Abstract
Crizotinib is a multi-targeted tyrosine kinase inhibitor (TKI) with activity against mesenchymal-epithelial transition factor (MET) and anaplastic lymphoma kinase (ALK). However, the concomitant oncogenic drivers may affect the sensitivity of crizotinib. Herein, we present a 69-year-old never-smoker Chinese male with advanced lung adenocarcinoma harboring concomitant spectrin beta non-erythrocytic 1 (SPTBN1)-ALK fusion, c-Met overexpression, and human epidermal growth factor receptor-2 (HER-2) amplification with inherent resistance to crizotinib, chemotherapy, and radiotherapy. Although the patient received timely and comprehensive treatment, the overall survival was only 8 months. Therefore, c-Met overexpression, HER-2 gene amplification, and SPTBN1-ALK gene fusion can coexist in lung adenocarcinoma and may become a potential biomarker of cancer refractory to crizotinib, chemotherapy, and radiotherapy as well as of a relatively poor prognosis. In addition, the novel SPTBN1-ALK fusion gene may become a potential target for anti-tumor therapy.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-016-0296-8) contains supplementary material, which is available to authorized users.
Keywords: NSCLC, Lung adenocarcinoma, Oncogenic drivers, SPTBN1-ALK, c-Met, HER-2, Crizotinib, Chemotherapy, Radiotherapy
To the editor
Crizotinib is a multi-targeted tyrosine kinase inhibitor (TKI) with activity against mesenchymal-epithelial transition factor (MET) and anaplastic lymphoma kinase (ALK) [1]. Driver oncogenes are conventionally considered mutually exclusive [2]. Here, we describe a rare case of lung adenocarcinoma harboring concomitant spectrin beta non-erythrocytic 1 (SPTBN1)-ALK fusion, c-Met overexpression, and human epidermal growth factor receptor-2 (HER-2) amplification with inherent resistance to crizotinib, chemotherapy, and radiotherapy.
In July 2015, a 69-year-old never-smoker man, whose brother died of lung cancer, experiencing pain in his low back and left lower extremity, underwent a total-body positron emission tomography-computed tomography (PET/CT) scan which showed a lung tumor and bone metastases (Fig. 1d–f). Then, a CT-guided percutaneous lung biopsy was performed (Fig. 1a), and a diagnosis of advanced lung adenocarcinoma was made. Based on the immunohistochemistry analysis, the tumor cells were negative for ALK (Fig. 1c) but extremely positive for c-Met (Fig. 1b). In addition, epidermal growth factor receptor (EGFR) mutation and repressor of silencing 1 (ROS-1) fusion detections revealed negative results.
Fig. 1.
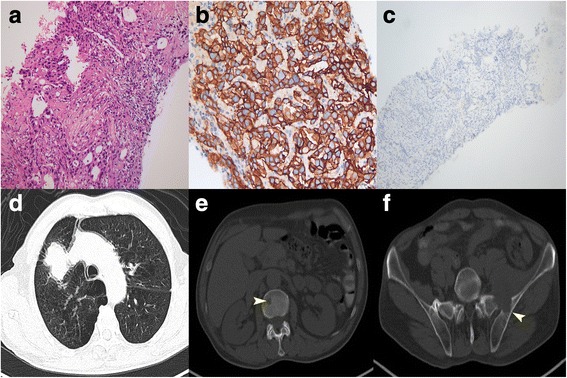
Lung biopsy specimens and total-body PET/CT scan before treatment. Hematoxylin and eosin (H & E) staining (magnification ×200, a), IHC of c-Met (magnification ×200, b), and ALK (magnification ×100, c) of a diagnostic biopsy specimen. Total-body PET/CT showed a 5.1 × 3.7 cm-sized tumor in the right upper lobe (d) with the second lumbar and left ilium metastases (arrowheads, e, f)
The patient initially received gemcitabine plus nedaplatin for one cycle in July 2015. Subsequent treatment included palliative radiation therapy to the left ilium. Additionally, the patient was treated with crizotinib based on c-Met overexpression. However, grade 3 thrombocytopenia occurred after chemotherapy, and he recovered with recombinant human thrombopoietin. Although the symptoms decreased, first restaging CT scans (September 2, 2015) showed marked worsening disease (Additional file 1: Figure S1a).
Then, the patient received two cycles of bevacizumab-based chemotherapy with pemetrexed, cisplatin, and bevacizumab from September to October in 2015. Additionally, he received local radiotherapy at the lumbar metastases. However, lumbar MRI and CT scan of the chest and abdomen (October 2015) showed progressive disease (Additional file 1: Figure S1b).
Given the patient’s reduced performance status, reduced paclitaxel liposome plus nedaplatin was administered (October 27, 2015). Subsequent treatments included iAPA DC-CIK and chest radiotherapy. However, the patient demonstrated evidence of progressive disease again in December 2015 (Additional file 1: Figure S1c). To re-evaluate the molecular characteristics, DNA extracted from the original biopsy was used for DNA sequencing with next-generation sequencing on December 2015. Interestingly, the patient had HER-2 amplification and a novel ALK rearrangement, namely SPTBN1-ALK fusion, which was created by an insertion between two breakpoints in exons 1 to 6 of the SPTBN1 gene and exons 20 to 29 of the ALK gene (Fig. 2). Given the patient’s performance interfered with starting chemotherapy, he was treated with whole-brain irradiation therapy. However, the patient’s performance status continuously deteriorated. On January 4, 2016, the patient died of brain and lung metastases. The patient’s overall survival was only 8 months.
