Abstract
Background
It is controversial for prognosis of invasive micropapillary carcinoma (IMPC) compared with invasive ductal carcinoma (IDC) of the breast. To better understand the difference between IMPC and IDC prognoses, we conducted this retrospective study.
Methods
Data from 33 patients with IMPC were retrospectively reviewed, and the clinicopathologic characteristics and survival status were compared with those of 347 patients with IDC who were treated during the same period.
Results
The IMPC cases were of larger tumor size, greater proportion of nodal involvement, and an increased incidence of lymphovascular invasion compared with IDC cases. The overall survival (OS), local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and failure-free survival (FFS) rates were not significantly different between IMPC and IDC. The 3-year OS rate was 97 vs 94.2 % for the IMPC and IDC patients, respectively. The 3-year FFS rate was 87.9 vs 86.2 % for the IMPC and IDC patients, respectively. For IMPC patients, the 3-year LRFS rate was 93.9 % and in IDC patients was 89.0 %. The 3-year DMFS rates of IMPC patients was 90.9 % and IDC patients was 89 %.
Conclusions
IMPC had poor clinical characteristics, but it showed no difference in OS, FFS, LRFS, and DMFS compare with IDC.
Keywords: Breast, Invasive micropapillary carcinoma, Invasive ductal carcinoma, Clinical characteristics, Survival, Retrospective study
Background
Breast cancer now represents the most common female malignancy in both the developing and developed world, and is the primary cause of death among women globally [1]. Invasive micropapillary carcinoma (IMPC) was first described by Siriaunkgul and Tavassoli as a rare variant of invasive breast carcinoma characterized by pseudopapillary and tubuloalveolar arrangement of tumor cell clusters in sponge-like, clear empty spaces, mimicking extensive lymphatic invasion [2].
As described by Luna More et al., IMPC is characterized by small, tightly cohesive groups of neoplastic cells within well-delineated clear spaces resembling lymphatic vessels [3].
The incidence of IMPC ranges from 3 to 6 % of all primary breast cancers [4]. Due to the low incidence of this breast cancer variant, most studies examining IMPC have small sample sizes; the clinicopathological characteristics and the clinical prognostic factors of invasive micropapillary carcinoma are therefore not well understood.
It is an important subtype due to its unique features such as high proclivity to lymphovascular invasion, lymph node metastasis, local recurrence, and distant metastasis, thus exhibiting a more aggressive behavior with a poorer prognosis than invasive ductal carcinoma (IDC) [4–8]. However, recently, it has been reported that this carcinoma IMPC has a similar or favorable prognosis compared with IDC [9, 10].
There is controversy about the prognosis of IMPC of the breast. Therefore, greater understanding about these rare tumors is urgent. The aims of this study were to investigate the clinicopathologic characteristics, treatment patterns, and the clinical outcomes compared with IDC in Hainan Island of South China. Moreover, the objective of this article is to draw attention to summarizing the survival rate of IMPC compared with IDC among the similar literatures.
Methods
We conducted a retrospective study of breast cancer patients who were treated at the Hainan Province People’s Hospital between January 2010 and December 2012. This study was approved by the institutional review board and ethics committee of our hospital. In these periods, a total of 525 patients had operations for breast cancers in this institute. Of them, 33 patients (6.3 %) were diagnosed with IMPC (including pure and mixed type) and 347 patients were diagnosed with pure IDC. All IMPC cases included in the study displayed a micropapillary tumor component that was in accordance with the morphological criteria described in the WHO histological classification of tumors of the breast [11]. These patients were compared with 347 patients with pure IDC who were treated during the same period. Of the 33 IMPC cases, 16 patients (48.5 %) were identified as having pure IMPC, whereas 17 patients (51.5 %) had mixed IMPC. We reviewed clinicopathologic factors, immunohistochemistries of biologic factors such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and treatment modalities (type of operation, use of chemotherapy, radiation therapy, and hormone therapy). The pathologic tumor stage was assessed according to the sixth American Joint Committee on Cancer (AJCC) staging system [12]. All patients were followed up by our department at 3-month intervals for the first 2 years, every 6 months for 3–5 years, and annually thereafter. All events were measured from the date of commencement of operation. The following end points (time to the first defining event) were assessed: overall survival (OS), failure-free survival (FFS), local relapse-free survival (LRFS), and distant metastasis-free survival (DMFS).
