Abstract
Background
The response to neoadjuvant chemotherapy has been proven to predict long-term clinical benefits for patients. Our research is to construct a nomogram to predict pathological complete response of human epidermal growth factor receptor 2 negative breast cancer patients.
Methods
We enrolled 815 patients who received neoadjuvant chemotherapy from 2003 to 2015 and divided them into a training set and a validation set. Univariate logistic regression was performed to screen for predictors and construct the nomogram; multivariate logistic regression was performed to identify independent predictors.
Results
After performing the univariate logistic regression analysis in the training set, tumor size, hormone receptor status, regimens of neoadjuvant chemotherapy and cycles of neoadjuvant chemotherapy were the final predictors for the construction of the nomogram. The multivariate logistic regression analysis demonstrated that T4 status, hormone receptor status and receiving regimen of paclitaxel and carboplatin were independent predictors of pathological complete response. The area under the receiver operating characteristic curve of the training set and the validation set was 0.779 and 0.701, respectively.
Conclusions
We constructed and validated a nomogram to predict pathological complete response in human epidermal growth factor receptor 2 negative breast cancer patients. We also identified tumor size, hormone receptor status and paclitaxel and carboplatin regimen as independent predictors of pathological complete response.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-016-2652-z) contains supplementary material, which is available to authorized users.
Keywords: HER2 negative breast cancer, Neoadjuvant chemotherapy, Nomogram, Pathological complete response
Background
Breast cancer is the most common malignant disease and the second most common cause of cancer death in women [1]. Neoadjuvant chemotherapy has several advantages compared with adjuvant chemotherapy [2]. It increases the rate of breast conservation and offers the opportunity for patients with locally advanced breast cancer to receive surgery. Moreover, sensitivity to different chemotherapy regimens can be assessed, thus helping to make decisions for subsequent treatment.
Pathological complete response (pCR) has been confirmed to predict long-term clinical benefit for patients receiving neoadjuvant chemotherapy and can serve as a dependable endpoint when investigating the efficiency of different treatment regimens [3]. With the application of human epidermal growth factor receptor 2 blockade using neoadjuvant treatments such as trastuzumab, pertuzumab and lapatinib in human epidermal growth factor receptor 2 (HER2) positive patients, the pCR rate of HER2 positive patients is high (16.8–66.2 %) [4]. However, the pCR rate of HER2 negative patients is relatively low (7.0–16.2 % for hormone receptor positive, HER2 negative patients and 33.6–35.0 % for triple negative patients) [3, 5]. Thus, predicting the response to neoadjuvant chemotherapy for HER2 negative patients is essential to optimizing the treatment for individual patients.
Anthracyclines used to be the most common chemotherapeutic agents for breast cancer [6]. However, as taxane-based [7] or platinum-based [8, 9] regimens showed their advantages, the use of anthracyclines has been declining in recent years [10]. The potential impact of this change is still unknown.
A nomogram is a simple graphical representation of a prediction model that helps oncologists assess the predictive information of individual patients [11]. Several earlier studies constructed nomograms to illustrate the impact of different variables on pCR probability [12–14], but none of them focused on HER2 negative patients and different neoadjuvant chemotherapy regimens.
Our current study aims to construct and validate a well-fitting nomogram based on multivariate logistic regression to evaluate the impact of different neoadjuvant chemotherapy regimens as well as the impact of several other variables on the pCR rate among HER2 negative patients in a prospective cohort.
Methods
Patient population
Relevant clinical data (age, menopausal status, tumor size, nodal status, regimens of chemotherapy and cycles of chemotherapy), core needle biopsy samples and surgical specimens were collected from Fudan University Shanghai Cancer Center between January 1, 2003 and April 31, 2015.
Overall, 1244 patients who were diagnosed with primary breast cancer and who received neoadjuvant chemotherapy followed by standard surgery were enrolled.
Patients with HER2 positive core needle biopsy samples, with metastatic disease, with missing data or with previous endocrine therapy were not eligible for this study. In total, 429 patients who had missing relevant information, who were HER2 positive or who had received neoadjuvant chemotherapy regimens other than cyclophosphamide, epirubicin and 5-fluorouracil, cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin, navelbine and epirubicin or paclitaxel and carboplatin or paclitaxel and cisplatin were excluded from our study.
The remaining 815 patients were randomized into a training set (N = 500, enrolled in the nomogram construction) or a validation set (N = 315, enrolled in the nomogram external validation) (Fig. 1).
Fig. 1.
