Abstract
Purpose
Diabetic retinopathy is one of the most common consequences of diabetes that affects millions of working-age adults worldwide and leads to progressive degeneration of the retina, visual loss, and blindness. Diabetes is associated with circadian disruption of the central and peripheral circadian clocks, but the mechanisms responsible for such alterations are unknown. Using a streptozotocin (STZ)-induced model of diabetes, we investigated whether diabetes alters 1) the circadian regulation of clock genes in the retina and in the central clocks, 2) the light response of clock genes in the retina, and/or 3) light-driven retinal dopamine (DA), a major output marker of the retinal clock.
Methods
To quantify circadian expression of clock and clock-controlled genes, retinas and suprachiasmatic nucleus (SCN) from the same animals were collected every 4 h in circadian conditions, 12 weeks post-diabetes. Induction of Per1, Per2, and c-fos mRNAs was quantified in the retina after the administration of a pulse of monochromatic light (480 nm, 1.17×1014 photons/cm2/s, 15 min) at circadian time 16. Gene expression was assessed with real-time reverse transcription PCR (RT–PCR). Pooled retinas from the control and STZ-diabetic mice were collected 2 h after light ON and light OFF (Zeitgeber time (ZT)2 and ZT14), and DA and its metabolite were analyzed with high-performance liquid chromatography (HPLC).
Results
We found variable effects of diabetes on the expression of clock genes in the retina and only slight differences in phase and/or amplitude in the SCN. c-fos and Per1 induction by a 480 nm light pulse was abolished in diabetic animals at 12 weeks post-induction of diabetes in comparison with the control mice, suggesting a deficit in light-induced neuronal activation of the retinal clock. Finally, we quantified a 56% reduction in the total number of tyrosine hydroxylase (TH) immunopositive cells, associated with a decrease in DA levels during the subjective day (ZT2).
Conclusions
These findings demonstrate that diabetes affects the molecular machinery and the light response of the retinal clock and alters the light-driven retinal DA level.
Introduction
Daily rhythms in behavior and physiology are regulated by the circadian system, a hierarchical group of biologic clocks widely distributed in mammalian tissues. The central clock of the circadian system, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, is synchronized by the day/night cycle and coordinates the phases of many peripheral clocks [1]. Photic entrainment of the SCN clock involves rods, cones, and melanopsin-containing light-sensitive retinal ganglion cells, or intrinsically photosensitive retinal ganglion cells (ipRGCs) [2-6]. The mammalian retina also contains a light synchronized circadian clock that controls many aspects of retinal physiology, including photoreceptor disc shedding [7], melatonin release [8-10], and dopamine synthesis [11,12].
The cell autonomous oscillations in both central and peripheral clocks involve autoregulatory positive and negative transcriptional and translational feedback loops that are composed of a set of clock genes including brain and muscle aryl-hydrocarbon receptor nuclear translocator-like protein-1 (Bmal1), circadian locomotor output cycles kaput (Clock), period (Per1, Per2 and Per3), and cryptochrome (Cry1 and Cry2) [13]. Additional regulatory loops involving nuclear hormone receptors such as Ror and Rev-erbα are also implicated in the control of circadian clock function [13]. These positive and negative feedback loops drive circadian physiologic outputs by regulating the expression of downstream clock-controlled genes, such as albumin D-site-binding protein (Dbp) and E4BP4 (a basic leucine zipper transcription factor). About 15–30% of the transcriptome in all tissues is under the control of clock genes depending on the tissue or the cell type [14-16].
Impairments in retinal function due to retinal diseases can potentially impact the central clocks and the circadian organization of the entire organism [17]. Diabetic retinopathy is one of the most common consequences of diabetes that affects millions of working-age adults worldwide and leads to progressive degeneration of the retina, visual loss, and blindness [18]. Studies in patients and animal models have shown that diabetes alters rhythmic clock gene expression in the central and peripheral clocks. In experimental or genetic models of type 1 and type 2 diabetes, pronounced phase advance or delay in the rhythms of several clock and clock-controlled genes have been reported in the heart, liver, and retina [19-22], whereas in the SCN and the cerebral cortex, no changes in expression were observed [22,23]. However, we previously reported that diabetic retinopathy affects clock genes and behavioral responses of the circadian timing system to light, possibly through direct alterations of ipRGCs [24].