Statistical analysis
The clinicopathological parameters of the different subgroups were compared using Pearson’s chi-square test; Fisher’s exact test was used when needed. Survival curves were determined and plotted using the Kaplan-Meier method and group differences in survival time were investigated by log-rank test. P values less than 0.05 was considered statistically significant. Hazard ratios (HR) were presented with 95 % confidence intervals. SPSS for Windows (version 16.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
The clinicopathological characteristics of patients with IMPC and IDC
A total of 33 patients with IMPC of the breast were identified in our database. At the same time, 347 patients with IDC were also identified. The clinico-pathologic characteristics of all patients are summarized in Table 1.
Table 1.
Baseline characteristics and treatment patterns for IMPC and IDC
| IMPC (n = 33) | IDC (n = 347) | P* | |
|---|---|---|---|
| Age (year) | 0.885 | ||
| ≤45 | 11 (33.3 %) | 120 (34.6 %) | |
| >45 | 22 (66.7 %) | 227 (65.4 %) | |
| Family history | 0.179 | ||
| Yes | 2 (6.1 %) | 7 (2.0 %) | |
| No | 17 (21.5 %) | 340 (98.0 %) | |
| ER status | 0.011 | ||
| Positive | 27 (81.8 %) | 206 (59.4 %) | |
| Negative | 6 (18.2 %) | 141 (40.6 %) | |
| PR status | 0.123 | ||
| Positive | 25 (75.8 %) | 209 (62.6 %) | |
| Negative | 8 (24.2 %) | 125 (37.4 %) | |
| Her2 status | 0.479 | ||
| Positive | 6 (18.8 %) | 82 (24.3 %) | |
| Negative | 26 (81.2 %) | 255 (75.7 %) | |
| Unknown | 1 | 10 | |
| Subtype | 0.006 | ||
| Luminal | 29 (87.9 %) | 223 (64.3 %) | |
| Non-luminal | 4 (12.1 %) | 124 (35.7 %) | |
| T classification | 0.044 | ||
| T1–T2 | 25 (75.8 %) | 309 (89.0 %) | |
| T3–T4 | 8 (24.2 %) | 38 (11.0 %) | |
| N classification | <0.001 | ||
| N0 | 7 (21.2 %) | 186 (53.8 %) | |
| N1–N3 | 26 (78.2 %) | 160 (46.2 %) | |
| Unknown | 0 | 1 | |
| Staging | <0.001 | ||
| I–II | 15 (45.5 %) | 262 (76.2 %) | |
| III | 18 (54.5 %) | 82 (23.8 %) | |
| Unknown | 0 | 3 | |
| Operation methods | 0.392 | ||
| BCS | 0 (0 %) | 19 (5.5 %) | |
| Mastectomy | 33 (100 %) | 328 (94.5 %) | |
| Adjuvant chemotherapy | 0.709 | ||
| Yes | 32 (97 %) | 321 (93.3 %) | |
| No | 1 (3.0 %) | 23 (6.7 %) | |
| Unknown | 0 | 3 | |
| Radiotherapy | 0.146 | ||
| Yes | 14 (42.4 %) | 104 (30.1 %) | |
| No | 19 (57.6 %) | 241 (69.9 %) | |
| Unknown | 0 | 2 | |
| Hormone therapy | 0.026 | ||
| Yes | 28 (84.8 %) | 227 (65.8 %) | |
| No | 5 (15.2 %) | 118 (34.2 %) | |
| Unknown | 0 | 2 | |
| Neoadjuvant chemotherapy | 0.128 | ||
| Yes | 4 (12.1 %) | 19 (5.5 %) | |
| No | 29 (87.9 %) | 328 (94.5 %) | |
| Trastuzumab | 0.754 | ||
| Yes | 2 (6.1 %) | 33 (9.5 %) | |
| No | 31 (93.9 %) | 314 (90.5 %) | |
| Lymphovascular invasion | <0.001 | ||
| Yes | 6 (18.2 %) | 2 (0.6 %) | |
| No | 27 (81.8 %) | 345 (99.4 %) | |
| Nerve invasion | 0.007 | ||
| Yes | 2 (6.1 %) | 0 (0 %) | |
| No | 3193.9 %) | 347 (100 %) | |
IMPC invasive micropapillary carcinoma, IDC invasive ductal carcinoma, ER estrogen receptor, PR progesterone receptor
* All P values calculated by two-sided x2 test
When comparing staging at presentation, IMPC patients had more T3 or T4 tumors (P = 0.044), a higher percentage of N1-3 nodal involvement (P < 0.001). IMPC patients had a higher incidence of lymphovascular invasion (P < 0.001) and nerve invasion (P = 0.007) compared with IDC patients.
