, economical and having minimum adverse effects is the need of the hour. For discovering such products, medicinal plants are considered as best choice, as they provide a wide range of bioactive compounds, making them a rich source of different types of medicines [1]. Majority of drugs currently used in the clinics are due to extensive research on isolation from the natural sources [2].
Monotheca buxifolia (Falc.) A. DC. is a member of genus Monotheca which belongs to family Sapotaceae. M. buxifolia is one of the important tree species of Pakistan that still exhibits dominance in some of the forests, particularly in the Dir District. It is also distributed in the mountains of Afghanistan, northern Oman, and in the south-east Saudi Arabia. M. buxifolia is mainly used for fuel, fodder, small timber, roof thatching materials, and notably used as fence around cultivated fields due to its thorny nature. This species also yields fruits, locally called Gurguri, which provides a source of income for the local inhabitants [3]. In folk medicine, M. buxifolia fruit is used as hematinic, laxative, purgative, vermicidal, antipyretic, and for the management of gastro-urinary disorders [4–7]. The leaves of M. buxifolia contain anthraquinones, flavonoids, terpenoids, cardiac glycosides, saponins, reducing sugars, tannins and poly-phenolic compounds [6]. The total phenolic compounds in M. buxifolia fruit fractions, ranged between 59.13 ± 2.6 mg and 16.66 ± 1.3 mg/g dry weight of fraction with the butanolic extract showed the highest total phenolics (59.13 ± 2.6 mg GAE/g fraction). Likewise, the flavonoid contents were in the range of 4.11 ± 0.51 to 48.68 ± 2.8 mg as rutin equivalents/g fraction with highest amount observed in the aqueous fraction (48.68 ± 2.8 mg/g) [8]. Recently, two new compounds, buxifoline-A as alkaloid and buxilide as pyrone were isolated from the ethylacetate fraction of M. buxifolia fruit [9]. Flavonoids and poly-phenolic compounds are reported to possess potent anti inflammatory and analgesic properties. Previously, the in vitro antioxidant activity of this fruit has been evaluated and has proved to exhibit potent antioxidant properties [8]. Moreover, M. buxifolia fruit also possess inhibitory potential against urease enzyme [9]. The family Sapotaceae is widely studied for antimicrobial [10, 11], antioxidant [8], antipyretic [12], CNS depressant [13], anti-inflammatory [14, 15], anthelmintic [16] and antinociceptive activities [12, 17] in various in vitro and in vivo experimental models.
Scientific studies concerning the therapeutic efficacy of M. buxifolia are lacking and, in an attempt to provide scientific evidence, we tested the M. buxifolia fruit hyrdo-ethanolic (30:70) extract (MBHE) for antinociceptive, anti-inflammatory and antipyretic activities in mice.
Methods
Chemicals and drugs
Tramadol (Tramal®, Searle Company (Pvt), Pakistan), diclofenac sodium (Voren®, AsianContinental (Pvt), Pakistan), carrageenan and brewer’s yeast (Sigma-Aldrich).
Animals
BALB/c mice of either sex (21–35 g) were purchased from the animal house of the Department of Pharmacy, University of Peshawar and were acclimatized at 25 ± 2 °C under a 12 h dark/light cycle for 10 days. Food and water were provided ad libitum. The experimental protocols for this study were approved by the Ethical Committee of the Department of Pharmacy, University of Peshawar, Pakistan (registration number: 04/EC-15/Pharm).
Plant material
Fruits of Monotheca buxifolia were collected from the northern areas (District Dir Lower) of Pakistan in August 2013. It was identified by Ghulam Jelani, taxonomist at the Department of Botany, University of Peshawar. Afterwards, a specimen was deposited in the herbarium under the voucher number Bot. 20061 (PUP).
Preparation of Monotheca buxifolia fruit extract
Fruits of Monotheca buxifolia (50 kg) were collected and washed to remove dust and other impurities. The seeds were separated and the collected fleshy fruit pulp was dried under shade at ambient temperature. The dried pulp (6.5 kg) was coarsely grinded and was subjected to extraction by adding hydro-ethanolic solvent (40 L) with occasional shaking for 14 days (2 × 7) according to a previous reported method [18]. It was then filtered using a Whatman-1 filter paper. The solvent was evaporated under reduced pressure in a rotary evaporator (BUCHI Rotavapor R-200, Switzerland) at 40 °C until a semisolid mass (2.419 kg) was obtained (yield 4.838 %). The obtained extract was kept in refrigerator till further analysis.