Because these changes in clock genes have been reported under diurnal conditions, it is not possible to unequivocally assess whether the effects described are due to effects on the circadian pacemaker or to complex masking processes of light. In the current study, we investigated the impact of diabetic retinopathy on the circadian expression and light response of clock and clock-controlled genes in the retina and in the SCN using a streptozotocin (STZ) mouse model of diabetes. In several models of diabetes, a dysfunction of the retinal dopaminergic system has been observed [25-27]. Since dopamine (DA) is involved in many physiologic aspects of retinal neuromodulation, mediation of light responsiveness, and clock gene regulation [28-30], we thus evaluated light-driven retinal DA as a major output marker of the retinal clock.
Methods
Animals
Wild-type male C57BL/6J mice (Janvier, Le-Gesest-St-Isle, France) were maintained in a temperature-controlled room (23±1 °C) under a 12 h: 12 h light-dark cycle with broadband white light at 300 lux, with food and water ad libitum. All treatments of animals were in strict accordance with current national and international regulations on animal care, housing, breeding and experimentation and are in agreement with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The specific protocols used in this study were approved the CELYNE Committee n° C2EA42-13-02-0402-005.
Induction of experimental diabetes in mice
Diabetes was chemically induced in 3-week-old fasted male mice as previously described [24,31]. Animals received an intraperitoneal injection of STZ (85 mg/kg, Calbiochem, San Diego, CA) dissolved in 0.01 M sodium citrate buffer (pH 4.5) during 3 successive days. The diabetic state was confirmed by measuring blood glucose levels. Mice with glucose levels higher than 2 g/l were considered to be diabetic. Age-matched non-diabetic control animals were injected with 0.01 M sodium citrate buffer. Animals were maintained until 12 weeks after the onset of diabetes. At this stage, the control group exhibited mean blood glucose of 1.66±0.17 g/l (p≤0.001), whereas the mean level in the diabetic mice was 4.99±0.3 g/l. This increase in the blood glucose level was correlated with a weight loss in the STZ group (21±1.05 g) compared to the control group (28.50±0.57 g; p≤0.001).
Circadian expression of clock and clock-controlled genes in the retina and the SCN
Wild-type (n = 16) and diabetic (n = 16) mice were initially maintained under a 12 h: 12 h light-dark cycle, and subsequently, at 10 weeks post-injection, the mice were kept in constant darkness (DD) for 15 days. To quantify the circadian expression of clock and clock-controlled genes (Clock, Bmal1, Per1–2-3, Cry1–2, Rev-erbα, Rorβ, Dbp, and E4BP4), retinas and SCN from the same animals were collected at 12 weeks post-diabetes during the first circadian cycle every 4 h (CT0, CT4, CT8, CT12, CT16, and CT20). Pooled retinas (n = 4 for each CT and each group) from the same animal and individual SCN (n = 4 for each CT and each group) were directly collected under dim red light (below 0.1 lux), frozen on dry ice, and stored at −80 °C.
Light induction of Per1, Per2, and c-fos in the retina
Mice were maintained under a 12 h: 12 h light-dark cycle (n = 11–12 for both groups) until 12 weeks after the onset of diabetes and subsequently maintained for one cycle under DD conditions. To analyze photic induction of Per1, Per2, and c-fos mRNAs, a pulse of monochromatic light (480 nm, 1.17×1014 photons/cm2/s, 15 min) was administered at circadian time 16 (CT16), the first day in DD. After the light pulse, the animals (n = 7 for each group) were returned to DD and were euthanized with cervical dislocation 30 min after the beginning of the light pulse. The dark control (n = 4–5 for each group) animals were handled identically but were not exposed to light. Two retinas from the same animal were pooled and stored at −80 °C until RNA was extracted and quantified. Handling and transfer of the animals and dissections were performed under dim red light.
