Abstract
Background
Air pollution can cause respiratory symptoms or exacerbate pre-existing respiratory diseases, especially in children. This study looked at the short-term association of air pollution concentrations with Emergency Room (ER) admissions for respiratory reasons in pediatric age (0–18 years).
Methods
Daily number of ER admissions in a children’s Hospital, concentrations of urban-background PM2.5, NO2, O3 and total aeroallergens (Corylaceae, Cupressaceae, Gramineae, Urticaceae, Ambrosia, Betula) were collected in Turin, northwestern Italy, for the period 1/08/2008 to 31/12/2010 (883 days). The associations between exposures and ER admissions were estimated, at time lags between 0 and 5 days, using generalized linear Poisson regression models, adjusted for non-meteorological potential confounders.
Results
In the study period, 21,793 ER admissions were observed, mainly (81 %) for upper respiratory tract infections. Median air pollution concentrations were 22.0, 42.5, 34.1 μg/m3 for urban-background PM2.5, NO2, and O3, respectively, and 2.9 grains/m3 for aeroallergens. We found that ER admissions increased by 1.3 % (95 % CI: 0.3-2.2 %) five days after a 10 μg/m3 increase in NO2, and by 0.7 % (95 % CI: 0.1-1.2 %) one day after a 10 grains/m3 increase in aeroallergens, while they were not associated with PM2.5 concentrations. ER admissions were negatively associated with O3 and aeroallergen concentrations at some time lags, but these association shifted to the null when meteorological confounders were adjusted for in the models.
Conclusions
Overall, these findings confirm adverse short-term health effects of air pollution on the risk of ER admission in children and encourage a careful management of the urban environment to health protection.
Electronic supplementary material
The online version of this article (doi:10.1186/s12889-016-3376-3) contains supplementary material, which is available to authorized users.
Keywords: Airborne pollutants, Pollens, Time-series analysis, Pediatric emergency room, Short-term respiratory effects
Background
Over the last decades, the prevalence of respiratory diseases, and in particular of asthma and allergies, has increased considerably, especially in industrialized countries [1, 2]. The etiology of respiratory diseases is multifactorial and includes, among others, interactions between genetic predisposition and environmental factors [3]. The environmental dynamics, characterized by climate change, qualitative and quantitative aspects of chemical air pollution and airborne pollens, may partially explain the increased incidence of respiratory symptoms and respiratory diseases during the last years [4]. The short-term respiratory effects of air pollution include decreases in pulmonary function [5], increases in inflammatory biomarkers [6] and respiratory symptoms [7, 8], exacerbations of chronic obstructive pulmonary disease (COPD), infections [9, 10], school absenteeism [11] and respiratory mortality [12, 13].
The respiratory system is a primary target of air pollution. In children, the small airway caliber allows for a higher chance of being affected by inflammation resulting from air pollution [14, 15]. Due to their respiratory rates, children breathe a proportionately greater volume of air than adults and their oxygen demand is significantly higher, as well as their respiration rates. Young people also spend more time engaged in intense activities than adults, often outdoors and during midday when air pollution levels tend to be higher. As a result, children inhale more pollutants per kilogram of body weight. Irritation caused by air pollutants that would produce only a slight response in an adult can result in potentially significant obstruction in the airways of a young child [16].
The environmental risk factors that may have an impact on children’s respiratory health, especially in urban areas, include chemical outdoor pollution, aeroallergens, indoor air pollution including environmental tobacco smoke, microorganisms such as virus and bacteria that infect the airways. The latter can exacerbate or re-exacerbate their manifestations in presence of other risk factors.
Several epidemiological studies have documented a positive association between exposure to particulate air pollution and respiratory symptoms of cough and wheeze, especially among children [17, 18]. In this regard, the findings from two Swiss studies showed that the reduction of exposure to particulate matter (PM) <10 μm in aerodynamic diameter (PM10) contributes to improved respiratory health, observed through fewer cases of chronic cough in children [19] and through fewer cough, wheezing and breathlessness in adults [20]. Exposure to ozone (O3) at environmental concentrations is associated with lung function decrease and respiratory symptoms including cough, shortness of breath and pain on deep inspiration [21]. Nitrogen dioxide (NO2) concentrations have also been associated with cough, wheeze and breath shortness in children. Residential traffic-related air pollution exposure is associated with reduced expiratory flows in schoolchildren [7, 22]. Variations in lung function that mirror changes in PM exposure have been reported in children who move to areas with different air pollution levels [23].
Pollen is a well know trigger of allergies and asthma aggravation, and actually has a changing profile; in fact new pollen types have emerged following the cultivation and spread of exotic ornamental plants in public and private places [24]. Moreover, global climate change has been linked to an earlier onset and an extended duration of the pollens season, to an increase in pollen production, and a stronger allergenicity for some pollen types [25]. Thunderstorm asthma epidemics may be triggered by pollen grains rupture in the atmosphere and the entrapment of respirable-size particles in the outflows of air masses at ground level [24, 25]. Increasing pollution is responsible for an increase in pollen-induced respiratory allergy, including asthma, because of airway inflammatory reaction and the passage of pollen grains into the lower respiratory tract [24].
The aim of this study was to analyze the short-term relationships between hospital emergency room (ER) admissions for respiratory diseases in children and concentrations of NO2, PM2.5, O3, and aeroallergens, in Turin, Italy, between 2008 and 2010.
Methods
Turin, the capital of Piedmont region (North-Western Italy) has 900,000 inhabitants, is located at 200 m above sea level and it is one of the most polluted Italian cities [26–29] Additional file 1: Figure S1. Daily data for the period 01/08/2008 to 31/12/2010 (883 days) for the city of Turin were collected or derived as described below. The locations of the data sources are shown in Additional file 1: Figure S1.