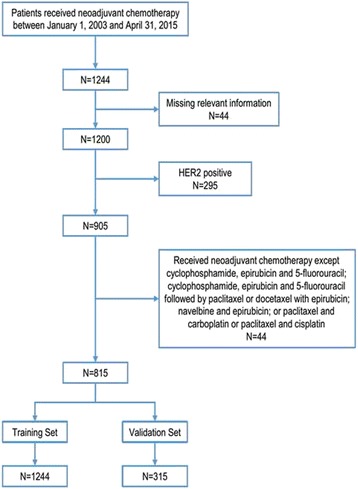
Flow diagram of the study design. A total of 815 Human Epidermal Growth Factor Receptor 2 (HER2) negative patients who received neoadjuvant chemotherapy with the regimen of cyclophosphamide, epirubicin and 5-fluorouracil; cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin; navelbine and epirubicin; or paclitaxel and carboplatin or paclitaxel and cisplatin were included in this study
Pathology and treatment
Estrogen receptor, progestogen receptor status and HER2 status were determined by immunohistochemical analysis, which was performed with formalin-fixed, paraffin-embedded tissue sections using standard protocols for core needle biopsy specimens by the pathology department of Fudan University Shanghai Cancer Center. The cut-off value for estrogen receptor positivity and progestogen receptor positivity was set at 1 %. Absence of both estrogen receptor and progestogen receptor was defined as hormone receptor negative (estrogen receptor negative and progestogen receptor negative); presence of either was defined as hormone receptor positive (estrogen receptor positive or progestogen receptor positive). HER2-positivity was defined as 3 (+) by immunohistochemical or amplification and was confirmed by fluorescence in situ hybridization. Each specimen was examined independently by two experienced pathologists.
The patients in our cohort received one of the following neoadjuvant chemotherapy regimens for a median of 4 cycles (range, 1–6 cycles): navelbine and epirubicin, cyclophosphamide, epirubicin and 5-fluorouracil, paclitaxel with carboplatin/paclitaxel with cisplatin or epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin. pCR was defined as complete disappearance of invasive carcinoma in the breast and regional lymph nodes [3].
Construction of the nomogram
To develop a well-calibrated and useful nomogram for predicting pCR, possible predictive variables were identified by univariate logistic regression (P < 0.05 in univariate logistic regression analysis). The Hosmer-Lemeshow test was used to assess the fitness of the nomogram (P > 0.05 indicating good fit) [15]. Multivariate logistic regression analysis was performed to screen independent variables predicting pCR. Odds ratios and 95 % confidence intervals (CI) were calculated.
Evaluating model performance
The internal validation of our model was performed by a calibration method and the area under the receiver operating characteristic (ROC) curve (AUC). Calibration [16] (visualized as the calibration plot) with a bootstrapping method [17] was used to illustrate the association between the actual probability and the predicted probability. The external validation was achieved by performing the ROC as well as the AUC in a separated population. The AUC ranged from 0 to 1, with the value of 1 indicating perfect concordance, 0.5 indicating no better than chance, and 0 indicating discordance. Statistical differences between different AUCs were investigated by the DeLong method [18].
Statistical analysis
Chi-square test was used to evaluate the relationship between neoadjuvant chemotherapy regimens and other characteristics. Fisher’s exact test was performed when necessary. All reported P-values are two-sided. The statistical analysis was carried out using SPSS (version 20.0; SPSS Company, Chicago, IL) and R software version 3.13 (http://www.r-project.org). The R package with rms, pROC, Hmisc and ggplot2 (available at URL: http://cran.r-project.org/web/packages/) was used (last accessed on March 9, 2015). All relevant R code were shown in Additional file 1.
Results
Patient characteristics
Of the 815 HER2 negative patients enrolled in this study, 111 (13.6 %) reached pCR (Table 1). Young patients (≤40 years) [19] had higher pCR rates than older patients (>40 years) (17.0 % versus 12.8 %). Pre-menopausal patients (14.2 %) had higher pCR rates than those who were post-menopausal (12.8 %). Patients with smaller tumor size and more positive lymph nodes reached pCR more easily. hormone receptor negative patients (23.0 %) had higher pCR rates than hormone receptor positive ones (9.8 %). Patients who received the paclitaxel with carboplatin/paclitaxel with cisplatin regimen had higher pCR rates than those who received the cyclophosphamide, epirubicin and 5-fluorouracil, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin or navelbine and epirubicin regimens (19.4 % versus 1.9 %, 7.8 and 9.8 %, respectively). Patients who received 3 to 4 cycles of neoadjuvant chemotherapy had higher pCR rates (16.1 %) than other subjects. These results were similar in the training and validation sets.
Table 1.