Real-time RT–PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Villebon sur Yvette, France). Total RNAs was reverse transcribed using random primers and MMLV Reverse Transcriptase (Invitrogen). Real time RT-PCR was then performed on a LightCycler™ system (Roche Diagnostics, Meylan, France). PCR reaction was performed with 1 µl of cDNA supplemented with 0.75 µl of LightCycler® FastStart Enzyme and LightCycler® FastStart Reaction Mix SYBR Green (Roche Diagnostic), 0.8 µl of forward and reverse primers (10 µM; Eurofins, Ebersberg, Germany), 1.2 µl of MgCl2 (4 mM; Roche Diagnostic) in a total volume of 10 µl. The thermal cycler conditions were as follows: 8 min at 95 °C, and then 45 cycles of denaturation at 95 °C for 15 s, annealing at 68 °C for 15 s and the temperature was reduced by 0.5 °C each cycle until 60 °C. Hypoxanthine ribosyl-transferase (Hprt) was used for normalization. Initially, two housekeeping genes were tested for RT–PCR, the Hprt and 36b4 genes. The Hprt gene was finally used for internal standardization of target gene expression as this gene exhibits only constitutively non-regulated expression in both groups and independently of the physiologic state or the experimental conditions. The efficiency and the specificity of the amplification were controlled by generating standard curves and carrying out melting curves. Relative transcript levels of each gene were calculated using the second derivative maximum values from the linear regression of cycle number versus log concentration of the amplified gene. Primer sequences are shown in Table 1.
Table 1. Primers.
| Primer | Sens | Reverse |
|---|---|---|
|
Hprt |
ATCAGTCAACGGGGGACATA |
AGAGGTCCTTTTCACCAGCA |
|
Clock |
GTTTGATCACAGCCCAACTC |
CTCCGCTGTGTCATCTTTTC |
|
Bmal1 |
CTCAGCTGCCTCGTTGCAAT |
GCTGTCGCCCTCTGATCTAC |
|
Per1 |
GCGTTGCAAACGGGATGTGT |
GAACCTGCAGAGGTGCCAG |
|
Per2 |
CCACACTTGCCTCCGAAATA |
ACTGCCTCTGGACTGGAAGA |
|
Per3 |
CAGTGGCAGAGACGTGCGT |
GACACTGTCGATACAGTTCAT |
|
Cry1 |
GCCAGCTGATGTATTTCCCAG |
CGCCAGCCTCAGTAGCCAG |
|
Cry2 |
GAGAGACCTCGGATGAATGC |
CTCGCCACAGGAGTTGTCCA |
|
Rev-erbα |
GCTCCATCGTTCGCATCAAT |
CTAGAGGGCACAGGCTGCT |
|
Rorβ |
GCGAGCACAAATTGAAGTGA |
AACGGTTCCTGTTGGTTCTG |
|
E4BP4 |
CGGAAGTTGCATCTCAGTCA |
GCAAAGCTCTCCAACTCCAC |
|
Dbp |
CGTGGAGGTGCTAATGACCT |
CGGCTCCAGTACTTCTCATC |
| c-fos | AGCGCCCCATCCTTACGGAC | TCAGCAGATTGGCAATCTCA |
Immunohistochemistry
Twelve weeks post-injection, at Zeitgeber time 4 (ZT4), the male mice were deeply anesthetized with ketamine (100 mg/kg) and perfused intracardially with saline followed by 4% paraformaldehyde in phosphate buffer (PB, pH 7.4). Flatmounted retinas (n = 6 for the control and n = 5 for the diabetic groups) were rinsed three times (10 min each) in 0.01 M saline PBS (0.01 M phosphate buffer, 0.09% NaCl, pH 7.4), and endogenous peroxidase activity was suppressed using a solution of 50% ethanol with 0.03% H2O2 for 1 h. Retinas were preincubated in 0.01 M PBS containing 0.3% triton and 0.1% sodium azide (PBSTA) and blocked with 1% bovine serum albumin for 2 h, and then in antityrosine hydroxylase (TH) primary antibody (1:1,000; Millipore, Fontenay ss Bois, France) for 2 days, at 4 °C. Retinas were then washed twice in PBST and reacted with the secondary goat anti-rabbit biotinylated immunoglobulin (IgG ; 1 :200 ; Vector Laboratories, Burlingame, CA) for 2 h. Immunoreactivity was visualized using a Vectastain ABC Elite kit (1:200; PK-6100, Vector Laboratories) for 90 min at room temperature, followed by incubation in 0.2% 3,3′-diaminobenzidine, 0.5% ammonium nickel sulfate, and 0.003% H2O2 in 0.05 M Tris buffer (pH 7.6). The flatmounted retinas were mounted on slides, dehydrated in graded ethanol, cleared in xylene, and coverslipped. The number of TH-positive cells was determined using the software package Mercator running on ExploraNova technology.