ER admissions for respiratory diseases
Daily data on ER admissions (date of admission, primary diagnosis and diagnostic code) for 19 respiratory diseases to “Regina Margherita” Pediatric Hospital of Turin were collected (age range 0–18 years). Diagnoses were coded according to the International Classification of Disease (ICD) 9th edition (Table 1).
Table 1.
Distribution of daily ER admissions, for the diagnoses of the respiratory diseases considered in the analysis, during the study period (883 days)
| Respiratory disease diagnoses | total n. of admissions | daily n. of admissions | ||||
|---|---|---|---|---|---|---|
| Group | Description | ICD-IX-CM code | count | mean ± SD | median | min-max |
| Upper respiratory tract infections | Acute rhino pharyngitis | 460 | 17684 | 18.1 ± 9.0 | 17 | 0-45 |
| Acute pharyngitis | 462 | |||||
| Acute tonsillitis | 463 | |||||
| Acute laryngitis without obstruction | 46400 | |||||
| Acute laryngitis with obstruction | 46401 | |||||
| Acute upper respiratory infections | 4658 | |||||
| Lower respiratory tract infections | Acute bronchitis | 4660 | 1989 | 3.0 ± 3.2 | 2 | 0-20 |
| Acute bronchiolitis other infectious agents | 46619 | |||||
| Flu with respiratory manifestations | 4871 | |||||
| Bronchitis | 490 | |||||
| Deep lung infections | Viral pneumonia | 4809 | 839 | 2.1 ± 2.1 | 2 | 0-8 |
| Bacterial pneumonia | 4829 | |||||
| Bronchopneumonia | 485 | |||||
| Pneumonia | 486 | |||||
| Asthma | Asthma, without status asthmatics | 49390 | 1281 | 1.5 ± 1.6 | 1 | 0-7 |
| Asthma, with status asthmatics | 49391 | |||||
| Total | 21793 | 24.7 ± 11.7 | 23 | 0-80 | ||
International classification of diseases, 9th edition, clinical modification (ICD-IX-CM) codes
Meteorological data
Meteorological data derived from the station placed on the roof of the Department of Physics of the University of Turin, located at about 1 km from the city center. The station is permanently active since 1989 in order to collect and display in real time the weather data in the urban surface layer of the city. The station is equipped with the instruments reported in Additional file 1: Table S1. Data are collected every 5 s by the acquisition system, and subsequently averaged every 5 min and stored in an electronic archive. Data acquired in this way were aggregated in a daily form for the subsequent analysis.
Chemical air pollution data
Daily concentrations of NO2, PM2.5 and O3 were derived from hourly data collected at the urban background monitoring station “Lingotto” located in Turin (viale Augusto Monti, 21) by the Local Environmental Protection Agency (ARPA Piemonte), coordinated by the regional air pollution service of Piedmont Region, according to the current European legislation (DIR 2008/50/ECX).
Aeroallergen data
Among the pollen taxa usually considered in aerobiological monitoring for being allergenic, Corylaceae, Cupressaceae, Gramineae, Urticaceae, Ambrosia, and Betula were quantified in this study. Daily data were derived from a station located 12 m above the ground, as required by the standard [30], on the flat roof of a building located in a semi-central area of the city of Turin. In this site, atmospheric circulation is local and not affected by surrounding obstacles such as walls or other types of protection. The station is equipped with a HIRST sampler, which consists of three main parts: a swivel head, a suction pump and a deposition drum (the sampling part), which rotates at 2 mm / h with 7 days of power reserve. Weekly, a specific adhesive tape is fixed on the drum. This tape captures the aeroallergens avoiding any loss for rebound or natural detachment. The air pump provides a constant airflow of 10 L / min inside the sampler, equivalent to 14.4 m3 each 24 h. Daily aeroallergen counts were carried out at the Department of Life Science and System Biology, University of Turin, and expressed as concentrations (grains/m3). For the statistical analysis, daily total aeroallergen concentrations were obtained as the sum of the concentrations of the single aeroallergen types.
Statistical Analysis
Quantitative variables were summarized with means ± SD, medians with interquartile ranges (IQR), and minimum and maximum values. The interquartile ratio (IQR/median ratio) was also computed in order to compare variability across different air pollutants. Linear correlations among exposure variables were evaluated using Pearson’s r coefficients.
The association between daily ER admission counts for all diagnoses in Table 1 combined (dependent variable) and air pollution exposure variables were analyzed using Generalized Linear Models (GLMs) fitting a non-stationary Poisson process [30, 31]. We used the following model:
Where λt denotes the count of daily ER admissions at day t, α is a constant, β is the vector of estimated parameters, Xi is the matrix of k independent variables (exposure and adjustment variables), and NS(Zt) is a natural spline smoothing function of calendar day Z with 14° of freedom (df), [31] which was included to take the medium/long term trend into account [30, 31]. The number of df of the smoothing function was chosen by minimizing the sum of the absolute values of the partial autocorrelation function (PACF) of the residuals [11, 30, 31]. Day of the week was included when estimating the smoothing function to remove the 7-day positive correlation across PACF residuals. To avoid overfitting, the maximum number of df allowed was 15, which corresponds to about 6 df per calendar year (60-day windows) [32].
The adjustment variables considered were: a) day of the week, b) influenza outbreaks, defined as days when influenza incidence was greater than 2‰ [33], which were computed by the Regional Reference Service of Epidemiology for the Surveillance, Prevention and Control of Infectious Diseases, ASL Alessandria, Italy Reference Service Regional Epidemiology and Infectious Disease (SeREMI), c) holidays (4-level variable coded as: Christmas and Easter; 3 days around Christmas and Easter; other holidays; other days), d) summer population decrease (from Saturday before Mid-August to the next Sunday for a total of 16 days/year; from 16 July to 31 August, except for the aforementioned period; all other days) [34], e) average daily temperature, f) average relative humidity, and g) cumulative daily precipitations. The following models were fit to the data:
-
A)
One exposure variable + medium/long trend function + non meteorological variables (day of the week, influenza outbreaks, holidays and summer population decrease) (single-pollutant models);
-
B)
Model A + meteorological variables (daily temperature, daily relative humidity, cumulative daily precipitations). Temperature and relative humidity were modeled using natural splines with 3 and 2° of freedom, respectively. The number of df was chosen using the PACF criterion as above. Daily precipitations were binary coded (present if ≥1 mm; absent otherwise);
-
C)
One chemical pollutant (PM2.5, NO2 or O3) + aeroallergens + medium/long trend function + non meteorological variables (two-pollutant models). To avoid multicollinearity, two-pollutant models only combined one chemical pollutant per time and aeroallergens, because correlations across chemical pollutants were very strong (Pearson’s coefficients of correlation in absolute value |r| >0.50).