Phytochemical analysis
MBHE was preliminary evaluated by qualitative phytochemical analysis [19] and was further screened by quantitative analysis of phenolic, flavonoid [20] and triterpenoid [21] contents. MBHE was also subjected to different analytical techniques for isolation and structural elucidation of natural compounds. MBHE (2.304 kg) was mixed with 2.5 L distilled water and soaked overnight, extracted successively with hexanes (3 × 5 L), chloroform (3 × 5 L), ethyl-acetate (3 × 5 L), and n-butanol (3 × 5 L) to obtain hexane soluble (56 g), chloroform soluble (57.7 g), ethylacetate soluble (34.9 g) and n-butanol soluble (54.5 g) fractions respectively. The ethyl-acetate fraction (30 g) was subjected to vacuum liquid chromatography on normal phase silica gel and eluted using hexane, hexanes-ethylacetate, ethylacetate, ethylacetate-methanol and methanol with increasing polarity to yield 18 fractions (Fr. 1–18). Compound 1 (13 mg) was obtained from fraction 2 (1.2 g) through repeated column chromatography using hexanes-ethylacetate (9:1 – 8:2) while fraction 15 (1.4 g) was re-chromatographed on silica gel column using hexanes-ethylacetate (8:2) to obtained compound 2 (15 mg). Purity of these two compounds was assessed using TLC followed by spraying with ceric sulphate and heating. Their chemical structure was elucidated using 1H-NMR, 13C-NMR, 2D-NMR, EI-MS, FAB-MS, UV, and IR analytical techniques. The data obtained for these compounds were unambiguously matched with reported data from the literature [22, 23].
Antinociceptive activity
The antinociceptive activity of MBHE was evaluated by acetic acid induced abdominal constriction assay [24] in BALB/c mice (21–24 g). The animals were withdrawn from food 2 h before the start of experiment. MBHE was administered orally at doses of 50, 100, and 150 mg/kg. Diclofenac sodium was administered at a dose of 50 mg/kg p.o and served as positive control. After 30 min of treatment, all animals were injected i.p with 1 % acetic acid. The number of writhes were counted after 5 min of acetic acid injection and the animals were observed for 20 min. The mean incidence of constrictions in treated groups was compared to untreated controls. Percent protection against nociception was calculated as:
Anti-inflammatory activity
The anti-inflammatory activity of MBHE was tested in the carrageenan induced paw edema assay [25] in BALB/c mice (25–30 g). The animals were starved for 4 h before the start of experiment. MBHE was administered orally at doses of 50, 100, and 150 mg/kg. Aspirin was used as standard and was administered at a dose of 150 mg/kg p.o. After 30 min, all animals were challenged with a 50 μL of 1 % solution of carrageenan, injected subcutaneously into the plantar region of the left hind paw. The paw volume was measured using a digital plethysmometer at 1 to 5 h after challenge with carrageenan. The mean paw swelling across the treated groups was compared to untreated control group. Percent inhibition of paw edema was determined as:
Where A is the paw volume of carrageenan alone treated group and B is the paw volume of tested group.
Antipyretic activity
The antipyretic potential of MBHE was screened against brewer’s yeast induced pyrexia [26] in BALB/c mice (30–35 g). The animals were deprived of food for 5 h before the start of experiment. Their normal rectal temperature was recorded using a digital thermometer, after which a 15 % solution of brewer’s yeast was injected subcutaneously at a dose of 10 ml/kg to each animal. The rise in body temperature was recorded after 24 h and animals showing at least 0.5 °C rise of their body temperature were included in the experiment. The pyrexic animals were orally administered with MBHE at doses of 50, 100 and 150 mg/kg or acetaminophen, which was used as standard in a dose of 150 mg/kg. The rectal temperature of animals was then recorded at 1 to 5 h duration. The mean elevation of body temperature in °F across treated groups was compared to that of untreated control.
Statistical analysis
Data were expressed as mean ± S.E.M or SD. Statistical analysis was performed by one way ANOVA followed by Dunnett’s or Tukey’s post hoc test where appropriate using GraphPad Prism 5 (GraphPad Software Inc. San Diego CA, USA). Statistical significance was deduced at P ≤ 0.05.