Clinicopathologic characteristics of patients
| Overall | Training set | Validation set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL (N) | pCR (N) | pCR rate | ALL (N) | pCR (N) | pCR rate | ALL (N) | pCR (N) | pCR rate | |
| Total | 815 | 111 | 13.6 % | 500 | 68 | 13.6 % | 315 | 43 | 13.7 % |
| Age | |||||||||
| ≤40 years | 165 | 28 | 17.0 % | 105 | 17 | 16.2 % | 60 | 11 | 18.3 % |
| >40 years | 650 | 83 | 12.8 % | 395 | 51 | 12.9 % | 255 | 32 | 12.5 % |
| Menopausal status | |||||||||
| Pre-menopausal | 457 | 65 | 14.2 % | 276 | 40 | 14.5 % | 181 | 25 | 13.8 % |
| Post-menopausal | 358 | 46 | 12.8 % | 224 | 28 | 12.5 % | 134 | 18 | 13.4 % |
| Tumor size | |||||||||
| T1 | 89 | 21 | 23.6 % | 60 | 15 | 25.0 % | 29 | 6 | 20.7 % |
| T2 | 346 | 47 | 13.6 % | 210 | 30 | 14.3 % | 136 | 17 | 12.5 % |
| T3 | 235 | 28 | 11.9 % | 137 | 15 | 10.9 % | 98 | 13 | 13.3 % |
| T4 | 145 | 15 | 10.3 % | 93 | 8 | 8.6 % | 52 | 7 | 13.5 % |
| Nodal status | |||||||||
| N0 | 170 | 22 | 12.9 % | 100 | 15 | 15.0 % | 70 | 7 | 10.0 % |
| N1 | 593 | 79 | 13.3 % | 363 | 45 | 12.4 % | 230 | 34 | 14.8 % |
| N2 | 23 | 4 | 17.4 % | 16 | 3 | 18.8 % | 7 | 1 | 14.3 % |
| N3 | 29 | 6 | 20.7 % | 21 | 5 | 23.8 % | 8 | 1 | 12.5 % |
| Hormone receptor status | |||||||||
| Negative | 235 | 54 | 23.0 % | 147 | 36 | 24.5 % | 88 | 18 | 20.5 % |
| Positive | 580 | 57 | 9.8 % | 353 | 32 | 9.1 % | 227 | 25 | 11.0 % |
| Regimens | |||||||||
| Cyclophosphamide, epirubicin and 5-fluorouracil | 107 | 2 | 1.9 % | 66 | 1 | 1.5 % | 41 | 1 | 2.4 % |
| Cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin | 116 | 9 | 7.8 % | 73 | 5 | 6.8 % | 43 | 4 | 9.3 % |
| Navelbine and epirubicin | 153 | 15 | 9.8 % | 94 | 8 | 8.5 % | 59 | 7 | 11.9 % |
| Paclitaxel and carboplatin or paclitaxel and cisplatin | 439 | 85 | 19.4 % | 267 | 54 | 20.2 % | 172 | 31 | 18.0 % |
| Cycles | |||||||||
| 1-2 | 97 | 3 | 3.1 % | 61 | 2 | 3.3 % | 36 | 1 | 2.8 % |
| 3-4 | 578 | 93 | 16.1 % | 359 | 58 | 16.2 % | 219 | 35 | 16.0 % |
| 5-6 | 140 | 15 | 10.7 % | 80 | 8 | 10.0 % | 60 | 7 | 11.7 % |
Abbreviations: pCR pathological complete response
Predictors for pCR
In the training set, univariate logistic regression was performed to analyze the association between response to chemotherapy and patient age, menopausal status, tumor size, nodal status, hormone receptor status, regimens of chemotherapy and cycles of chemotherapy (Table 2). Tumor size (P = 0.029), hormone receptor status (<0.001), and neoadjuvant chemotherapy regimens (P < 0.001) and cycles (P = 0.029) were identified to be statistically significant predictors of pCR. No significant differences in pCR rate were observed among patients with different ages, menopausal statuses or nodal statuses.
Table 2.