Exposure variables were included in the models at single time lags, from the same day when ER admissions were evaluated (Lag 0) to 5 days before (Lag 5). Associations between exposure variables (10 μg/m3 increase in PM2.5, NO2, O3 concentrations; 10 grains/m3 increase in aeroallergen concentrations) and ER admissions were reported with rate ratios (RR) with 95 % confidence intervals (CI).
Results
In the study period, 21,793 pediatric ER admissions for respiratory diseases were observed, mainly (81 %) for infections of the upper airways (Table 1). Figure 1 shows average daily ER admissions by month of the year. The cold months showed the highest frequency of ER admissions, probably due to the more frequent outbreaks of colds. Table 2 shows a general description of the daily concentrations of airborne pollutants during the study period. NO2 concentration showed the highest mean absolute levels and, overall, the air pollution concentrations observed underline the poor air condition in Turin as compared to the rest of Europe [26, 35].
Fig. 1.
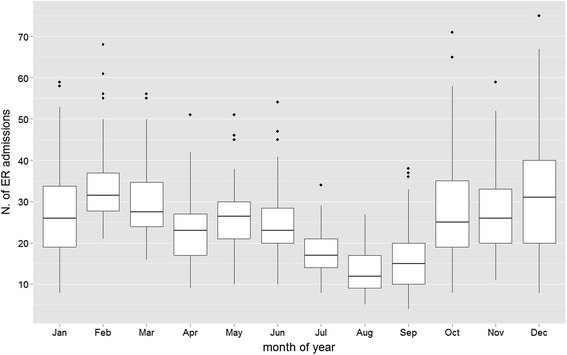
Monthly distribution of daily ER admissions for respiratory diseases during the study period
Table 2.
Distribution of daily concentrations of air pollution and aeroallergens at the Lingotto urban background monitoring station during the study period (883 days)
| Exposure variable | available data (days) | median (IQR) | interquartile ratio | min-max | mean ± SD | EU annual reference valueb |
|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 833 | 22.0 (30.0) | 1.4 | 4–157 | 32.0 ± 26.2 | 25 |
| NO2 (μg/m3) | 851 | 42.5 (32.0) | 0.8 | 7.4–192.9 | 48.3 ± 25.0 | 40 |
| O3 (μg/m3) | 858 | 34.1 (53.6) | 1.6 | 1.8–123.3 | 9.6 ± 29.1 | |
| aeroallergens (grains/m3)a | 826 | 2.9 (19.6) | 6.7 | 0–271.9 | 16.1 ± 29.6 |
aincludes Corylaceae, Cupressaceae, Gramineae, Urticaceae, Ambrosia, Betula
bEuropean Union (EU) Directive 2008/50/CE10 (http://ec.europa.eu/environment/air/quality/legislation/directive.htm)
Aeroallergen concentrations showed larger variability than chemical air pollution concentrations: the interquartile ratio for aeroallergens was 4 (O3) to 8-fold (NO2) the interquartile ratio of the chemical pollutants. Both PM2.5 and NO2 (Fig. 2a and b) showed a prevailing maximum level during the coldest months, which is a typical behavior of primary pollutants. An opposite trend was shown by O3, with higher concentrations in summertime (Fig. 2c). The concentrations of aeroallergens were high in the warm season, and virtually absent in winter (Fig. 2d).
Fig. 2.
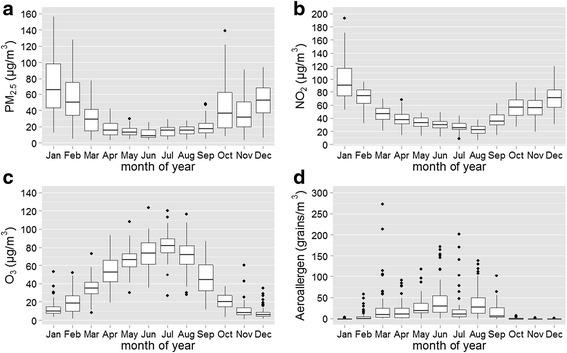
Monthly distribution of daily PM2.5, NO2, O3 and aeroallergen concentrations (panels a − d, respectively) during the study period
There was a strong positive linear correlation between PM2.5 and NO2 (r = 0.762, p < 0.001). The negative correlations of O3 with PM2.5 and NO2 were also strong (r = −0.591 and −0.695, respectively, all p < 0.001) (Fig. 3). The correlations between chemical pollutants and aeroallergens were weaker (|r| between 0.257 and 0.459).
Fig. 3.