Results
Phytochemical analysis of Monotheca buxifolia
Preliminary qualitative analysis of MBHE disclosed the presence of alkaloids, phenolics, flavonoids, athraquinones glycosides, saponins, and triterpenoids (Table 1), while quantitative analysis revealed (mean ± SEM, n = 5) the presence of phenolics (49.60 ± 1.93 as mg gallic acid equivalent/g dry weight), flavonoids (44.80 ± 1.65 as mg rutin equivalent/g dry weight) and triterpenoids (32.20 ± 2.05 as mg ursolic acid equivalent/g dry weight). Subsequent more detailed isolation and structural determination techniques led to the isolation of compound 1 as oleanolic acid (Fig. 1) and compound 2 as isoquercetin (Fig. 2) from MBHE. Oleanolic acid showed molecular ion peak at m/z at 456, and having a molecular formula of C30H48O3 at m/z 456.7003 (Calc. 456.6840) (Additional file 1: Figure S1–S6). Isoquercetin showed a pseudo-molecular ion peak [M-H] at m/z 463 and having a molecular formula of C21H20O12 at m/z 463.0012 (Additional file 1: Figure S7-S16).
Table 1.
Qualitative phytochemical analysis of Monotheca buxifolia
| Phytochemical class | Protocol | Result |
|---|---|---|
| Alkaloids | Mayer’s test | + |
| Dragendorff’s test | + | |
| Wagner’s test | + | |
| Phenolics | Ferric chloride test | + |
| Flavonoids | Lead acetate test | + |
| Ethyl acetate test | + | |
| Sodium hydroxide test | + | |
| Anthraquinone glycosides | Borntrager’s test | + |
| Saponins | Foam test | + |
| Triterpenoids | Liebermann-Burchard test | + |
Fig. 1.
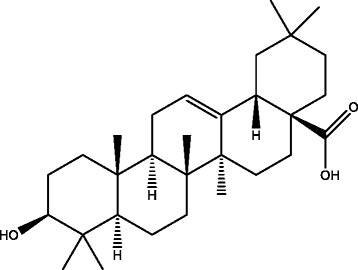
Structure of compound 1 (Oleanolic acid)
Fig. 2.
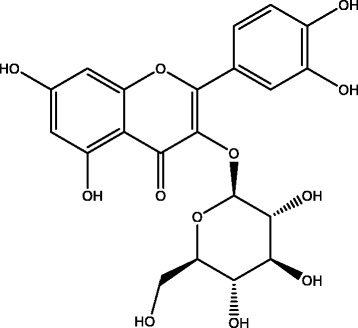
Structure of compound 2 (Isoquercetin)
Monotheca buxifolia attenuates tonic visceral chemical induced nociception
As shown in Fig. 3, significant attenuation (P < 0.01) of acetic acid induced visceral pain was demonstrated by MBHE at doses of 50 mg/kg (68.87 %) and 100 mg/kg (68.87 %). However, a highly significant (P < 0.001) antinociceptive effect was observed at 150 mg/kg (83.02 %). Similarly, the standard diclofenac sodium at 50 mg/kg produced significant amelioration (P < 0.001, 83.97 %) of acetic acid induced writhes.
Fig. 3.
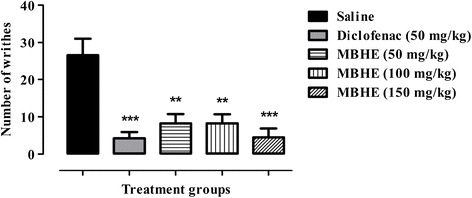
Antinociceptive activity of Monotheca buxifolia hydro-ethanolic extract (MBHE) in acetic acid induced abdominal constriction assay. Values expressed as mean number of writhes ± SEM. ANOVA followed by Dunnett’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline treated group. n = 6 animals per group
Monotheca buxifolia alleviates carrageenan induced paw edema
As shown in Table 2, carrageenan alone treated animals exhibited significant (P < 0.001) paw edema after 1 h of administration and this edema remained persistent for 5 h. Pretreatment with MBHE significantly alleviated the carrageenan elicited paw edema after 1 and 2 h at doses of 50 mg/kg (P < 0.01, 22.94 and 23.78 %), 100 mg/kg (P < 0.001 and P < 0.01, 33.23 and 25.13 %) and 150 mg/kg (P < 0.001, 38.23 and 30.54 %). After 3 and 4 h, high significant reduction (P < 0.001) of paw edema was produced by all the tested doses i.e. 50 mg/kg (27.34 and 40.41 %), 100 mg/kg (26.58 and 41.43 %) and 150 mg/kg (27.34 and 44.49 %), however; a less significant reduction (P < 0.01) was observed with these doses after 5 h (20, 21.13 and 25 %) of carrageenan administration. Moreover, the standard aspirin at a dose of 150 mg/kg produced moderate to high significant alleviation of paw edema after 1 h (P < 0.01, 25 %) and 2–5 h (P < 0.001, 32.43, 36.20, 33.67 and 30.68 %) duration of challenge with carrageenan.
Table 2.