Biochemistry
Control and diabetic mice were maintained under a 12 h: 12 h light-dark cycle. Twelve weeks after the onset of diabetes, pooled retinas from the same animal were directly collected at ZT2 and ZT14 (n = 7 for control and n = 8 for diabetic mice for each ZT time), frozen on dry ice, and stored at −80 °C until analysis. Frozen retinas were homogenized by sonication in 60 µl buffered perchloric acid 0.1 M and centrifuged at 5,000 ×g for 15 min at 4 °C. The supernatants were injected into the high-performance liquid chromatography (HPLC) separation system (column SpheriSorb RP18–5UM, mobile phase 50 mM KH2PO4, 15 mg/ml EDTA-Na, 0.26 mM sodium octyle sulfate, methanol 8%, adjusted to pH 4.5, flow rate 0.3 ml/min). DA and 3,4-dihydroxyphenylacetic acid (DOPAC) peaks were identified based on retention time, and concentrations were estimated by rationing the peak areas of each substance and their respective external standard (analytical software AZUR, Datalys, St Martin d’Heres, France). The DA and DOPAC concentrations are expressed as pg/mg tissue.
Statistical analysis
To evaluate circadian rhythmicity in gene expression, data were analyzed with the cosinor method (Sigmaplot Systat software). Rhythmicity in gene expression was assessed by fitting the 24 h data to a cosine curve using the following equation:
| f(x) = M+A x cos x (2π x (T- φ)/24) |
T represents the time (h), M represents the mean value of the cosine curve (mesor), A represents the amplitude of the curve (half of the sinusoid), and φ the acrophase (h). Two-way ANOVA followed by the post-hoc Newman-Keuls test were used to compare the Per1, Per2, and c-fos mRNAs, DA and DOPAC concentrations, and the DOPAC/DA ratio between the control and diabetic groups. One-way ANOVA followed by the post-hoc Newman-Keuls test was used to compare the number of TH-positive cells between the control and diabetic animals.