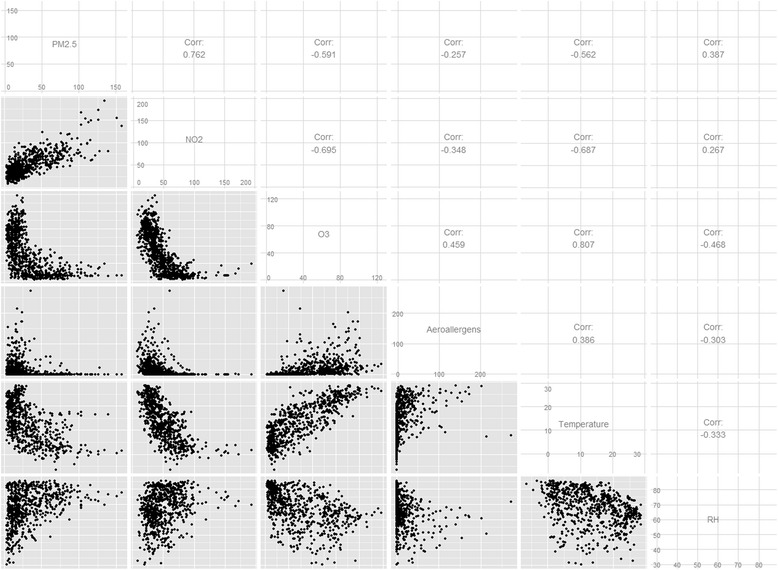
Pairwise distributions and Pearson’s r correlation coefficients of air pollutant concentrations and meteorological variables. All the hypothesis tests of no correlation were statistically significant (all p <0.001)
Table 3 shows the mean number of daily ER admissions, air pollution and aeroallergen concentrations according to the potential confounders considered. ER admissions were more frequent during weekends, holidays, and during influenza outbreaks than during the other days. They were also more likely in days with lower temperatures and more extreme (low and high) relative humidity levels. PM2.5 and NO2 were positively associated with temperature, whereas the opposite was true for O3 and aeroallergens. As expected, airborne pollution was lower during rainy days.
Table 3.
Distribution of the daily number of ER admissions for respiratory diseases and daily concentrations of chemical air pollution and aeroallergens during the study period, according to the potential confounders considered in the analysisa
| ER admissions (counts) | PM2.5 (μg/m3) | NO2 (μg/m3) | O3 (μg/m3) | Aeroallergens (grains/m3) | |
|---|---|---|---|---|---|
| Day of the week | |||||
| Monday | 22.7 ± 10.2 | 30.4 ± 26.5 | 47.2 ± 25.1 | 38.2 ± 27.7 | 12.7 ± 20.9 |
| Tuesday | 21.4 ± 9.8 | 30.8 ± 25.4 | 50.3 ± 24.5 | 37.8 ± 28.6 | 14.9 ± 28.8 |
| Wednesday | 20.4 ± 9.6 | 32.5 ± 25.4 | 51.0 ± 23.8 | 38.9 ± 29.2 | 18.8 ± 38.1 |
| Thursday | 21.6 ± 9.7 | 35.1 ± 27.8 | 52.9 ± 25.9 | 38.9 ± 30.2 | 15.1 ± 23.0 |
| Friday | 23.4 ± 11.1 | 33.3 ± 25.0 | 50.9 ± 24.7 | 39.9 ± 29.7 | 17.2 ± 32.1 |
| Saturday | 31.1 ± 12.4 | 31.5 ± 26.0 | 45.0 ± 25.1 | 42.2 ± 29.8 | 18.2 ± 33.4 |
| Sunday | 32.2 ± 12.8 | 30.4 ± 27.4 | 40.9 ± 24.0 | 41.3 ± 28.5 | 15.8 ± 27.4 |
| p | <0.001 | 0.79 | 0.002 | 0.87 | 0.73 |
| Holidays | |||||
| Christmas and Easter | 47.6 ± 17.5 | 25.4 ± 15.2 | 45.1 ± 27.5 | 32.0 ± 29.9 | 1.0 ± 1.9 |
| 3 days before/after Christmas and Easter | 34.9 ± 16.3 | 37.9 ± 21.7 | 58.6 ± 25.8 | 25.7 ± 23.7 | 6.4 ± 15.8 |
| Other holidays | 35.6 ± 15.6 | 37.4 ± 27.7 | 49.3 ± 22.7 | 32.2 ± 30.4 | 12.6 ± 1.9 |
| Other days | 23.8 ± 10.9 | 31.6 ± 26.4 | 48.0 ± 25.0 | 40.4 ± 29.1 | 16.6 ± 30.2 |
| p | <0.001 | 0.39 | 0.23 | 0.02 | 0.20 |
| Influenza outbreaks | |||||
| No | 19.9 ± 9.0 | 22.8 ± 21.0 | 36.8 ± 16.9 | 55.2 ± 27.4 | 23.8 ± 31.9 |
| Yes | 31.0 ± 12.0 | 43.9 ± 27.5 | 63.0 ± 25.8 | 19.9 ± 16.4 | 6.3 ± 23.0 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Summer population decrease | |||||
| 2 weeks around 15 August | 13.4 ± 4.71 | 15.8 ± 5.6 | 20.7 ± 6.3 | 66.1 ± 15.4 | 34.7 ± 28.5 |
| From 16/7 to 31/8 (except 2 weeks around 15 August) | 14.4 ± 5.8 | 14.5 ± 6.4 | 26.1 ± 8.5 | 78.0 ± 19.3 | 29.1 ± 31.6 |
| Other days | 26.4 ± 11.6 | 34.5 ± 27.1 | 52.5 ± 24.5 | 33.7 ± 26.3 | 13.4 ± 28.7 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Temperatureb (°C) | |||||
| −6.4, 6.4 | 29.1 ± 11.6 | 57.3 ± 28.3 | 78.9 ± 27.6 | 12.1 ± 8.8 | 0.8 ± 2.5 |
| 6.5, 12.8 | 28.1 ± 11.3 | 31.7 ± 21.5 | 51.7 ± 15.2 | 23.3 ± 17.3 | 10.1 ± 31.9 |