Anti-inflammatory activity of Monotheca buxifolia hydro-ethanolic extract (MBHE) in carrageenan induced paw edema in mice
| Treatment | 1st h | 2nd h | 3rd h | 4th h | 5th h |
|---|---|---|---|---|---|
| Group 1 Saline |
0.235 ± 0.012 | 0.227 ± 0.026 | 0.240 ± 0.011 | 0.210 ± 0.016 | 0.230 ± 0.036 |
| Group 2 Carrageenan |
0.340 ± 0.016### | 0.370 ± 0.028### | 0.395 ± 0.025### | 0.490 ± 0.057### | 0.440 ± 0.028### |
| Group 3 Aspirin (150 mg/kg) |
0.255 ± 0.028** | 0.250 ± 0.035*** | 0.252 ± 0.020*** | 0.325 ± 0.052*** | 0.305 ± 0.019*** |
| Group 4 (MBHE 50 mg/kg) |
0.262 ± 0.022** | 0.282 ± 0.012** | 0.287 ± 0.025*** | 0.292 ± 0.015*** | 0.352 ± 0.029** |
| Group 5 (MBHE 100 mg/kg) |
0.227 ± 0.040*** | 0.277 ± 0.035** | 0.290 ± 0.046*** | 0.287 ± 0.037*** | 0.347 ± 0.029** |
| Group 6 (MBHE 150 mg/kg) |
0.210 ± 0.028*** | 0.257 ± 0.026*** | 0.287 ± 0.022*** | 0.272 ± 0.044*** | 0.330 ± 0.038** |
Values are expressed as mean paw volume in ml ± SD. ANOVA followed by Tukey’s post hoc test. ### P < 0.001 compared to group 1. **P < 0.01, ***P < 0.001 compared to group 2. n = 6 animals per group
Monotheca buxifolia relieves brewer’s yeast induced pyrexia
As shown in Table 3, brewer’s yeast induced significant increase (P < 0.001) of body temperature which remained elevated throughout the 5 h study period. After 1 h of treatment, significant reduction (P < 0.001) in pyrexia was noticed with MBHE at doses of 100 and 150 mg/kg. Almost, similar protective effect was observed after 2 h of treatment with MBHE at 100 mg/kg (P < 0.01) and 150 mg/kg (P < 0.001). After 3 h, less significant antipyretic effect was observed with the 100 mg/kg (P < 0.05) and 150 mg/kg (P < 0.01) doses of MBHE and this effect persisted for the 5 h. The 50 mg/kg dose of MBHE was only effective at 2nd hour, at which a less significant reduction (P < 0.05) of pyrexic response was observed. The standard acetaminophen at 150 mg/kg produced favorable antipyretic effect (P < 0.001) throughout the study period (5 h).
Table 3.
Antipyretic activity of Monotheca buxifolia hydro-ethanolic extract (MBHE) in brewer’s yeast induced pyrexia in mice
| Treatment | 1st h | 2nd h | 3rd h | 4th h | 5th h |
|---|---|---|---|---|---|
| Group 1 (Brewer’s yeast + Saline) |
100.4 ± 0.358 | 100.5 ± 0.346 | 100.0 ± 0.335 | 100.4 ± 0.265 | 100.5 ± 0.341 |
| Group 2 (Acetaminophen 150 mg/kg) |
96.18 ± 0.757*** | 96.62 ± 0.755*** | 95.50 ± 1.023*** | 96.07 ± 0.720*** | 95.23 ± 0.731*** |
| Group 3 (MBHE 50 mg/kg) |
97.93 ± 0.449 | 98.20 ± 0.634* | 98.58 ± 0.838 | 99.27 ± 0.860 | 99.88 ± 0.812 |
| Group 4 (MBHE 100 mg/kg) |
96.02 ± 0.884*** | 97.18 ± 0.570** | 97.68 ± 0.244* | 97.72 ± 0.342* | 97.42 ± 0.709* |
| Group 5 (MBHE 150 mg/kg) |
93.70 ± 0.753*** | 96.93 ± 0.584*** | 96.48 ± 0.303** | 97.33 ± 0.547** | 96.35 ± 0.837** |
Values are expressed as mean rectal temperature in °F ± SD. ANOVA followed by Dunnett’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 compared to group 1. n = 6 animals per group
Discussion
Fruit of Monitica buxifollia was screened for its potential antinociceptive, anti-inflammatory, and antipyretic activities. The nociceptive response in the acetic acid induced writhing test results from the production of prostaglandins through the action of cyclooxygenases. The liberated prostaglandins stimulate sensory pathways in the mouse peritoneum and incite viscero-somatic y reflexes manifested as strong abdominal constrictions or writhes. The acetic acid induced writhes are sensitive to various analgesics. MBHE when administered at doses of 50, 100 and 150 mg/kg depressed the acetic acid induced writhes and the antinociceptive effect was analogous to that of the standard diclofenac sodium (50 mg/kg).