Furthermore, IMPC patients had a larger proportion with luminal subtype than IDC patients (P = 0.006). Expressions of ER were significantly higher (P = 0.011), and expressions of PR were slight higher (P = 0.123) in IMPC than in IDC. Expressions of Her2 were not statistically significantly different in the IMPC and IDC cases. Hormone therapy was significantly higher in IMPC patients. Chemotherapy and radiotherapy were slightly higher in the IMPC group but not statistically significant. In addition, there were no significant differences in age, family history, and Her2 status.
Survival of patients with IMPC and IDC
The median follow-up duration was 39 months for all patients (range 6 to 66). The OS, FFS, LRFS, and DMFS rates were not significantly different between IMPC and IDC. The 3-year OS rate was 97 vs 94.2 % for the IMPC and IDC patients, respectively (P = 0.78) (Fig. 1a). The 3-year FFS rate was 87.9 vs 86.2 % for the IMPC and IDC patients, respectively (P = 0.88) (Fig. 1b). For IMPC patients, the 3-year LRFS rate was 93.9 %, and in IDC patients, it was 89.0 % (P = 0.88) (Fig. 1c). The 3-year DMFS rates of IMPC patients was 90.9 % and IDC patients was 89.0 % (P = 0.97) (Fig. 1d).
Fig. 1.
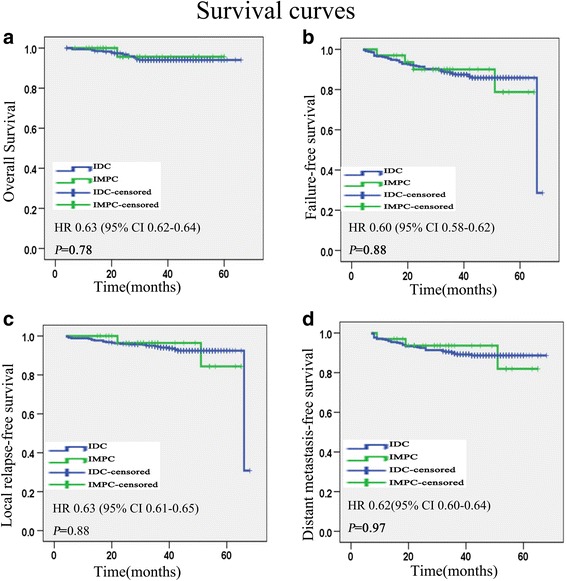
Kaplan-Meier survival curves for 33 patients with IMPC and 347 patients with IDC. Overall survival (a), failure-free survival (b), local relapse-free survival (c), and distant metastasis-free survival (d). P values were calculated with the unadjusted log-rank test. IMPC invasive micropapillary carcinoma, IDC invasive ductal carcinoma
Failure pattern
The 55 patients with treatment failure are listed in Table 2. The IMPC group had four patients that developed treatment failure: there was relapsing event in one patient, distant metastatic event in two patients (both of them had developed multi-organ metastasis), and both distant metastasis and recurrence in one patient.
Table 2.