| 12.9, 20.3 | 24.8 ± 11.5 | 27.5 ± 23.9 | 43.4 ± 17.1 | 41.2 ± 20.2 | 14.9 ± 21.6 |
| 20.4, 28.5 | 19.5 ± 8.2 | 14.9 ± 6.8 | 29.4 ± 9.5 | 73.7 ± 18.4 | 32.5 ± 39.7 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cumulative precipitation | |||||
| <1 mm | 25.4 ± 11.4 | 35.5 ± 28.0 | 51.5 ± 26.2 | 38.0 ± 33.7 | 16.0 ± 29.9 |
| ≥1 mm | 25.2 ± 11.5 | 25.5 ± 19.2 | 45.0 ± 20.1 | 33.7 ± 26.0 | 9.5 ± 27.3 |
| p | 0.87 | <0.001 | 0.01 | 0.10 | 0.02 |
| Relative humidity b(%) | |||||
| 30.4, 60.4 | 26.4 ± 10.3 | 17.8 ± 13.1 | 41.6 ± 17.9 | 52.1 ± 24.6 | 25.1 ± 34.3 |
| 60.5, 68.6 | 23.1 ± 11.6 | 25.5 ± 18.9 | 43.2 ± 22.2 | 50.9 ± 31.3 | 21.8 ± 37.5 |
| 68.7, 76.4 | 25.3 ± 11.3 | 44.7 ± 30.5 | 56.9 ± 32.1 | 28.3 ± 23.0 | 9.6 ± 25.4 |
| 76.5, 85.5 | 26.7 ± 12.1 | 42.5 ± 28.8 | 58.4 ± 22.1 | 16.7 ± 15.9 | 1.9 ± 6.0 |
| p | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 |
amean ± SD reported; overall p-values were calculated using non-parametric Kruskall-Wallis tests, under the null hypothesis that the distribution of a variables is homogeneous among the strata of a potential confounder
b coded in groups according to the quartiles of their frequency distribution
The associations between exposure variables and ER admissions for respiratory diseases, adjusted for non-meteorological potential confounders (model A), is described in Fig. 4. There was no statistically significant association of PM2.5 and ER admissions at any time lag (Fig. 4a). Instead, an increase of 10 μg/m3 of NO2 concentrations (Fig. 4b) was associated with a significant 1.3 % (95 % CI: 0.3-2.2 %) increase of ER admissions after 5 days (lag 5). O3 concentrations were significantly negatively associated with ER admissions for respiratory diseases, starting from lag 4 (Fig. 4c). Finally, a 0.7 % (95 % CI: 0.1–1.2 %) increase of ER admissions was observed 1 day after (lag 1) an increase of 10 grains/m3 of aeroallergens (Fig. 4d). When meteorological variables were also included as adjustment variables in the analyses, the results were consistent, with the exception that the negative associations between O3 (lags 4–5) and aeroallergen (lag 4) concentrations and ER admissions shifted to the null (Fig. 5). Joint models including individual chemical air pollutants and aeroallergens confirmed the main models results completely (Additional file 1: Figure S2), suggesting that the observed associations of chemical pollutants and aeroallergens with ER admissions were independent.
Fig. 4.
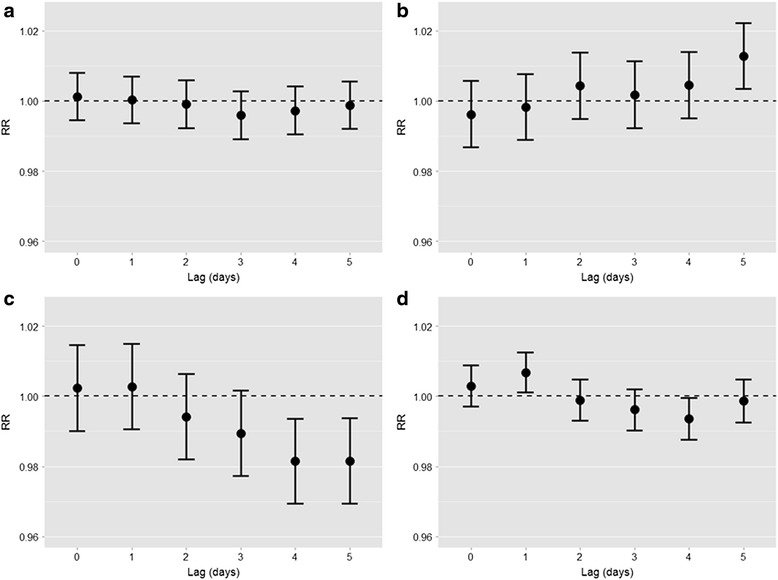
Estimates of association of daily PM2,5, NO2, O3, and aeroallergen concentrations (panels a − d, respectively) with ER admissions for respiratory diseases at different time lags, adjusted for medium/long-term trend function, day of the week, influenza outbreaks, holidays and summer population decrease.* *Single-pollutant models, see “model A” in the Statistical analysis section. Relative risks (RR) with 95%CIs are given for a 10 μg/m3 increase in PM2,5, NO2, O3 concentrations or a 10 grains/m3 increase in aeroallergen concentrations
Fig. 5.
