Abstract
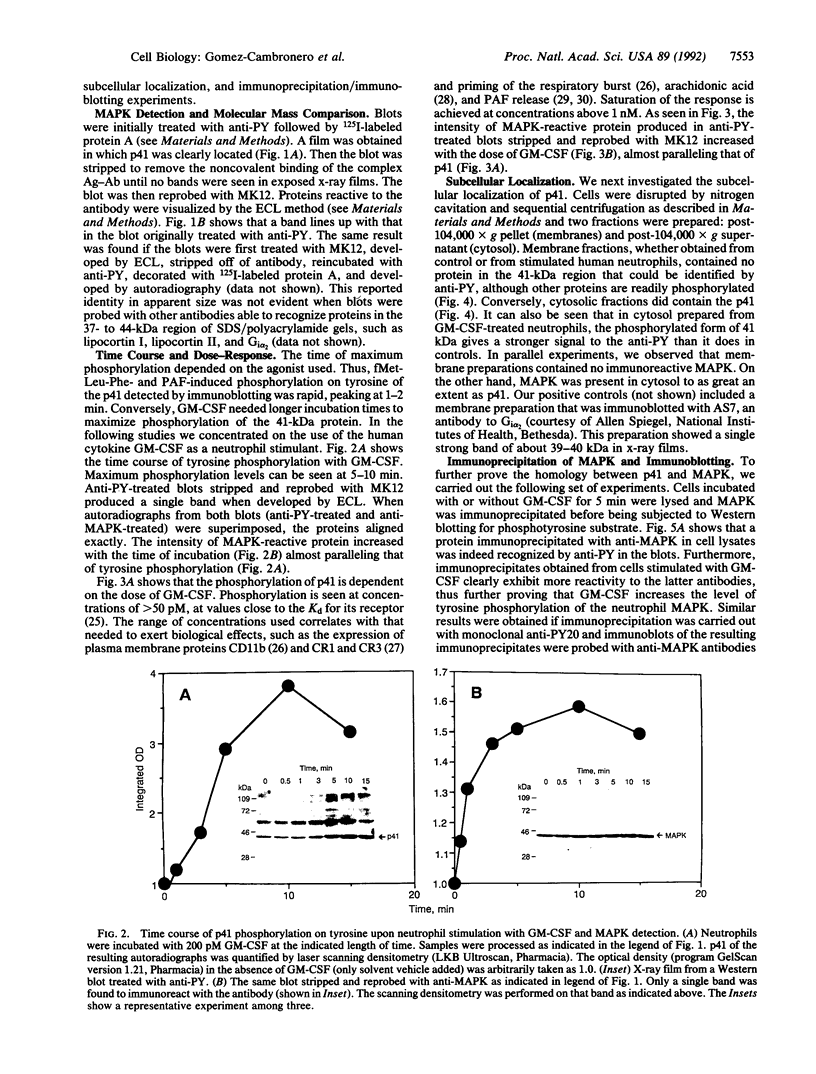
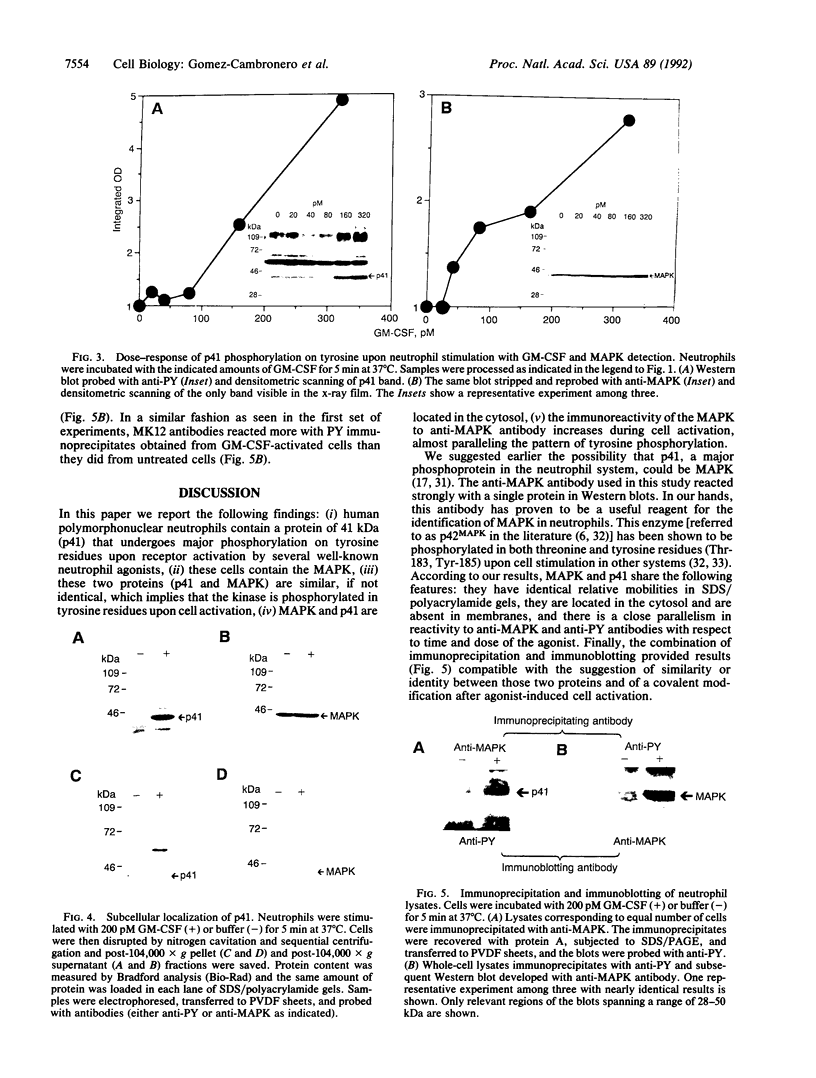
Granulocyte-macrophage colony-stimulating factor (GM-CSF), formylmethionylleucylphenylalanine, tumor necrosis factor alpha, platelet-activating factor, phorbol ester (phorbol 12-myristate 13-acetate), and calcium ionophore A23187 are able to increase the level of tyrosine phosphorylation of different protein substrates, as demonstrated by Western blotting with anti-phosphotyrosine antibody (anti-PY). A protein of 41 kDa (p41) consistently showed more intense reactivity to anti-PY than controls. Blots treated with anti-PY, stripped of the antibody, and reblotted with microtubule-associated protein kinase (MAPK, p42MAPK) antibody show only one band. The molecular mass of that band exactly matches that of p41. MAPK-reactive protein is present in control and stimulated cells, although the intensity of the band is greater in the latter. GM-CSF-stimulated phosphorylation of p41 is time- and dose-dependent. Anti-MAPK antibody detects a single band of 41 kDa, whose intensity increases with time of incubation and concentration of the agonist. Thus, the anti-MAPK antibody appears to react better to the phosphorylated form of p41 from GM-CSF-stimulated cells than to the dephosphorylated form. The p41 and MAPK proteins are localized in the cytosol. Finally, MAPK immunoprecipitates were probed with anti-PY in Western blots and a band of 41 kDa was found. In summary, these results suggest that this 41-kDa protein in neutrophils that is tyrosine phosphorylated in response to GM-CSF and other stimuli is MAPK. Its phosphorylation may represent an early and crucial signal associated with the GM-CSF neutrophil stimulation cascade.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Li P., Marsden L. A., Williams N., Roberts T. M., Sturgill T. W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991 Jul 15;277(Pt 2):573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Yancopoulos G. D., Gregory J. S., Slaughter C., Moomaw C., Hsu J., Cobb M. H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990 Jul 6;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Campos-González R., Glenney J. R., Jr Temperature-dependent tyrosine phosphorylation of microtubule-associated protein kinase in epidermal growth factor-stimulated human fibroblasts. Cell Regul. 