Patterns of failure in the IMPC and IDC patients after treatment
| Patterns of failure | IMPC (n = 33) | IDC (n = 347) |
|---|---|---|
| Recurrence | n (%) | n (%) |
| Primary recurrence | 1 (3 %) | 9(2.6 %) |
| Nodal recurrence | 0 (0 %) | 2 (0.6 %) |
| Distant metastasis | n (%) | n (%) |
| Bone metastasis | 0 (0 %) | 10 (2.9 %) |
| Lung metastasis | 0 (0 %) | 5 (1.4 %) |
| Liver metastasis | 0 (0 %) | 2 (0.6 %) |
| Mediastina metastasis | 0 (0 %) | 0 (0.0 %) |
| Multiple metastasis | 2 (6.1 %) | 9 (2.6 %) |
| Distant metastasis, primary and/or nodal recurrence | 1 (3 %) | 14 (4 %) |
IMPC invasive micropapillary carcinoma, IDC invasive ductal carcinoma
In IDC group, 51 patients in all had developed treatment failure: there was relapsing event in 11 patients, distant metastatic event in 26 patients and 14 patients had both distant metastasis and recurrence. Seventeen patients had developed distant metastasis in a single organ: ten cases in bone, five cases in lung, and two cases in liver. Nine patients had developed multi-organ metastasis.
Discussion
IMPC is a rare pathological subtype of breast cancer, and the pure variant of IMPC is even rarer. Previous studies have shown that most patients (80~86 %) had mixed IMPC [7, 13]. In our study, most patients (57.6 %) had pure IMPC, whereas only 42.4 % had the mixed form.
Previous studies have shown that IMPC usually presents with a higher TNM stage and is associated with lymphovascular invasion and a higher propensity for lymph node metastases [4–8]. In our study, IMPC patients had more T3 or T4 tumors, a higher percentage of N1–N3 nodal involvement, and a higher incidence of lymphovascular invasion and nerve invasion compared with IDC patients, which is consistent with previous reports.
Most studies reported higher rate of ER positivity than the IDC comparison group (Table 3), and only two studies showed lower or similar rate of ER positivity (Table 3). In our study, the high percentages of ER and PR positivity in IMPCs (81.8 and 75.8 %, respectively) are in accordance with other reports [13–15]. Recently, Rodrigues’ study demonstrated the nuclear localization of epidermal growth factor receptor (EGFR) in the canine spontaneous model of IMPC of the mammary gland. This finding could be useful for EGFR as a predictive biomarker of therapeutic response for IMPC [16].
Table 3.
Characteristics on breast IMPC and IDC in previous seven reports and the present study
| Author | Published time | No. of cases | Component of IMPC | Criteria of eligible patients | ER and PR | Nodal metastases | Lymphovascular invasion | |
|---|---|---|---|---|---|---|---|---|
| IMPC | IDC | |||||||
| Chen [4] | 2008 | 100 | 100 | Mixed | Randomly selected | Lower | Lower | \ |
| Yu [8] | 2010 | 72 | 144 | Pure or more than 70 % | Age, pathologic tumor and node stage, treatment methods | Higher | Higher | Higher |
| Vingiani [14] | 2013 | 49 | 98 | Pure | Age, tumor size and grade | Higher | Higher | Higher |
| Liu [9] | 2014 | 51 | 102 | Pure | Nodal status and age | Higher | Higher | Higher |
| Shi [7] | 2014 | 188 | 1289 | mixed | Simple random sampling | Higher | Higher | Higher |
| Chen [10] | 2014 | 636 | 297735 | unknown | The same study period | Higher | Higher | \ |
| Yu [16] | 2015 | 267 | 267 | Mixed | Age, pathologic tumor and node stage, treatment method | Similar | Similar | Higher |
| Present study | \ | 33 | 347 | Mixed | The same study period | Higher | Higher | Higher |
NO number, IMPC invasive micropapillary carcinoma, IDC invasive ductal carcinoma, ER estrogen receptor, PR progesterone receptor
There were no prospective study and only seven studies for comparative analysis between IMPC and IDC in nearly 20 years, but the criteria of eligible patients were diverse (Table 3). For example, the criteria of eligible patients in Chen’s report was “randomly selected,” the criteria of eligible patients in Yu’s report was “age, pathologic tumor and node stage, treatment method,” and so on. The criteria of eligible patients in Chen’s report was “the same study period” which was the same as our study.