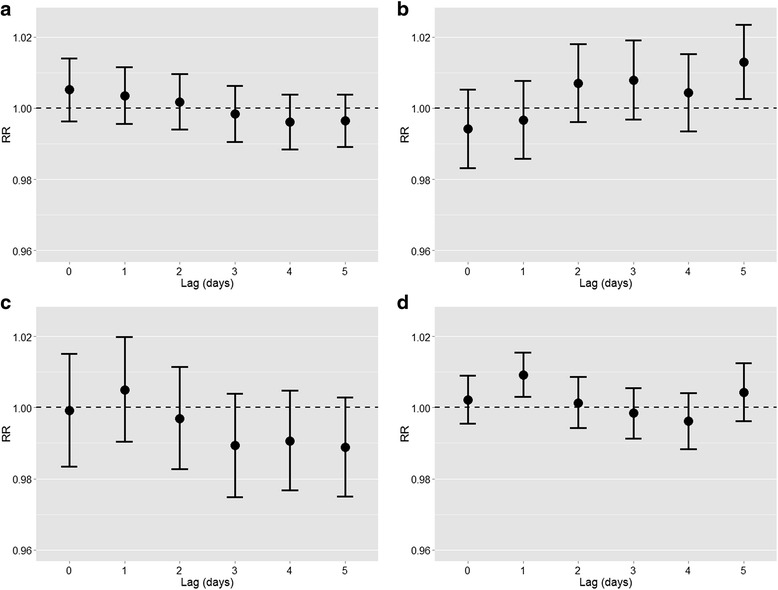
Estimates of association of daily PM2,5, NO2, O3, and aeroallergen concentrations (panels a − d, respectively) with ER admissions for respiratory diseases at different time lags, adjusted for medium/long-term trend function, day of the week, influenza outbreaks, holidays, summer population decrease, and for the meteorological variables (daily temperature, daily relative humidity, cumulative daily precipitations).* * Single-pollutant models, see “model B” in the Statistical analysis section of the article. Relative risks (RR) with 95%CIs are given for a 10 μg/m3 increase in PM2,5, NO2, O3 concentrations or a 10 grains/m3 increase in aeroallergen concentrations
Discussion and conclusion
The main purpose of this study was to analyze in the selected period the trend of ER admissions for respiratory reasons in a Children’s Hospital in Turin and the relationships of ER admissions with urban background chemical air pollutants and aeroallergens.
To achieve this objective, we considered the monitoring site of chemical air pollution where the data were the most complete for the study period and which was the closest to the pediatric emergency room (Additional file 1: Figure S1).
Among the 19 diseases diagnosed at the moment of the access to pediatric emergency room, as expected the upper respiratory tract infections was the most frequent reason for access to the emergency room. These infectious diseases of viral etiology are very common in pediatric age also because children generally attend kindergarten, nurseries and schools and they are therefore more exposed both to the etiological agents and to environmental risk factors such as poor air quality. Air pollution in fact, as well as tobacco smoke, can inhibit defensive mechanisms against oxidative stress [7, 15] and inflammation of the upper respiratory tract, which can favor the development of respiratory virus infections.
Despite the fact that the concentration of PM2.5 appears quite high when compared to European levels [29], this pollutant did not reveal any significant short-term association with ER pediatric admission, as documented by other authors [36]. Instead, NO2 showed a positive significant association with ER admissions, but only after 5 days (lag 5). Also Li et al. have shown a positive association between NO2 air pollution at lag 5 and ER admission in children of Detroit (MI, U.S.A.) in 2011 [37]. However, in contrast to what we observed, they have also shown a positive and significant association between PM2.5 concentrations and ER admissions. This does not seem to depend primarily on the average concentrations of PM2.5, which were much lower than in our study area, and it may be due to a different composition of particulate, perhaps more toxic in the City of Detroit than in Turin.
The adjusted estimates of relative risk for the effect of O3 were significantly less than one, seemingly suggesting a little protective effect. In 2009, Jerrett et al. also showed how relative risk for the effect of ozone on the risk of death from cardiovascular causes were significantly less than 1.0 [38].
Such beneficial influence of ozone, however, is currently completely to exclude from toxicological point of view. In experimental studies, O3 can increase airway inflammation [39] and can worsen pulmonary function and gas exchange [40]. In addition, exposure to elevated concentrations of tropospheric O3 has been associated with numerous adverse health effects, including the induction [26] and exacerbation [27, 28] of asthma, pulmonary dysfunction [33, 34] and hospitalization for respiratory reasons [31]. In our study, the apparent protective effect of O3 seems to be due to a confounding by meteorology, or to the fact that O3 acts as a mediator of the effect of temperature. In fact, when temperature, relative humidity and precipitations were included in the models as adjustment factors, the associations between O3 and ER admissions shifted to the null. Measurements of PM2.5 and NOx obtained using background monitoring stations are probably more representative of population’s exposure than measurements of O3. In fact, O3 concentrations tend to vary within cities more than PM2.5, because of the scavenging of O3 by NO near roadways and principally, for its photochemical origin [37]. Thus, in the presence of a high density of local traffic, the measurement error is probably higher for exposure to O3 than for exposure to PM2.5. The effects of O3 could therefore be confounded by the presence of PM2.5 because of collinearity between the measurements of the two pollutants and the higher precision of measurements of PM2.5 [38].
Finally, a 0.7 % (95 % CI: 0.1–1.2 %) increase of ER admissions for every 10 grains/m3 increase of aeroallergens was observed at lag 1. This indicates a lower latency between the stimulus and the effect, compared to chemical pollutants. An apparent protective effect of aeroallergens at lag 4 shifted to the null when the models included meteorological adjustment variables. Adverse short-term effects of aeroallergens are supported by other studies [41, 42], although the time lags when excesses of ER admissions are observed vary according to a number of reasons, including differences in study populations, air pollutant mixtures, as well as exposure assessment and statistical methodologies applied.
A limitation in our study is that we used only one monitoring site to estimate air pollution concentrations. However, both chemical pollution and aeroallergen monitoring stations were located close to the children’s hospital. It is likely that children are referred to the closest hospital, especially in the case of acute health events that are captured by ER admissions, and we can therefore hypothesize that children lived at relative close distance to the monitoring area. Any measurement error in exposures due to spatial heterogeneity in airborne air pollution concentrations is more likely to bias risk estimates toward the null than in the opposite direction [43].
In conclusion, we observed consistent and positive associations of background NO2 and aeroallergen concentrations with ER admissions in children in a populated and heavily polluted city in western Italy. Our findings add to the existing evidence and call for urgent public health policies especially in the Po valley in northern Italy, one of the most polluted areas in Europe because of high emissions but also poor ventilation and precipitation especially in winter. Moreover, replacement of non-allergenic cultivated plant species and their management (for example frequent grassland mowing which limit the production of flowers and consequently of pollens) can reduce the concentrations of allergenic pollens in the air [44]. Air pollution reduction policies are also recommended in the protection and promotion of public health, especially in children.