Carrageenan-induced mice paw edema assay is a widely used test to determine the anti-inflammatory activity of both natural and synthetic compounds. Edema formation due to carrageenan administration in mouse paw is a biphasic event. The initial phase lasting about 1–5 h is predominately characterized by a non-phagocytic edema and has been attributed to the action of various mediators including histamine, serotonin and bradykinin on vascular permeability [27]. The initial phase is followed by a second phase having a duration of 2–5 h and results from overproduction of prostaglandins [28]. It has been reported that the second phase of edema is sensitive to drugs like hydrocortisone, phenylbutazone and indomethacin. In the present study, we have observed potential anti-inflammatory activity of MBHE at all the tested doses (50, 100 and 150 mg/kg) and was comparable to the classical cyclooxygenase inhibitor, aspirin (150 mg/kg).
Brewer’s yeast is an exogenous pyrogen which produces pathogenic fever by binding to lipopolysaccharide binding protein and results in the release of different cytokines like interleukin 1, 6, tumor necrosis factor alpha and prostaglandins. These pro-inflammatory mediators cross the blood brain barrier and act on hypothalamus causing the release of prostaglandin E2 which is produced through the action of cyclo-oxygenase-2 and thus increases the body temperature [29]. In this study, potential antipyretic effect was observed for the 100 and 150 mg/kg doses of MBHE and the effect was similar to that of acetaminophen (150 mg/kg).
Biologically active molecules derived from herbal extracts can be used to treat acute and chronic diseases [30]. In the current study, the chromatographically isolated compounds i.e. isoquercetin have been reported to possess neuroprotective [31], cardioprotective [32], hepatoprotective [33], nephroprotective [34], chemopreventive [35], analgesic [36], antioxidant [37] and anti-inflammatory [38] properties. In addition, oleanolic acid has anti-tumor [39], anti-inflammatory [40], antioxidant [41], anti-hyperlipidemic [42], anti-ulcer [43], anti-microbial [44] and analgesic [45, 46] activities. Keeping in view, the potent antinociceptive, anti-inflammatory, and antipyretic activity of MBHE, it can be argued that these beneficial effects can be attributed at least to the presence of isoquercetin and oleanolic acid contents in M. buxifolia. However, the involvement of other phytochemical constituents cannot be ignored in the mediation of pain, inflammation and pyrexia relieving properties as M. buxifolia has been reported to contain high amount of phenolics and flavonoids [8], β-sitosterol [47], tannins, tri-terpenoids, glycosides, anthraquinones and alkaloids [48] which is further corroborated in this study. Flavonoids are polyphenolic compounds that occur ubiquitously in plants and having a variety of biological effects including antioxidant, anti-inflammatory, antihypertensive, anti-atherosclerotic, anti-tumor, anti-thrombogenic, anti-osteoporotic, antimicrobial, antiviral, anti-ulcerogenic, and antihepatotoxic activities [49, 50]. Naturally occurring anthraquinones are a group of secondary metabolites that possess anti-inflammatory, anti-cancer, antidiabetic, antimicrobial and laxative properties [51]. Similarly, other phytochemical constituents like glycosides and alkaloids also have known beneficial pharmacological effects both in vitro and in vivo [52, 53].
Conclusion
Monotheca buxifolia hydroethanolic extract attenuated the tonic visceral chemical induced nociception and alleviated a phlogistic agent induced inflammatory response. It also relieved a pyrogenic substance induced elevation of body temperature. Phytochemical analysis revealed that M. buxifolia contained isoquercetin and oleanolic acid that might be involved in allay of pain, inflammation and pyrexia. Though, the involvement of other phytochemical constituents cannot be ignored in mediating the antinociceptive, anti-inflammatory and antipyretic effects, as M. buxifolia also have sufficient amount of flavonoids, anthraquinones glycosides, triterpenoids, and alkaloids. Our findings suggest that M. buxifolia is able to mend disease conditions afflicted with pain, inflammation and pyrexia. Further studies are warranted like HPLC-DAD profile to elucidate the exact chemical constituents responsible for these beneficial pharmacological effects.
Acknowledgements
The authors are grateful to the H.E.J. Research Institute of Chemistry, University of Karachi, Pakistan and COMSATS Institute of Information Technology, Abbottabad, Pakistan for providing necessary facilities related to phytochemical analysis.