It is widely agreed IMPC has its unique features such as high proclivity to lymphovascular invasion (LVI) and axillary lymph node (ALN) metastasis [7–10, 14]. However, it is controversial for survival rate of IMPC compared with IDC (Table 4).
Table 4.
Survival on breast IMPC and IDC in previous seven reports and the present study
| Author | Median follow-up (month) | OS (IMPC vs IDC) | FFS (IMPC vs IDC) | LRFS (IMPC vs IDC) | DMFS (IMPC vs IDC) |
|---|---|---|---|---|---|
| Chen [4] | 60.1 | 59 vs 77 % P = 0.004 |
/ | 88.8 vs 96 % P = 0.055 |
61.2 vs 72 % P = 0.108 |
| Yu [8] | 45.0 | 86 vs 87.7 % P = 0.18 |
/ | 68.2 vs 81.4 % P = 0.045 |
78.1 vs 79.3 % P = 0.86 |
| Vingiani [14] | 51.0 | 89.8 vs 90.8 % P = 0.8 |
75.5 vs 79.6 % P = 0.47 |
/ | / |
| Liu [9] | 68.4 | / | 84.3 vs 78.4 % P = 0.518 |
/ | / |
| Shi [7] | 40.5 | 75.9 vs 89.5 % P = 0.001 |
67.1 vs 84.5 % P < 0.001 |
/ | / |
| Chen [10] | 48.0 | 82.9 vs 80.5 % P = 0.52 |
/ | / | / |
| Yu [16] | 59.0 | 97.7 vs 95.7 % P = 0.67 |
/ | 91.8 vs 96.3 % P = 0.03 |
/ |
| Present study | 39.0 | 97 vs 94.2 % P = 0.78 |
87.9 vs 86.2 % P = 0.91 |
93.9 vs 89.0 % P = 0.82 |
90.9 vs 89 % P = 0.97 |
IMPC invasive micropapillary carcinoma, IDC invasive ductal carcinoma, OS overall survival, LRFS local relapse-free survival, DMFS distant metastasis-free survival, FFS failure-free survival
In 2008, Chen [4] reported that the survival at 5 and 10 years in the IMPC group was significantly lower than the survival rates in the IDC group. In 2010, Yu [8] showed that the locoregional recurrence-free survival at 5 years in IMPC patients was significantly lower than that in IDC patients, but the 5-year OS and DMFS was no different between two groups. Vingiani [14] reported that disease-free survival (DFS) and OS from breast cancer for MPC and IDC patients were not statistically different in 2013. However, Vingiani’s report did not compare with Chen’s report. Vingiani’s report also did not analyze the detail between their study and Yu’s study.
In 2014, three larger retrospective studies have been reported. Liu et al. [9] showed that no difference in DFS was observed between IMPC and LN-matched IDC patients, but IMPC patients demonstrated significantly reduced survival compared to IDC patients in the T1N2–3 subpopulation, whereas IDC patients demonstrated significantly increased recurrence and metastasis compared to IMPC patients in the T2N2–3 subgroup. Chen et al. [10] showed that despite IMPC’s higher propensity for lymph node metastasis, IMPC has disease-specific survival (DSS) and overall survival (OS) that compare favorably with IDC (the 5-year rates comparing DSS and OS for IMPC was 91.8 and 82.9 %, respectively, compared with 88.6 and 80.5 % for IDC, respectively). However, Shi’s [7] results were different from Liu’s and Chen’s. Shi’s report revealed that patients with IMPC had poorer 5-year BCSS and RFS rates (75.9 and 67.1 %, respectively) than patients with IDC (89.5 %, P = 0.001 and 84.5 %, P < 0.001, respectively).
Recently, a retrospective multicenter study by Yu et al. [17] showed that the rate of distant metastasis (P = 0.52) and overall survival (P = 0.67) did not differ between the two groups. However, LRR-free survival (P = 0.03) and recurrence-free survival (P = 0.007) were significantly different between the two groups. These results were in line with their previous results in 2010 [8].