1991 Aug;2(8):663–673. doi: 10.1091/mbc.2.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. F., Billing P., Williams R., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J Immunol. 1988 Jun 15;140(12):4315–4322. [PubMed] [Google Scholar]
- DiPersio J., Billing P., Kaufman S., Eghtesady P., Williams R. E., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1988 Feb 5;263(4):1834–1841. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Bonak V. A., Wang E., Casnellie J. E., Shiraishi T., Sha'afi R. I. Tyrosine phosphorylation in human neutrophil. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Yamazaki M., Wang E., Molski T. F., Becker E. L., Sha'afi R. I. Phorbol ester inhibits granulocyte-macrophage colony-stimulating factor binding and tyrosine phosphorylation. Am J Physiol. 1992 Feb;262(2 Pt 1):C276–C281. doi: 10.1152/ajpcell.1992.262.2.C276. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Wang E., Johnson G., Huang C. K., Sha'afi R. I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991 Apr 5;266(10):6240–6245. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Yamazaki M., Metwally F., Molski T. F., Bonak V. A., Huang C. K., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor and human neutrophils: role of guanine nucleotide regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3569–3573. doi: 10.1073/pnas.86.10.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Translocation of the FGR protein-tyrosine kinase as a consequence of neutrophil activation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8783–8787. doi: 10.1073/pnas.86.22.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Her J. H., Wu J., Rall T. B., Sturgill T. W., Weber M. J. Sequence of pp42/MAP kinase, a serine/threonine kinase regulated by tyrosine phosphorylation. Nucleic Acids Res. 1991 Jul 11;19(13):3743–3743. doi: 10.1093/nar/19.13.