Funding
The authors have not received any funding for this study.
Availability of data and materials
All data and materials related to this study are available with the corresponding author. Moreover, Additional file 1 is available along with the manuscript.
Authors’ contribution
IU conducted the experiments and prepared the initial draft of manuscript. JAK guided the research group as supervisor throughout the research project. MS helped in the analysis and interpretation of data as well as in preparing the final version of manuscript. AK assisted in the analytical work. AA helped in the structure elucidation of compounds. PAH provided his help in the collection and extraction procedures. IJ assisted in manuscript writing. FS attributed necessary experimental materials. UF performed qualitative and quantitative analysis of MBHE. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Not relevant.
Ethics approval and consent to participate
The experimental procedures on laboratory animals were approved by the Ethical Committee of the Department of Pharmacy, University of Peshawar, Pakistan (04/EC-15/Pharm).
Additional file
Supporting material. (DOCX 12960 kb)
Contributor Information
Irfan Ullah, Phone: +923459122675, Email: irfanullah@upesh.edu.pk.
Jamshaid Ali Khan, Phone: +92919216750, Email: jamshaidkhan@upesh.edu.pk.
Muhammad Shahid, Email: shahidsalim_2002@hotmail.com.
Ajmal Khan, Email: ajmalchemist@yahoo.com.
Achyut Adhikari, Email: adhikarimine@yahoo.com.
Peer Abdul Hannan, Email: peer_hannan@yahoo.com.
Ibrahim Javed, Email: ibrahim.javed@lums.edu.pk.
Faisal Shakeel, Email: faisalshakeel1@gmail.com.
Umar Farooq, Email: umarf@ciit.net.pk.
References
- 1.Munuswamy H, Thirunavukkarasu T, Rajamani S, Elumalai EK, Ernest D. A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu. J Acute Dis. 2013;2(2):99–105. doi: 10.1016/S2221-6189(13)60107-9. [DOI] [Google Scholar]
- 2.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan N, Ahmed M, Shaukat SS, Wahab M, Siddiqui MF. Structure, diversity, and regeneration potential of Monotheca buxifolia (Falc.) A. DC. dominated forests of Lower Dir District, Pakistan. Front Agric China. 2011;5(1):106–21. doi: 10.1007/s11703-011-1062-x. [DOI] [Google Scholar]
- 4.Shah AM SK, Gohar F, Khan A, Bhatti KH, Amin M, Din NU, Ahmad M, Zafar M. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am J Plant Sci. 2013;4:98–116. doi: 10.4236/ajps.2013.41015. [DOI] [Google Scholar]
- 5.Ullah R, Hussain Z, Iqbal Z, Hussain J, Khan FU, Khan N, et al. Traditional uses of medicinal plants in Darra Adam Khel NWFP Pakistan. J Med Plants Res. 2010;17:1815–21. [Google Scholar]
- 6.Rehman J, Khan IU, Farid S, Kamal S, Aslam N. Phytochemical screening and evaluation of in-vitro antioxidant potential of Monotheca buxifolia. E3 J Biotechnol Pharm Res. 2013;4(4):54–60. [Google Scholar]
- 7.Murad W, Azizullah A, Adnan M, Tariq A, Khan KU, Waheed S, et al. Ethnobotanical assessment of plant resources of Banda Daud Shah, District Karak, Pakistan. J Ethnobiol Ethnomed. 2013;9(1):1. doi: 10.1186/1746-4269-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jan S, Khan MR, Rashid U, Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca Buxifolia fruit. Osong Public Health Res Perspect. 2013;4(5):246–54. doi: 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah I, Khan JA, Adhikari A, Khan A, Hannan PA, Wadood A, et al. Bioassay- guided isolation of new urease inhibitory constituents from Monotheca buxifolia (Falc.) fruit and their molecular docking studies. Rec Nat Prod. 2016;10(6):744–9. [Google Scholar]
- 10.Kuete V, Tangmouo JG, Penlap Beng V, Ngounou FN, Lontsi D. Antimicrobial activity of the methanolic extract from the stem bark of tridesmostemon omphalocarpoides (Sapotaceae) J Ethnopharmacol. 2006;104(1–2):5–11. doi: 10.1016/j.jep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Marwat SK, Rehman F, Usman K, Khakwani A, Ghulam S, Anwar N, et al. Medico-ethnobotanical studies of edible wild fruit plants species from the flora of north western Pakistan (DI Khan district) J Med Plants Res. 2011;5:3679–86. [Google Scholar]