Results
Circadian expression of clock genes and clock-controlled genes in the retina and the SCN of control and diabetic animals
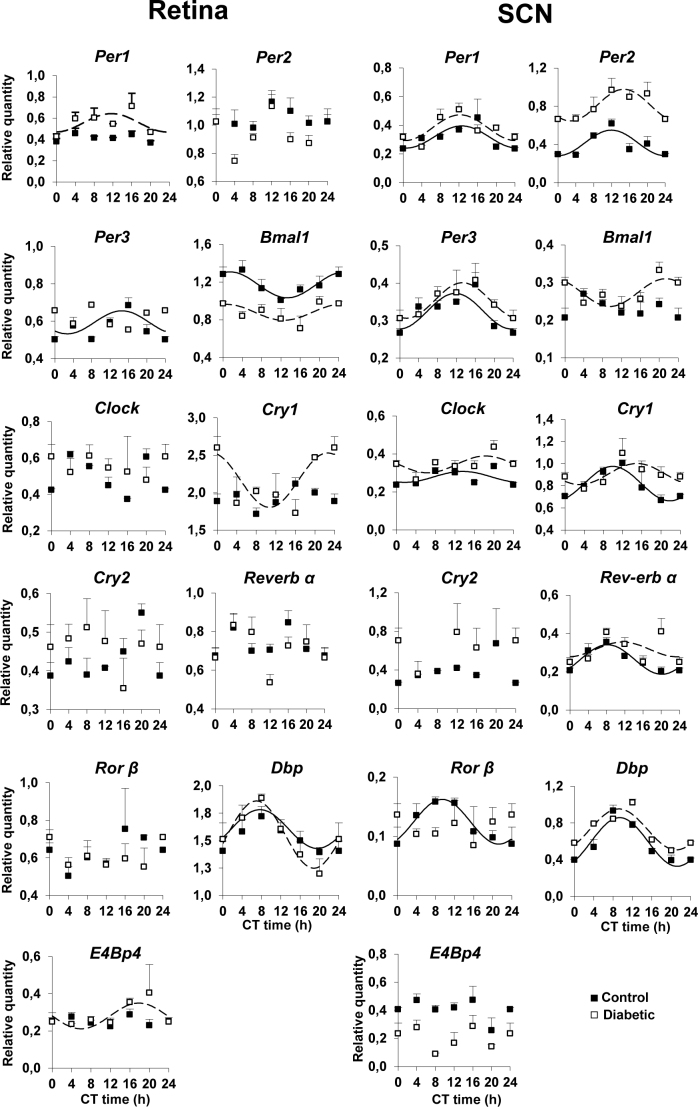
The circadian expression of clock genes (Clock, Bmal1, Per1–3, Cry1–2, Rev-erbα, and Rorβ) was analyzed in the retina and the SCN and compared in diabetic mice to the age-matched control animals 12 weeks post-onset of diabetes (Figure 1). In the DD conditions, cosinor analysis showed that in the whole retina, a significant rhythmicity was found in a few clock genes in the control and diabetic mice. In the control mice, only Bmal1 (p = 0.00016) and Per3 (p = 0.006) were rhythmic, while Clock, Per1–2, Cry1–2, Rev-erbα, and Rorβ did not show a significant 24 h rhythmic component (p≥0.05). In diabetic mice, Bmal1 remained rhythmic (p≤0.05) with a significantly reduced amplitude (0.16±0.07) compared to the control mice (0.27±0.06; p≤0.05) but with a similar acrophase between the control (1.4±1.04 h) and diabetic mice (0.68±2.3 h). Per1 (p = 0.30) and Cry1 (p = 0.11) that were non-rhythmic in the control mice exhibited circadian expression in the diabetic mice with an acrophase of 11.79±1.5 h for Per1 and 22.8±1.1 h for Cry1 (Figure 1, Table 2).
Figure 1.
Circadian expression of clock genes (Per1–3, Bmal1, Clock, Cry1–2, Rev-erbα, and Rorβ) and clock-controlled genes (Dbp and E4BP4) in the retina and the SCN of the wild-type and STZ-diabetic mice at 12 weeks post-diabetes. Animals were euthanized in constant darkness (DD). Pooled retinas (n = 4 for each circadian time (CT) and each group) and individual suprachiasmatic nucleus (SCN; n = 4 for each CT and each group) from the same animals were isolated every 4 h (CT0, CT4, CT8, CT12, CT16, and CT20), and mRNA levels were measured using real-time reverse transcription PCR (RT–PCR). Results are expressed as mean ± standard error of the mean (SEM). Data from CT0 are double plotted at CT24. Continuous (wild-type) and dashed (diabetic) lines represent the periodic sinusoidal function determined by Cosinor analysis. Only periodic sinusoidal functions with amplitude significantly different from zero are represented (p≤0.05, zero amplitude test).