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Laramee G. R., Casnellie J. E. Chemotactic factor induced tyrosine phosphorylation of membrane associated proteins in rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1988 Mar 15;151(2):794–801. doi: 10.1016/s0006-291x(88)80351-6. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Khwaja A., Roberts P. J., Jones H. M., Yong K., Jaswon M. S., Linch D. C. Isoquinolinesulfonamide protein kinase inhibitors H7 and H8 enhance the effects of granulocyte-macrophage colony-stimulating factor (GM-CSE) on neutrophil function and inhibit GM-CSF receptor internalization. Blood. 1990 Sep 1;76(5):996–1003. [PubMed] [Google Scholar]
- Kraft A. S., Berkow R. L. Tyrosine kinase and phosphotyrosine phosphatase activity in human promyelocytic leukemia cells and human polymorphonuclear leukocytes. Blood. 1987 Aug;70(2):356–362. [PubMed] [Google Scholar]
- McColl S. R., DiPersio J. F., Caon A. C., Ho P., Naccache P. H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991 Oct 1;78(7):1842–1852. [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Nakano H., Kobayashi E., Takahashi I., Tamaoki T., Kuzuu Y., Iba H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 1987 May;40(5):706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- Neuman E., Huleatt J. W., Jack R. M. Granulocyte-macrophage colony-stimulating factor increases synthesis and expression of CR1 and CR3 by human peripheral blood neutrophils. J Immunol. 1990 Nov 15;145(10):3325–3332. [PubMed] [Google Scholar]
- Pelz C., Matsumoto T., Molski T. F., Becker E. L., Sha'afi R. I. Characterization of the membrane-associated GTPase activity: effects of chemotactic factors and toxins. J Cell Biochem. 1989 Feb;39(2):197–206. doi: 10.1002/jcb.240390211. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Wirthmueller U., De Weck A. L., Dahinden C. A. Platelet-activating factor production in human neutrophils by sequential stimulation with granulocyte-macrophage colony-stimulating factor and the chemotactic factors C5A or formyl-methionyl-leucyl-phenylalanine. J Immunol. 1989 May 1;142(9):3213–3218. [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]