Fig. 2.
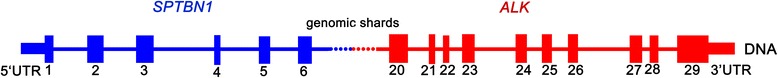
Hypothetical structure of SPTBN1-ALK fusion gene. SPTBN1-ALK fusion gene was formed by placing SPTBN1 exons 1–6 upstream of ALK exons 20–29, which were separated by small genomic shards
The SPTBN1-ALK fusion gene was first identified in colorectal cancer, which was formed by the fusion of exon 7 of the SPTBN1 gene with exon 20 of the ALK gene [3]. In this case, we first identified a novel SPTBN1-ALK fusion in lung cancer. The frequency of c-Met overexpression is 31.9 % in NSCLC, and it potentially causes intrinsic resistance to EGFR-TKIs without causing resistance to crizotinib [4]. Nonetheless, the present patient gained inherent crizotinib resistance despite harboring both c-Met overexpression and the SPTBN1-ALK fusion gene. HER-2 is noted in 10 to 20 % of NSCLC patients [5] and confers relative resistance to conventional chemotherapy [6]. The novel ALK rearrangement and interactive crosstalk between Met and HER2 may have been responsible for the failed response to crizotinib treatment. In this context, inhibition of ALK, Met, and Her-2 was required for efficient inhibition of tumor growth.
To the best of our knowledge, this is the first case of lung cancer with a novel SPTBN1-ALK fusion gene, which may become a potential target for anti-tumor therapy. This interesting case demonstrates that c-Met overexpression, HER-2 gene amplification, and SPTBN1-ALK gene fusion can coexist in lung adenocarcinoma, and their combination might be a biomarker for resistance to crizotinib, traditional chemotherapy, and radiotherapy as well as for a relatively poor prognosis. Further evidence is required to validate these preliminary data.
Abbreviations
ALK, anaplastic lymphoma kinase; CIK, cytokine-induced killers; DC, dendritic cell; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor-2; iAPA, inhibition of antigen presentation attenuators; MET, mesenchymal-epithelial transition factor; MRI, magnetic resonance imaging; NSCLC, non-small-cell lung cancer; PET/CT, positron emission tomography-computed tomography; ROS1, repressor of silencing 1; SPTBN1, spectrin beta non-erythrocytic 1; TKI, tyrosine kinase inhibitor
Acknowledgements
Not applicable.
Funding
This work was supported by the grant from National Natural Science Foundations of China (NO.81372260).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
LL and JSY contributed to the design of the study. YYL and JYL acquired the data. FT and XHH conducted the data analyses and interpretation. FFG and YZ were in charge of manuscript writing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The authors did not obtain written consent of the patient to describe his illness and publish this case report because the patient died before we started work on the case study. However, we did not use patient data that would allow identifying him.
Ethics approval and consent to participate
Ethics committee approval is not included as it is commonly accepted that case reports do not require such approval. In our work, we did not use patient data that would allow identifying him. Patient agreed to all above diagnostic tests and treatment that was used.
Additional file
Multiple metastases after three treatment approaches (a) CT scans of the chest and abdomen showed mediastinal lymph nodes metastases (left, arrowhead), a soft tissue tumor located at the axillary segment of the right sixth rib with bone destruction (middle, arrowhead) and left adrenal metastasis (right, arrowhead) following the first-line treatment. (b) After the second-line treatment, CT scans of the chest and abdomen showed a soft tissue tumor at the right scapula with bone destruction (left, arrowhead) and bilateral adrenal metastases (right, arrowheads). Lumbar MRI showed the third lumbar metastasis (middle, arrowhead). (c) After the third-line treatment, chest CT scans showed multiple bilateral pulmonary nodules and axillary lymph nodes metastases (upper, arrowheads) and brain MRI showed multiple brain metastases (lower, arrowheads). (TIF 13959 kb)
Footnotes
Fei-fei Gu and Yong Zhang are co-first authors.
References
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gainor JF, Varghese AM, Ou SHI, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ying J, Lin C, Wu J, et al. Anaplastic lymphoma kinase rearrangement in digestive tract cancer: implication for targeted therapy in Chinese population. PLoS One. 2015;10:e0144731. doi: 10.1371/journal.pone.0144731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou NN, Yang JJ, Zhang XC, et al. Response to tyrosine kinase inhibitors in non-small-cell lung cancer with concomitant c-MET overexpression and driver genes. J Clin Oncol. 2015;33(suppl; abstr 8089):15s. [Google Scholar]
- 5.Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 6.Kuyama S, Hotta K, Tabata M, et al. Impact of HER2 gene and protein status on the treatment outcome of cisplatin-based chemoradiotherapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:477–482. doi: 10.1097/JTO.0b013e31816e2ea3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