Table 2. Acrophase (± SD) of mRNA levels of clock and clock-controlled genes in the retina and the SCN of control and diabetic animals at 12 weeks post-diabetes.
| Gene | Acrophase (h) |
|||
|---|---|---|---|---|
| Retina |
SCN |
|||
| Control | SZT-diabetic | Control | SZT-diabetic | |
|
Per1 |
- |
11.79±1.5 |
12.7±1.14 |
12.32±1.02 NS |
|
Per2 |
- |
- |
12.07±1.2 |
15.02±1.05 NS |
|
Per3 |
14.7±1.3 |
- |
11.8±1.22 |
13.43±1.17 NS |
|
Bmal1 |
1.4±1.04 |
0.68±2.3 NS |
- |
21.6±1.16 |
|
Clock |
- |
- |
13,7±2.09 |
18.53±1.42 NS |
|
Cry1 |
- |
22.8±1.1 |
10.04±0.8 |
15.10±1.32 * |
|
Rev-erb α |
- |
- |
8.3±0.9 |
11.6±2.09 NS |
|
Ror β |
- |
- |
9.48±1.13 |
- |
|
Dbp |
7.7±1.4 |
7.01±0.9 NS |
- |
- |
| E4Bp4 | - | 18.02±1.52 | - | - |
Acrophase values are determined using Cosinor analysis and test of amplitude to assess goodness of fit. Only rhythms with a significant value are shown. * p≤0.05.
In the SCN, all the genes studied (Per1–3, Clock, Cry1, Rev-erbα, and Rorβ) showed circadian expression, except Cry2 in the control and diabetic mice, Bmal1 in the control mice, and Rorβ in the diabetic mice. The expression of Per2 showed greater amplitude in diabetic animals (0.33±0.007) compared to the control animals (0.26±0.007, p≤0.05). However, no significant difference in acrophases was observed between the diabetic and age-matched control mice except Cry1 (Figure 1, Table 2) that exhibited around a 5 h phase delay in the diabetic group (15.10±1.32 h) compared to 10.04±0.8 h in the control group (p≤0.05).
Cosinor analysis of the clock-controlled genes showed that in the retina and the SCN, Dbp exhibited a circadian rhythm with a similar acrophase in the control and diabetic groups (Figure 1, Table 2). The acrophase was 7.7±1.4 h for the control mice and 7.07±0.9 h for the diabetic mice (p≥0.05) in the retina and 9.43±0.64 h for the control mice and 9.23±0.67 h for the diabetic mice in the SCN (p≥0.05). In the retina, E4BP4 showed a circadian variation with an acrophase of 18.02±1.5 h only in the diabetic mice (p = 0.0002), whereas in the SCN no significant circadian rhythm was found for either group (p≥0.05).
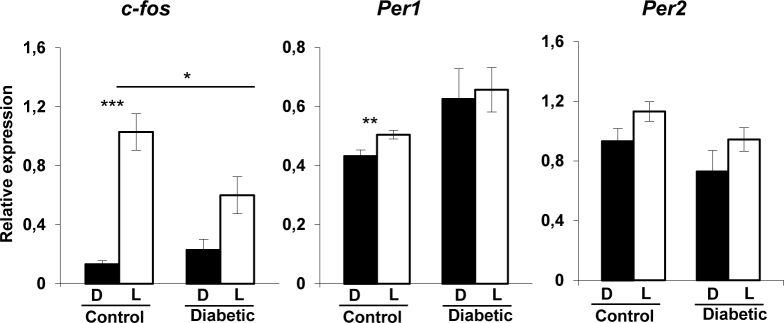
Altered light induction of c-fos, Per1 and Per2 in the retina of diabetic mice
Our previous work has shown that diabetes induces morphological changes in ipRGCs, including soma swelling and dendritic varicosities, associated with decreased c-fos and clock genes induction by light in the SCN at 12 weeks post-onset of diabetes [24]. Here, we analyzed the effect of light stimulation on the induction of c-fos and Period genes in the retina to assess the role of ipRGCs in the light response of the retinal clock. Mice were exposed to a 480 nm monochromatic light pulse of constant irradiance and duration (1.17×1014 photons/cm2/s; 15 min) at CT16, known to induce significant Per1 and Per2 expression [32]. c-fos and Per1 were significantly induced by light in the control animals compared to the respective dark-exposed mice (F(1–10) = 7.9; p≤0.001 for c-fos and F(1–10) = 8.36; p≤0.001 for Per1), whereas no significant induction by light was observed in the diabetic mice for both genes (Figure 2). A reduction in c-fos induction by light was observed between the control and diabetic mice (p≤0.05). ANOVA analysis showed that the expression of the Per2 gene was not induced by light in either the control (F(1–10) = 3.58; p≥0.05) or diabetic (F(1–10) = 2.04; p≥0.05) mice in comparison to the respective dark control animals.