In brief, six of seven studies referred to the OS, and four of the six studies suggested that the OS of IMPC is not inferior to that of IDC. Just scattered studies provided information about RFS and DMS in the IMPC and matched series of IDC patients. Only Yu’s and Chen’s studies mentioned RFS and DMS; they revealed that IMPC showed a tendency for a higher recurrence rate and had a risk of distant metastasis similar to that observed in the matched series of IDC patients. In our study, IMPC has FFS and OS that compare similarly with IDC which is consistent with Chen’s study. However, Chen’s study did not provided information about LRFS and DMFS. We also analyzed the failure pattern and found that IMPC has LRFS and DMFS that compare similarly with IDC.
Nodal status, tumor size, tumor characteristics, and choice of surgery will dictate additional adjuvant therapies like chemotherapy, radiation, and hormonal therapy [18]. Patients with IMPC who had high percentages of ER and PR positivity, larger tumor size, greater proportion of nodal involvement, and an increased incidence of lymphovascular invasion showed no difference in survival. We thought maybe it is largely attributable to getting much more endocrine therapy, adjuvant chemotherapy, and radiotherapy than those with IDC. There are some limitations in this study. First, the number of IMPC patients was small. Second, the retrospective nature of the data introduces bias. Third, Ki-67 pathological data were not routinely obtained from patients, while Ki-67 was commonly used as a prognostic factor. In addition, longer follow-up is needed to verify the prognosis of IMPC in our study.
We believe that there will be more large-scale retrospective studies or clinical trials about IMPC of the breast to understand the prognosis. Why did the IMPC show no difference in prognosis compared with IDC though it had inferior clinical characteristics? Is it largely attributable to getting much more endocrine therapy, adjuvant chemotherapy, and radiotherapy than those with IDC? Or did IMPC have unique features of the molecular mechanisms that underlie its pathology and progression? These issues deserve our further study.
Conclusions
Patients with IMPC had high percentages of ER and PR positivity, larger tumor size, greater proportion of nodal involvement, and an increased incidence of lymphovascular invasion. IMPC showed no difference in OS, FFS, LRFS, and DMFS compare with IDC.
Abbreviations
DMFS, distant metastasis-free survival; ER, estrogen receptor; FFS, failure-free survival; IDC, invasive ductal carcinoma; IMPC, invasive micropapillary carcinoma; LRFS, local relapse-free survival; OS, overall survival; PR, progesterone receptor
Acknowledgements
We are very grateful to Dean Moody who helped us in improving the English writing.
Funding
This work was supported by Hainan Province Natural Science funding (20158293), Hainan Province Medical and Health Research Project (200309).
Availability of data and materials
Our data will not be shared temporarily because the data will be used in further study about IMPC.
Authors’ contributions
YC designed and edited the manuscript of the study. LG and YS drafted the manuscript, collected and interpreted the data, and drafted the manuscript. YJ carried out the statistical analysis and critically revised the manuscript. WZ, YG, and LM was involved in the study design, statistical analysis, and data interpretation. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Consent to publish was not obtained because an individual person’s information is not included in this study.
Ethics approval and consent to participate
This study was approved by the ethics committee of Hainan Province People’s Hospital. Informed consent was not obtained from each participant because this was a retrospective study.
Contributor Information
Guanqiao Li, Email: guanqiaoli1981@163.com.
Shiping Yang, Email: shipingyang1982@sina.com.
Jia Yao, Email: javance@sina.com.
Zhenping Wang, Email: wang_zp2013@163.com.
Guangyu Yao, Email: ygy531@163.com.
Mingfeng Liu, Email: matthewliu007@163.com.
Changsheng Ye, Email: yechsh@smu.edu.cn.