Univariate logistic regression analysis of different variables predicting pCR in the training set
| P | OR | 95 % CI | |
|---|---|---|---|
| Total | |||
| Age | 0.385 | ||
| ≤40 years | 1 | ||
| >40 years | 0.385 | 0.767 | 0.423-1.394 |
| Menopausal status | 0.518 | ||
| Pre-menopausal | 1 | ||
| Post-menopausal | 0.518 | 0.843 | 0.502-1.416 |
| Tumor Size | 0.029 | ||
| T1 | 1 | ||
| T2 | 0.052 | 0.500 | 0.248-1.007 |
| T3 | 0.014 | 0.369 | 0.167-0.815 |
| T4 | 0.008 | 0.282 | 0.111-0.716 |
| Nodal status | 0.432 | ||
| N0 | 1 | ||
| N1 | 0.493 | 0.802 | 0.426-1.508 |
| N2 | 0.701 | 1.308 | 0.332-5.147 |
| N3 | 0.328 | 1.171 | 0.564-5.561 |
| Hormone receptor status | <0.001 | ||
| Negative | 1 | ||
| Positive | <0.001 | 0.307 | 0.182-0.518 |
| Regimens | <0.001 | ||
| Cyclophosphamide, epirubicin and 5-fluorouracil | 1 | ||
| Cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin | 0.158 | 4.779 | 0.544-42.018 |
| Navelbine and epirubicin | 0.094 | 6.047 | 0.738-49.558 |
| Paclitaxel and carboplatin or paclitaxel and cisplatin | 0.006 | 16.479 | 2.236-121.451 |
| Cycles | 0.029 | ||
| 3-4 | 1 | ||
| 1-2 | 0.018 | 0.176 | 0.042-0.740 |
| 5-6 | 0.143 | 0.577 | 0.264-1.261 |
Abbreviations: pCR pathological complete response, OR odds ratio, CI confidence interval
Given that the baseline patient characteristics of different neoadjuvant chemotherapy regimens were not in concordance (Additional file 2), we performed multivariate logistic regression analysis to screen for the independent predictors of pCR (Table 3). Relative to T1 patients, T4 patients were less likely to achieve pCR [P = 0.015, odds ratio =0.281 (95 % CI: 0.101–0779)]. The odds ratio of hormone receptor positive patients was 0.224 (95 % CI: 0.125–0.400); for hormone receptor negative patients, it was 1 (P < 0.001). After adjustment for tumor size, hormone receptor status and neoadjuvant chemotherapy cycles, those who received paclitaxel with carboplatin/paclitaxel with cisplatin had a statistically significant higher rate of pCR Compared with patients who received cyclophosphamide, epirubicin and 5-fluorouracil [P = 0.003, odds ratio =27.696 (95 % CI: 3.131–245.030)]. Patients who received epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin, navelbine and epirubicin had higher odds ratio than those who received cyclophosphamide, epirubicin and 5-fluorouracil (6.973 and 4.701 versus 1), but the difference was not statistically significant. Although we found out the trends that patients receiving only 1–2 cycles neoadjuvant chemotherapy showed lower probability for pCR (odds ratio: 0.579) while patients receiving 5–6 cycles neoadjuvant chemotherapy showed higher probability for pCR (odds ratio: 2.338) than those who received 3–4 cycles of neoadjuvant chemotherapy, different neoadjuvant chemotherapy cycles were not statistically significant for predicting pCR.
Table 3.
Multivariable logistic regression analysis of possible variables (P<0.05 in univariate logistic regression analysis) predicting pCR
| P | OR | 95 % CI | |
|---|---|---|---|
| Tumor size | |||
| T1 | 1 | ||
| T2 | 0.186 | 0.576 | 0.255-1.304 |
| T3 | 0.544 | 0.737 | 0.275-1.975 |
| T4 | 0.015 | 0.281 | 0.101-0.779 |
| Hormone receptor status | |||
| Negative | 1 | ||
| Positive | <0.001 | 0.224 | 0.125-0.400 |
| Regimens | |||
| Cyclophosphamide, epirubicin and 5-fluorouracil | 1 | ||
| Cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin | 0.208 | 4.673 | 0.423-51.590 |
| Navelbine and epirubicin | 0.078 | 6.999 | 0.804-60.897 |
| Paclitaxel and carboplatin or paclitaxel and cisplatin | 0.003 | 27.696 | 3.131-245.030 |
| Cycles | |||
| 3-4 | 1 | ||
| 1-2 | 0.500 | 0.579 | 0.118-2.834 |
| 5-6 | 0.217 | 2.338 | 0.606-9.017 |
Abbreviations: pCR pathological complete response, OR odds ratio, CI confidence interval
We performed logistic regression to explore the predictors for pCR separately both in hormone receptor positive and negative cohort. Tumor status (T3 vs T1, T4 vs T1) was only statistically significant in hormone receptor positive patients and not in hormone receptor negative patients. Nodal status was not statistically significant in either group. Epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel with epirubicin and navelbine with epirubicin showed statistically significant superiority to cyclophosphamide, epirubicin and 5-fluorouracil regimens in hormone receptor negative patients, but not in hormone receptor positive patients, while paclitaxel with carboplatin/paclitaxel with cisplatin regimen treated patients had statistically significant higher pCR in overall patients. Only hormone receptor negative patients who received 1–2 cycles had statistically significant lower pCR rate than those receiving 3–4 cycles (Additional file 3). In addition, we found that among paclitaxel with carboplatin/paclitaxel with cisplatin treated patients, hormone receptor negative (triple negative) patients had higher rate of pCR rate (38.9 %, Chi-square test P < 0.001) than hormone receptor positive patients (13.0 %) (Additional file 4).