Abbreviations
COPD, chronic obstructive pulmonary disease; ER: emergency room: GAMs, generalized additive models; ICD, international classification of diseases; IQR: interquartile ranges, PACF partial autocorrelation function of the residuals; PM, particulate matter
Acknowledgments
The authors are grateful to all the Institutions that have contributed to the construction of the database. In particular: the Pediatrics Emergency of Regina Margherita Children’s Hospital, Turin, Italy; the Agency of Environmental Protection of Piedmont (A.R.P.A. Piemonte), 5 T S.r.L., Botanical Garden of University of Torino, Italy.
Authors’ contributions
RB has conceived, designed, and coordinated the study, performed the analysis and wrote the manuscript; VR and VB have made substantial contributions to acquisition of data and to their analysis and interpretation; RT and GT have made substantial intellectual contribution and have contributed to the design of the study and to organization and quality control of database; AU, CC, and CS have made substantial intellectual contribution in the execution of the work and have collected and processed data related to their profession (admission data, aeroallergens, and meteorological data); PM and AM have contributed to the design of the study, revised the manuscript, performed statistical analysis and contributed to the interpretation and discussion of data.
All authors have made substantive intellectual contributions in the study, read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional file
Characteristics of the instruments of the meteorological station. Figure S1. Map of the city of Turin (grey line). The dots indicate the locations of the “Regina Margherita” Children’s Hospital (red), the aeroallergen sampling station (green), and the chemical air pollution monitoring and weather station (blue) (© 2015 Google maps). Figure S2. Estimates of the association of daily PM2,5 + aeroallergens (panel A), NO2 + aeroallergens (panel B), O3 + aeroallergens (panel C) with ER admissions for respiratory diseases at different time lags, adjusted for medium/long trend function, day of the week, influenza outbreaks, holidays and summer population decrease.* (DOCX 931 kb)
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.de Marco R, Cappa V, Accordini S, Rava M, Antonicelli L, Bortolami O, Braggion M, Bugiani M, Casali L, Cazzoletti L, Cerveri I, Fois AG, Girardi P, Locatelli F, Marcon A, Marinoni A, Panico MG, Pirina P, Villani S, Zanolin ME, Verlato G, GEIRD Study Group Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet Lond Engl. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh BT, Tual S, Turbelin C, Pelat C, Cecchi L, D’Amato G, Blanchon T, Annesi-Maesano I. Short-term effects of airborne pollens on asthma attacks as seen by general practitioners in the Greater Paris area, 2003–2007. Prim Care Respir J J Gen Pract Airw Group. 2010;19:254–259. doi: 10.4104/pcrj.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G, Ostro BD. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health Glob Access Sci Source. 2006;5:11. doi: 10.1186/1476-069X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163:849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- 7.Bono R, Tassinari R, Bellisario V, Gilli G, Pazzi M, Pirro V, Mengozzi G, Bugiani M, Piccioni P. Urban air and tobacco smoke as conditions that increase the risk of oxidative stress and respiratory response in youth. Environ Res. 2015;137C:141–146. doi: 10.1016/j.envres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Delfino RJ, Gong H, Linn WS, Pellizzari ED, Hu Y. Asthma symptoms in Hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ Health Perspect. 2003;111:647–656. doi: 10.1289/ehp.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz J. Air pollution and hospital admissions for respiratory disease. Epidemiol Camb Mass. 1996;7:20–28. doi: 10.1097/00001648-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Marcon A, Pesce G, Girardi P, Marchetti P, Blengio G, de Zolt SS, Falcone S, Frapporti G, Predicatori F, de Marco R. Association between PM10 concentrations and school absences in proximity of a cement plant in northern Italy. Int J Hyg Environ Health. 2014;217:386–391. doi: 10.1016/j.ijheh.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised Analyses of the National Morbidity, Mortality, and Air Pollution Study: Mortality Among Residents Of 90 Cities. J Toxicol Environ Health A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- 13.Ostro B, Feng W-Y, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in california: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babin SM, Burkom HS, Holtry RS, Tabernero NR, Stokes LD, Davies-Cole JO, DeHaan K, Lee DH. Pediatric patient asthma-related emergency department visits and admissions in Washington, DC, from 2001–2004, and associations with air quality, socio-economic status and age group. Environ Health Glob Access Sci Source. 2007;6:9. doi: 10.1186/1476-069X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang I-J, Tung T-H, Tang C-S, Zhao Z-H. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. 2016;219:66–71. doi: 10.1016/j.ijheh.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Bono R, Bellisario V, Romanazzi V, Pirro V, Piccioni P, Pazzi M, Bugiani M, Vincenti M. Oxidative stress in adolescent passive smokers living in urban and rural environments. Int J Hyg Environ Health. 2014;217:287–293. doi: 10.1016/j.ijheh.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Pierse N, Rushton L, Harris RS, Kuehni CE, Silverman M, Grigg J. Locally generated particulate pollution and respiratory symptoms in young children. Thorax. 2006;61:216–220. doi: 10.1136/thx.2004.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z, Chapman RS, Hu W, Wei F, Korn LR, Zhang JJ. Using air pollution based community clusters to explore air pollution health effects in children. Environ Int. 2004;30:611–620. doi: 10.1016/j.envint.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Bayer-Oglesby L, Grize L, Gassner M, Takken-Sahli K, Sennhauser FH, Neu U, Schindler C, Braun-Fahrländer C. Decline of ambient air pollution levels and improved respiratory health in Swiss children. Environ Health Perspect. 2005;113:1632–1637. doi: 10.1289/ehp.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler C, Keidel D, Gerbase MW, Zemp E, Bettschart R, Brändli O, Brutsche MH, Burdet L, Karrer W, Knöpfli B, Pons M, Rapp R, Bayer-Oglesby L, Künzli N, Schwartz J, Liu L-JS, Ackermann-Liebrich U, Rochat T, SAPALDIA Team Improvements in PM10 exposure and reduced rates of respiratory symptoms in a cohort of Swiss adults (SAPALDIA) Am J Respir Crit Care Med. 2009;179:579–587. doi: 10.1164/rccm.200803-388OC. [DOI] [PubMed] [Google Scholar]