References
- 1.Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;8(6):697–702. doi: 10.2217/fon.12.61. [DOI] [PubMed] [Google Scholar]
- 2.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660e2. [PubMed] [Google Scholar]
- 3.Luna-More S, delosSantos F, Breton JJ, Candas MA. Estrogen and progesterone receptors, c-erbB-2, p53, and Bcl-2 in thirty-three invasive micropapillary breast carcinomas. Pathol ResPract. 1996;192:27–32. doi: 10.1016/S0344-0338(96)80126-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Fan Y, Lang RG, Guo XJ, Sun YL, Cui LF, Liu FF, Wei J, Zhang XM, Fu L. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16:155–163. doi: 10.1177/1066896907307047. [DOI] [PubMed] [Google Scholar]
- 5.De la Cruz C1, Moriya T, Endoh M, Watanabe M, Takeyama J, Yang M, Oguma M, Sakamoto K, Suzuki T, Hirakawa H, Orita Y, Ohuchi N, Sasano H. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54(2):90–6. doi: 10.1111/j.1440-1827.2004.01590.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda H, Sakamoto G, Ohnisi K, Itoyama S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer. 2004;11(2):169–74. doi: 10.1007/BF02968297. [DOI] [PubMed] [Google Scholar]
- 7.Shi WB, Yang LJ, Hu X, Zhou J, Zhang Q, Shao ZM. Clinico-pathological featuresand prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from China. PLoS One. 2014;9(6):e101390. doi: 10.1371/journal.pone.0101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JI, Choi DH, Park W, Huh SJ, Cho EY, Lim YH, Ahn JS, Yang JH, Nam SJ. Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive ductal carcinoma of the breast: matched case-control study. Breast. 2010;19:231–237. doi: 10.1016/j.breast.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Huang X, Bi R, Yang W, Shao Z. Similar prognoses for invasive micropapillary breast carcinoma and pure invasive ductal carcinoma: a retrospectively matched cohort study in China. PLoS One. 2014;9(9):e106564. doi: 10.1371/journal.pone.0106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC, Teh BS. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer. 2014;111(3):619–22. doi: 10.1038/bjc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol. 2002;86:116–9. [PubMed] [Google Scholar]
- 12.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–19. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 13.Gokce H, Durak MG, Akin MM, Canda T, Balci P, Ellidokuz H, Demirkan B, Gorken IB, Sevinc AI, Kocdor MA, Saydam S, Harmancioglu O. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013;19(4):374–81. doi: 10.1111/tbj.12128. [DOI] [PubMed] [Google Scholar]
- 14.Vingiani A, Maisonneuve P, Dell’orto P, Farante G, Rotmensz N, Lissidini G, Del Castillo A, Renne G, Luini A, Colleoni M, Viale G, Pruneri G. The clinical relevance of micropapillary carcinoma of the breast: a case-control study. Histopathology. 2013;63:217–224. doi: 10.1111/his.12147. [DOI] [PubMed] [Google Scholar]
- 15.Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC, Teh BS. Prognostic markers for invasive micropapillary carcinoma of the breast: a population-based analysis. Clin Breast Cancer. 2013;13(2):133–139. doi: 10.1016/j.clbc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues MA, Gamba CO, Faria JA, Ferreira Ê, Goes AM, Gomes DA, Cassali GD. Inner nuclear membrane localization of epidermal growth factor receptor (EGFR) in spontaneous canine model of invasive micropapillary carcinoma of the mammary gland. Pathol Res Pract. 2016;212(4):340–4. doi: 10.1016/j.prp.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JI, Choi DH, Huh SJ, Cho EY, Kim K, Chie EK, Ha SW, Park IA, Ahn SJ, Lee JS, Shin KH, Kwon Y, Kim YB, Suh CO, Koo JS, Kim JH, Jeong BG, Kim IA, Lee JH, Park W. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case-control study (KROG 13-06) Clin Breast Cancer. 2015;15(5):353–361. doi: 10.1016/j.clbc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Graham LJ, Shupe MP, Schneble EJ, Flynt FL, Clemenshaw MN, Kirkpatrick AD, Gallagher C, Nissan A, Henry L, Stojadinovic A, Peoples GE, Shumway NM. Current approaches and challenges in monitoring treatment responses in breast cancer. J Cancer. 2014;5(1):58–68. doi: 10.7150/jca.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data will not be shared temporarily because the data will be used in further study about IMPC.