Construction and validation of the nomogram
Statistically significant predictors in univariate logistic regression analysis (tumor size, hormone receptor status, neoadjuvant chemotherapy regimens and cycles) were included into the nomogram construction (Fig. 2). The total points were added up by the points of each variable (top scale). The pCR probability depended on the total points (bottom scale). The P-value for the Hosmer-Lemeshow test was 0.817, indicating good fit of the model.
Fig. 2.
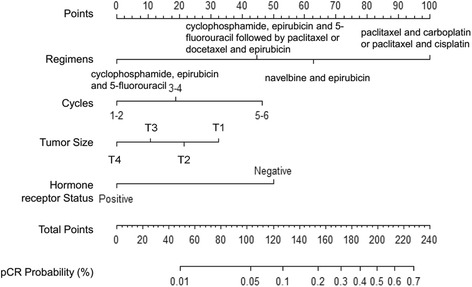
Nomogram predicting the probability of pathological complete response (pCR) after neoadjuvant chemotherapywith the regimen of cyclophosphamide, epirubicin and 5-fluorouracil; cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin; navelbine and epirubicin; or paclitaxel and carboplatin or paclitaxel and cisplatin
The calibration of the nomogram was performed internally by a calibration plot with bootstrap sampling (n = 1000) (Fig. 3). The calibration plot illustrated that the nomogram was well calibrated.
Fig. 3.
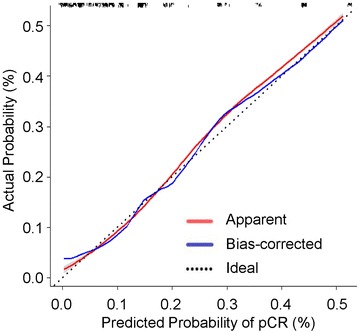
Calibration plot of the nomogram for the probability of pathological complete response (pCR) (bootstrap 1000 repetitions)
Next, we constructed the ROC to further validate the nomogram internally in the training set (Fig. 4a) and externally in the validation set (Fig. 4b). In the training set, the AUC was 0.779 (95 % CI: 0.718–0.839). In the validation set, the AUC was slightly lower: 0.703 (95 % CI: 0.624–0.782). The difference between two AUCs was not statistical significant (P = 0.132). These results illustrated that the predicted and observed pCR probabilities were concordant.
Fig. 4.
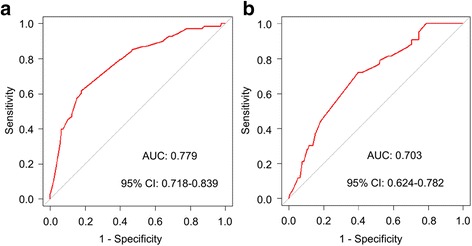
Validation of the Nomogram. a Internal validation using receiver operating characteristic (ROC) curve. The area under the ROC curve (AUC) is 0.779, 95 % confidence intervals (CI): 0.718–0.839. b External validation using ROC. The AUC is 0.703, 95 % CI: 0.622–0.780
Nomogram performance in individual patients
To display the application of the nomogram, we took two breast cancer patients who had received neoadjuvant chemotherapy as examples. The first patient was to receive epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin as an neoadjuvant chemotherapy regimen (45 points) for four cycles (19 points); his tumor size was T2 (22 points) and his hormone receptor status was positive (0 points). According to the nomogram, his probability of reaching pCR was approximately 0.01 to 0.05 (total points: 86). The second patient was to receive paclitaxel with carboplatin/paclitaxel with cisplatin as an neoadjuvant chemotherapy regimen (100 points) for four cycles (19 points); his tumor size was T4 (0 points) and his hormone receptor status was negative (50 points). According to the nomogram, his probability of reaching pCR was approximately 0.2 to 0.3 (total points: 169). As a result of using this nomogram, clinicians can obtain an overview of the response of different treatments for individual patients.
Discussion
Based on the logistic regression, we screened for predictors and constructed a concise and well fitted nomogram containing the variables of tumor size, hormone receptor status, regimens of neoadjuvant chemotherapy and cycles of neoadjuvant chemotherapy to predict the pCR rate of HER2 negative patients. This would be a convenient application for clinicians. Using the method of calibration plot with bootstrap sampling, as well as internal and external validation by AUC and ROC, the nomogram proved to be of good fitness.