- 21.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H, Thomas DC. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159:768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 22.Rosenlund M, Forastiere F, Porta D, De Sario M, Badaloni C, Perucci CA. Traffic-related air pollution in relation to respiratory symptoms, allergic sensitisation and lung function in schoolchildren. Thorax. 2009;64:573–580. doi: 10.1136/thx.2007.094953. [DOI] [PubMed] [Google Scholar]
- 23.Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 24.D’Amato G, Cecchi L. Effects of climate change on environmental factors in respiratory allergic diseases. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2008;38:1264–1274. doi: 10.1111/j.1365-2222.2008.03033.x. [DOI] [PubMed] [Google Scholar]
- 25.D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 26.Bono R, Degan R, Pazzi M, Romanazzi V, Rovere R. Benzene and formaldehyde in air of two Winter Olympic venues of “Torino 2006.”. Environ Int. 2010;36:269–275. doi: 10.1016/j.envint.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Bono R, Bugliosi EH, Schiliro T, Gilli G. The Lagrange Street story: the prevention of aromatics air pollution during the last nine years in a European city. Atmos Environ. 2001;35:107–113. doi: 10.1016/S1352-2310(01)00085-1. [DOI] [Google Scholar]
- 28.Gilli G, Scursatone E, Bono R. Geographical distribution of benzene in air in northwestern Italy and personal exposure. Environ Health Perspect. 1996;104(Suppl 6):1137–1140. doi: 10.1289/ehp.961041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazenkamp-Von Arx ME, Gotschi T, Ackermann-Liebrich U, Bono R, Burney P, Cyrys J, Jarvis D, Lillienberg L, Luczynska C, Maldonado JA, Jaen A, de Marco R, Mi YH, Modig L, Bayer-Oglesby L, Payo F, Soon A, Sunyer J, Villani S, Weyler J, Kunzli N. PM2.5 and NO2 assessment in 21 European study centres of ECRHS II: annual means and seasonal differences. London: Research Portal, King’s College; 2004. [Google Scholar]
- 30.Mandrioli, P. The Italian Aeroallergen Network: report 1990. Aerobiologia, 1990;6(2/1):2–59.
- 31.Katsouyanni K, Samet JM, Anderson HR, Atkinson R, Le Tertre A, Medina S, Samoli E, Touloumi G, Burnett RT, Krewski D, Ramsay T, Dominici F, Peng RD, Schwartz J, Zanobetti A, HEI Health Review Committee Air pollution and health: a European and North American approach (APHENA) Res Rep Health Eff Inst. 2009;142:5–90. [PubMed] [Google Scholar]
- 32.Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. J Royal Stat Soc Series A Stat Soc 2006. 2006;169:179–203. doi: 10.1111/j.1467-985X.2006.00410.x. [DOI] [Google Scholar]
- 33.Biggeri A, Baccini M, Bellini P, Terracini B. Meta-analysis of the Italian studies of short-term effects of air pollution (MISA), 1990–1999. Int J Occup Environ Health. 2005;11:107–122. doi: 10.1179/oeh.2005.11.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Stafoggia M, Colais P, Serinelli M, Gruppo collaborativo EpiAir [Methods of statistical analysis to evaluate the short term effects of air pollution in the EpiAir Project] Epidemiol Prev. 2009;33(6 Suppl 1):53–63. [PubMed] [Google Scholar]
- 35.Traversi D, Degan R, De Marco R, Gilli G, Pignata C, Ponzio M, Rava M, Sessarego F, Villani S, Bono R. Mutagenic properties of PM2.5 air pollution in the Padana Plain (Italy) before and in the course of XX Winter Olympic Games of “Torino 2006.”. Environ Int. 2008;34:966–970. doi: 10.1016/j.envint.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Linares C, Diaz J. Short-term effect of PM(2.5) on daily hospital admissions in Madrid (2003–2005) Int J Environ Health Res. 2010;20:129–140. doi: 10.1080/09603120903456810. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Batterman S, Wasilevich E, Wahl R, Wirth J, Su F-C, Mukherjee B. Association of daily asthma emergency department visits and hospital admissions with ambient air pollutants among the pediatric Medicaid population in Detroit: time-series and time-stratified case-crossover analyses with threshold effects. Environ Res. 2011;111:1137–1147. doi: 10.1016/j.envres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Jerrett M, Burnett RT, Pope CA, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Emberlin J, Strachan DP. Air pollution, pollens, and daily admissions for asthma in London 1987–92. Thorax. 1998;53:842–848. doi: 10.1136/thx.53.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galán I, Tobías A, Banegas JR, Aránguez E. Short-term effects of air pollution on daily asthma emergency room admissions. Eur Respir J. 2003;22:802–808. doi: 10.1183/09031936.03.00013003. [DOI] [PubMed] [Google Scholar]
- 41.Gleason JA, Bielory L, Fagliano JA. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ Res. 2014;132:421–429. doi: 10.1016/j.envres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Allergenicity of the ornamental urban flora: ecological and aerobiological analyses in Cordoba (Spain) and Ascoli Piceno (Italy) [http://www.uco.es/investiga/grupos/rea/publicaciones/andalucia/cordoba/Staffolani_2011.pdf]
- 43.Darrow LA, Hess J, Rogers CA, Tolbert PE, Klein M, Sarnat SE. Ambient pollen concentrations and emergency department visits for asthma and wheeze. J Allergy Clin Immunol. 2012;130:630–638. doi: 10.1016/j.jaci.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aerobiological and ecological study of the potentially allergenic ornamental plants in south Spain - Springer [http://link.springer.com/article/10.1007/s10453-013-9311-5#/page-1]