- 12.Shekhawat N, Vijayvergia R. Investigation of anti-inflammatory, analgesic and antipyretic properties of Madhuca indica GMEL. Int J Mol Med Adv Sci. 2010;6(2):26–30. [Google Scholar]
- 13.Nasrin M, Dash PR, Saha MR. Investigation of analgesic and neuropharmacological activities of methanolic bark extract of Mimusops elengi. Int J Pharm Sci Res. 2011;2:2050–5. [Google Scholar]
- 14.Araujo-Neto V, Bomfim RR, Oliveira VO, Passos AM, Oliveira JP, Lima CA, et al. Therapeutic benefits of Sideroxylon obtusifolium (Humb. ex Roem. & Schult.) TD Penn., Sapotaceae, in experimental models of pain and inflammation. Rev Bras Farmacogn. 2010;20(6):933–8. doi: 10.1590/S0102-695X2010005000043. [DOI] [Google Scholar]
- 15.Purnima A, Koti B, Thippeswamy A, Jaji M, Swamy AV, Kurhe Y, et al. Antiinflammatory, analgesic and antipyretic activities of Mimusops elengi Linn. Indian J Pharm Sci. 2010;72(4):480. doi: 10.4103/0250-474X.73908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koné WM, Vargas M, Keiser J. Anthelmintic activity of medicinal plants used in Côte d’Ivoire for treating parasitic diseases. Parasitol Res. 2012;110(6):2351–62. doi: 10.1007/s00436-011-2771-z. [DOI] [PubMed] [Google Scholar]
- 17.Karmakar UK, Sultana R, Biswas NN. Antioxidant, analgesic and cytotoxic activities of Mimusops elengi Linn. leaves. Int J Pharm Sci Res. 2011;2(11):2791. [Google Scholar]
- 18.Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012;12(1):59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raaman N. Qualitative phytochemical screening. In: Phytochemical techniques. New India Publishing; 2006. pp. 19–24. [Google Scholar]
- 20.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12(1):221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei L, Zhang W, Yin L, Yan F, Xu Y, Chen F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron J Biotechnol. 2015;18(2):88–95. doi: 10.1016/j.ejbt.2014.12.005. [DOI] [Google Scholar]
- 22.Eldahshan O. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr Res J Biol Sci. 2011;3(1):52–5. [Google Scholar]
- 23.Seo S, Tomita Y, Tori K. Biosynthesis of oleanene-and ursene-type triterpenes from [4-13C] mevalonolactone and sodium [1, 2-13C2] acetate in tissue cultures of Isodon japonicus Hara. J Am Chem Soc. 1981;103(8):2075–80. doi: 10.1021/ja00398a034. [DOI] [Google Scholar]
- 24.Collier H, Dinneen L, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Inflammation Protocols. USA: Springer; 2003. p. 115–21. [DOI] [PubMed]
- 26.Gabriel EI, Chidiebere OF, Ota AA. Evaluation of the methanolic rhizome extract of Anchomanes difformis for analgesic and antipyretic activities. Int J Basic Appl Sci. 2013;2(4):289–96. [Google Scholar]
- 27.Maity TK, Mandal SC, Mukherjee PK, Saha K, Das J, Pal M, et al. Studies on antiinflammatory effect of Cassia tora leaf extract (fam. Leguminosae) Phytother Res. 1998;12(3):221–3. doi: 10.1002/(SICI)1099-1573(199805)12:3<221::AID-PTR221>3.0.CO;2-L. [DOI] [Google Scholar]
- 28.Pérez-Guerrero C, Herrera MD, Ortiz R, de Sotomayor MA, Fernández MA. A pharmacological study of Cecropia obtusifolia Bertol aqueous extract. J Ethnopharmacol. 2001;76(3):279–84. doi: 10.1016/S0378-8741(01)00253-7. [DOI] [PubMed] [Google Scholar]
- 29.Math P, Mishra D, Prajapati P, Roshy J, Jha P. Antipyretic activity of madhukadi and madhukadi Ghana-an experimental study. Int J Pharm Biol Arch. 2011;2(1):572–6. [Google Scholar]
- 30.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–95. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C-P, Li J-L, Zhang L-Z, Zhang X-C, Yu S, Liang X-M, et al. Isoquercetin protects cortical neurons from oxygen–glucose deprivation–reperfusion induced injury via suppression of TLR4–NF-кB signal pathway. Neurochem Int. 2013;63(8):741–9. doi: 10.1016/j.neuint.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Annapurna A, Reddy CS, Akondi RB, Rao SR. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009;61(10):1365–74. doi: 10.1211/jpp.61.10.0014. [DOI] [PubMed] [Google Scholar]
- 33.Gilani A, Janbaz K, Shah B. Quercetin exhibits hepatoprotective activity in rats. Biochem Soc Trans. 1997;25(4):S619-S. doi: 10.1042/bst025s619. [DOI] [PubMed] [Google Scholar]