In this study, we first screened variables that could predict the response to neoadjuvant chemotherapy by univariate logistic regression. Tumor size, hormone receptor status, and neoadjuvant chemotherapy regimens and cycles were included in the construction of the nomogram. Next, we intended to identify several independent predictors of the pCR rate. In the multivariate logistic regression analysis, we found that T4 status (P = 0.015, odds ratio: 0.281, 95 % CI: 0.101–0.779), hormone receptor positivity (P < 0.001, odds ratio: 0.224, 95 % CI: 0.125–0.400) and receiving the paclitaxel with carboplatin/paclitaxel with cisplatin regimen (P = 0.003, odds ratio: 27.696, 95 % CI: 3.131–245.030) were the most important predictors of pCR in this model. Compared with T1 patients, T4 patients had worse responses to chemotherapy, which is consistent with previous research [20]. Hormone receptor status was another independent predictor, and hormone receptor positive patients had lower pCR rates than hormone receptor negative patients. Our findings are concordant with previous studies [20–22] that show that hormone receptor positive tumor cells are less sensitive to chemotherapy compared with hormone receptor negative cells. Patients treated with paclitaxel with carboplatin/paclitaxel with cisplatin had better neoadjuvant chemotherapy responses compared with those treated with cyclophosphamide, epirubicin and 5-fluorouracil. Anthracyclines such as epirubicin and doxorubicin were once considered to be the most effective agents in the treatment of breast cancer, but the use of them has been declining recently [10]. In our current study, the anthracycline-based regimens included cyclophosphamide, epirubicin and 5-fluorouracil, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel with epirubicin and navelbine with epirubicin. Cyclophosphamide, epirubicin and 5-fluorouracil was the standard anthracycline-based regimen, and the pCR rate after 6 cycles of cyclophosphamide, epirubicin and 5-fluorouracil was reported to be 14–15 % [23, 24]. However, only 1.9 % of patients who received cyclophosphamide, epirubicin and 5-fluorouracil in our study reached pCR, which may be partially due to the relatively higher proportion of larger tumor size (T3: 50.5 %; T4: 13.1 %) and fewer neoadjuvant chemotherapy cycles received (1–2 cycles: 49.5 %) in the cyclophosphamide, epirubicin and 5-fluorouracil cohort. The total pCR rate for epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin patients was low (7.8 %) which may due to the relatively higher proportion of hormone receptor positive patients (87.1 %). The cumulative cardiac toxicity of anthracyclines has also limited its use, especially in older patients or in those with cardiovascular comorbidities. Therefore, non-anthracycline based regimens are required. Paclitaxel, a mitotic inhibitor and anti-microtubule agent, results in a G2-M phase arrest [25]. Carboplatin and cisplatin share similar anti-cancer mechanisms, as they are both DNA alkylating agents [26]. The combination of paclitaxel and platinum is now widely used in breast cancer patients, and the agents have no overlapping toxicities [27]. Previous research has already assessed the efficacy and the toxicity of the paclitaxel with carboplatin/paclitaxel with cisplatin regimen in adjuvant therapy and in neoadjuvant chemotherapy. The pCR rate of patients who received paclitaxel with carboplatin/paclitaxel with cisplatin as neoadjuvant chemotherapy ranged from 9.5 to 19.4 % [28, 29]. The data from our center is 19.4 %, similar to previous studies. The paclitaxel with carboplatin/paclitaxel with cisplatin regimen achieved greater therapeutic effect than any anthracycline-based regimens, especially in triple negative breast cancer patients. Triple negative breast cancer patients have higher rate of BRCA1/2 (Breast Cancer 1/2) mutation and are sensitive to platinum (because of the deficiencies in the DNA repair mechanism) [30, 31]. In aggregate, these results suggested that platinum contained therapy is recommend for triple negative breast cancer patients.
The nomogram provides a simple graphical representation of sophisticated statistical prediction models and has been accepted as a reliable tool for predicting clinical events. It is especially widely used in oncology [11]. Previously, several studies constructed nomograms to predict the pCR rate of neoadjuvant chemotherapy. The first of these studies appeared in 2005 [12]. Rouzier et al. constructed two nomograms to predict the responses to anthracycline-based neoadjuvant chemotherapy and to combined anthracycline and paclitaxel neoadjuvant chemotherapy. The nomograms were validated externally. Colleoni et al. constructed a nomogram to predict pCR probability based on a population of 783 patients [13]. The nomogram proved to be well fitted after external validation by 101 patients. However, the HER2 status was not mentioned in these two studies. Keam et al. constructed another nomogram to predict pCR and predict which patients would not relapse [14]. Overall, 370 patients who received 3 cycles of neoadjuvant docetaxel or doxorubicin were included in this study. However, the HER2 status was not stratified and the validation of the nomogram was only performed internally. The advantage of our research is that we first constructed a nomogram for predicting the pCR rate among HER2 negative patients, and the nomogram was proven to be well fitted by internal and external validation. We selected HER2 negative patients as our target population for two reasons. First, the pCR rates of these patients were relatively low, so individualized therapy for each patient was required. Second, confounding variables such as HER2 blockade treatment were limited in our cohort. Additionally, we discovered that paclitaxel with carboplatin/paclitaxel with cisplatin was the more favored neoadjuvant chemotherapy regimen compared with cyclophosphamide, epirubicin and 5-fluorouracil in HER2 negative patients.
One limitation of our study was that the design was a single center analysis. Applying the nomogram in another database will greatly improve the power of our current result, and we have carefully searched through existing public databases. Unfortunately, we were unable to find a proper database containing all of the variables analyzed in our current study (age, menopause status, tumor size, nodal status, hormone receptor status, neoadjuvant chemotherapy regimens, neoadjuvant chemotherapy cycles and response to neoadjuvant chemotherapy). We expect to assess the nomogram with large-scale randomized prospective clinical trials. The efficacy and safety of the paclitaxel with carboplatin/paclitaxel with cisplatin regimen used in neoadjuvant chemotherapy also needs to be assessed. Another limitation was that the molecular mechanisms of the paclitaxel with carboplatin/paclitaxel with cisplatin regimen (more so than the cyclophosphamide, epirubicin and 5-fluorouracil regimen) were unclear so further research is required in the future to study these mechanisms.
Conclusion
Our current study screened for several predictors and constructed a well fitted nomogram based on those predictors to predict the pCR rate among HER2 negative breast cancer patients.
Abbreviations
AUC, the area under the ROC curve; CI, confidence interval; HER2, human epidermal growth factor receptor 2; pCR, pathological complete response; ROC, receiver operating characteristic
Acknowledgements
The authors are grateful to Jiong Wu, Guang-Yu Liu and Zhen-Zhou Shen for their excellent data management.
Funding
This work was supported by grants from the Research Project of Fudan University Shanghai Cancer Center (YJ201401); the National Natural Science Foundation of China (81572583, 81502278, 81372848, 81370075); the Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals (SHDC12010116); the Cooperation Project of Conquering Major Diseases in Shanghai Municipality Health System (2013ZYJB0302); the Innovation Team of the Ministry of Education (IRT1223); and the Shanghai Key Laboratory of Breast Cancer (12DZ2260100). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions
JX and YZJ contributed to the conception of the study, data analysis and interpretation, and writing the manuscript. JX, SW and MD helped in the nomogram construction. CS made tissue sections and participated in immunohistochemical analysis. YKD, DGH and SZM contributed to the collection and assembly of data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All the procedures followed were in accordance with the Helsinki Declaration (1964, amended in 1975, 1983, 1989, 1996 and 2000) of the World Medical Association. This study was approved by the Ethics Committee of Fudan University shanghai Cancer Center, and each participant signed an informed consent document.
Additional files
R running of Nomogram for predqicting pCR. (DOC 16 kb)
Patient baseline characteristics of different NCT regimens. (DOC 16 kb)
Univariate logistic regression analysis of different variables predicting pathological complete response (pCR) in hormone receptor (HR) positive and negative cohorts. CEF: cyclophosphamide, epirubicin and 5-fluorouracil; CI: Confidence interval; E + P: cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin; NE: navelbine and epirubicin; OR: odds ratios; PC: paclitaxel and carboplatin or paclitaxel and cisplatin. (DOC 1415 kb)
Pathological complete response (pCR) of different neoadjuvant chemotherapy (NCT) regimens in hormone receptor (HR) positive and negative cohorts. (DOC 152 kb)
Contributor Information
Xi Jin, Email: 13211230023@fudan.edu.cn.
Yi-Zhou Jiang, Phone: +86-64175590, Email: yizhoujiang@fudan.edu.cn.
Sheng Chen, Email: 656790628@qq.com.
Ke-Da Yu, Email: yukeda@163.com.
Ding Ma, Email: 369128696@qq.com.
Wei Sun, Email: wsun14@fudan.edu.cn.
Zhi-Min Shao, Email: zhimingshao@yahoo.com.