- 34.Nabavi SM, Nabavi SF, Habtemariam S, Moghaddam AH, Latifi AM. Ameliorative effects of quercetin on sodium fluoride-induced oxidative stress in rat’s kidney. Ren Fail. 2012;34(7):901–6. doi: 10.3109/0886022X.2012.687347. [DOI] [PubMed] [Google Scholar]
- 35.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269(2):315–25. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Qiu X-C, Zhang J, Wang Y, Han J-S, He S-P, Wei M-C. Isoquercitrin increases brain cGMP levels and potentiates electroacupuncture (EA) analgesia. Yao Xue Xue Bao. 1985;20(12):891–5. [PubMed] [Google Scholar]
- 37.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2):325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, et al. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003;74(6):709–21. doi: 10.1016/j.lfs.2003.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Hsu H-Y, Yang J-J, Lin C-C. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111(1):7–13. doi: 10.1016/S0304-3835(96)04481-3. [DOI] [PubMed] [Google Scholar]
- 40.Singh G, Singh S, Bani S, Gupta B, Banerjee S. Anti-inflammatory activity of oleanolic acid in rats and mice. J Pharm Pharmacol. 1992;44(5):456–8. doi: 10.1111/j.2042-7158.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Ye X-l, Liu R, Chen H-L, Bai H, Liang X, et al. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact. 2010;184(3):328–37. doi: 10.1016/j.cbi.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Sun H, Wang X, Mu D, Liao H, Zhang L. Effects of oleanolic acid and maslinic acid on hyperlipidemia. Drug Dev Res. 2007;68(5):261–6. doi: 10.1002/ddr.20187. [DOI] [Google Scholar]
- 43.Rodríguez JA, Astudillo L, Schmeda-Hirschmann G. Oleanolic acid promotes healing of acetic acid-induced chronic gastric lesions in rats. Pharmacol Res. 2003;48(3):291–4. doi: 10.1016/S1043-6618(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 44.Hichri F, Jannet HB, Cheriaa J, Jegham S, Mighri Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. Comptes Rendus Chimie. 2003;6(4):473–83. doi: 10.1016/S1631-0748(03)00066-3. [DOI] [Google Scholar]
- 45.Vasconcelos MAL, Royo VA, Ferreira DS, Crotti AEM, Carvalho JCT, Bastos JK, et al. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae) Z Naturforsch C. 2006;61(7–8):477–82. doi: 10.1515/znc-2006-7-803. [DOI] [PubMed] [Google Scholar]
- 46.Szakiel A, Ruszkowski D, Grudniak A, Kurek A, Wolska KI, Doligalska M, et al. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis) Planta Med. 2008;74(14):1709–15. doi: 10.1055/s-0028-1088315. [DOI] [PubMed] [Google Scholar]
- 47.Nazir M, Khan S, Bhatty M. The constituents of unsaponifiable from Monotheca buxifolia seed oil. Fette, Seifen, Anstrichmittel. 1986;88(7):266–8. doi: 10.1002/lipi.19860880707. [DOI] [Google Scholar]
- 48.Ullah I, Khan JA, Adhikari A, Shahid M. Hepatoprotective effect of Monotheca buxifolia fruit against antitubercular drugs-induced hepatotoxicity in rats. Bangladesh J Pharmacol. 2016;11(1):248–56. doi: 10.3329/bjp.v11i1.25289. [DOI] [Google Scholar]
- 49.Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 50.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8(3):229–35. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 51.Chien S-C, Wu Y-C, Chen Z-W, Yang W-C. Naturally occurring anthraquinones: chemistry and therapeutic potential in autoimmune diabetes. Evid Based Complement Alternat Med. 2015;2015:357357. doi: 10.1155/2015/357357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan J, Yuan C, Lin C, Jia Z, Zheng R. Pharmacological activities and mechanisms of natural phenylpropanoid glycosides. Pharmazie. 2003;58(11):767–75. [PubMed] [Google Scholar]
- 53.Martha Perez Gutierrez R, Maria Neira Gonzalez A, Hoyo-Vadillo C. Alkaloids from piper: a review of its phytochemistry and pharmacology. Mini Rev Med Chem. 2013;13(2):163–93. doi: 10.2174/138955713804805148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials related to this study are available with the corresponding author. Moreover, Additional file 1 is available along with the manuscript.