Gen-Hong Di, Phone: +86-64175590, Email: didy@medmail.com.cn.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Generali D, Ardine M, Strina C, Milani M, Cappelletti MR, Zanotti L, Forti M, Bedussi F, Martinotti M, Amoroso V, et al. Neoadjuvant treatment approach: the Rosetta stone for breast cancer? J Natl Cancer Inst Monogr. 2015;2015(51):32–5. doi: 10.1093/jncimonographs/lgv019. [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 4.Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med. 2015;66:31–48. doi: 10.1146/annurev-med-051413-024741. [DOI] [PubMed] [Google Scholar]
- 5.Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N, Vrancken Peeters MT. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–8. doi: 10.1245/s10434-010-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlan LC, Clegg LX, Abrams J, Stevens JL, Ballard-Barbash R. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987–2000. J Clin Oncol. 2006;24(6):872–7. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 7.Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381–7. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 8.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 10.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–9. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 12.Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23(33):8331–9. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- 13.Colleoni M, Bagnardi V, Rotmensz N, Viale G, Mastropasqua M, Veronesi P, Cardillo A, Torrisi R, Luini A, Goldhirsch A. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer. 2010;46(12):2216–24. doi: 10.1016/j.ejca.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Keam B, Im SA, Park S, Nam BH, Han SW, Oh DY, Kim JH, Lee SH, Han W, Kim DW, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011;137(9):1301–8. doi: 10.1007/s00432-011-0991-3. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer DW, Lemeshow S. Assessing the fit of the model. In: Applied logistic regression. Wiley; 2005: 143–202. doi: 10.1002/0471722146.
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81. doi: 10.1016/S0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 19.Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131(3):1061–6. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 20.Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11):1821–8. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Precht LM, Lowe KA, Atwood M, Beatty JD. Neoadjuvant chemotherapy of breast cancer: tumor markers as predictors of pathologic response, recurrence, and survival. Breast J. 2010;16(4):362–8. doi: 10.1111/j.1524-4741.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 22.Tan MC, Al Mushawah F, Gao F, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Predictors of complete pathological response after neoadjuvant systemic therapy for breast cancer. Am J Surg. 2009;198(4):520–5. doi: 10.1016/j.amjsurg.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouret-Reynier M-A, Abrial CJ, Ferrière J-P, Amat S, Curé HD, Kwiatkowski FG, Feillel VA, Lebouëdec G, Penault-Llorca FM, Chollet PJM. Neoadjuvant FEC 100 for operable breast cancer: eight-year experience at centre jean Perrin. Clin Breast Cancer. 2004;5(4):303–7. doi: 10.3816/CBC.2004.n.035. [DOI] [PubMed] [Google Scholar]
- 24.Buzdar AU. Preoperative chemotherapy treatment of breast cancer--a review. Cancer. 2007;110(11):2394–407. doi: 10.1002/cncr.23083. [DOI] [PubMed] [Google Scholar]
- 25.Jiang YZ, Yu KD, Peng WT, Di GH, Wu J, Liu GY, Shao ZM. Enriched variations in TEKT4 and breast cancer resistance to paclitaxel. Nat Commun. 2014;5:3802. doi: 10.1038/ncomms4802. [DOI] [PubMed] [Google Scholar]
- 26.Xiong X, Sui M, Fan W, Kraft AS. Cell-cycle dependent antagonistic interactions between paclitaxel and carboplatin in combination therapy. Cancer Biol Ther. 2014;6(7):1067–73. doi: 10.4161/cbt.6.7.4323. [DOI] [PubMed] [Google Scholar]
- 27.Pentheroudakis G, Razis E, Athanassiadis A, Pavlidis N, Fountzilas G. Paclitaxel-carboplatin combination chemotherapy in advanced breast cancer: accumulating evidence for synergy, efficacy, and safety. Med Oncol. 2006;23(2):147–60. doi: 10.1385/MO:23:2:147. [DOI] [PubMed] [Google Scholar]
- 28.Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu JS, Shao ZM, Shen ZZ, Shen KW. Weekly paclitaxel plus carboplatin is an effective nonanthracycline-containing regimen as neoadjuvant chemotherapy for breast cancer. Ann Oncol. 2010;21(5):961–7. doi: 10.1093/annonc/mdq041. [DOI] [PubMed] [Google Scholar]
- 29.Gogas H, Pectasides D, Kostopoulos I, Lianos E, Skarlos D, Papaxoinis G, Bobos M, Kalofonos HP, Petraki K, Pavlakis K, et al. Paclitaxel and carboplatin as neoadjuvant chemotherapy in patients with locally advanced breast cancer: a phase II trial of the Hellenic cooperative oncology group. Clin Breast Cancer. 2010;10(3):230–7. doi: 10.3816/CBC.2010.n.031. [DOI] [PubMed] [Google Scholar]
- 30.Hurley J, Reis IM, Rodgers SE, Gomez-Fernandez C, Wright J, Leone JP, Larrieu R, Pegram MD. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138(3):783–94. doi: 10.1007/s10549-013-2497-y. [DOI] [PubMed] [Google Scholar]
- 31.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.