Figure 2.
Relative light-induced c-fos, Per1 and Per2 mRNA levels in the retinas of the control and diabetic mice at 12 weeks post-diabetes. The light stimulus is a 480 nm monochromatic light pulse (1.17× 1014 photons/cm2/s, 15 min duration) delivered at circadian time 16 (CT16). White bars represent animals that received the light pulse (L, n = 7 for each group). The dark control mice (D, black bars) were handled in the same way but did not received a light stimulus (n = 4–5 for each group). Data are represented as mean ± standard error of the mean (SEM). Asterisk indicates a statistically significant difference (* p≤0.05; ** p≤0.01; *** p≤0.001).
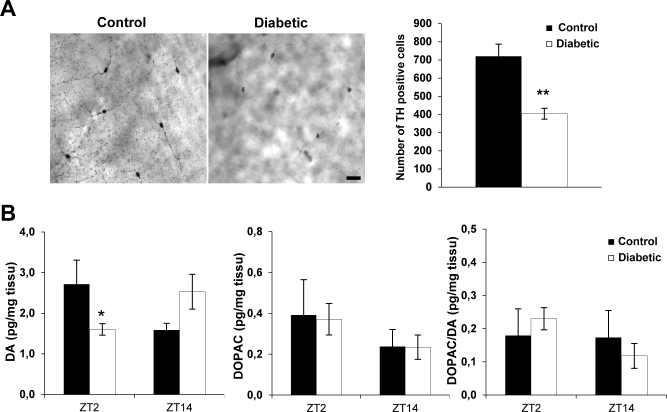
Diabetes induced a significant reduction in the total number of TH-positive neurons and the retinal dopamine content
DA is a crucial neurotransmitter involved in many physiological aspects of retinal neuromodulation, mediation of light responsiveness, and clock gene regulation [29]. We first evaluated the impact of diabetes on the total number of TH-positive cells in whole-mounted retinas at 12 weeks post-onset of diabetes (Figure 3A). A reduction of 56% in the total number of TH-positive cells was found in the STZ-diabetic mice compared to the control animals (controls: 719.5±67.11 cells/retina and STZ-diabetic: 404.2±66.48 cells/retina with F(1–10) = 15.69; p≤0.001). In the diabetic mice, the DA level was decreased at ZT2 (p = 0.04) but not at ZT14 (p≥0.05). Diabetes did not alter the DOPAC levels (F(1–27) = 0.0016; p≥0.05) or the ratio of DOPAC to DA at the two zeitgeber times ((F(1–27) = 0.036; p≥0.05; Figure 3).
Figure 3.
Alteration of the dopaminergic system in diabetic mice at 12 weeks post-diabetes. A: Right panel: representative flatmounted retinas from a control and a streptozotocin (STZ)-diabetic animal, immunostained with tyrosine hydroxylase (TH) antibody. Scale bar = 40 µm. Left panel: total number of TH-positive cells in the control and diabetic mice at 12 weeks post-diabetes. A significant reduction in the total number of TH-positive cells was observed in the diabetic group in comparison to the age-matched control animals. Data are represented as mean ± standard error of the mean (SEM; n = 6 for the control group and n = 5 for the diabetic mice group). B: Dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) levels, and the DOPAC/DA ratio in wild-type and diabetic mice at 12 weeks post-diabetes and at two different Zeitgeber time (ZT) points (ZT2 and ZT14; n = 7 for control and n = 8 for diabetic mice for each ZT point). The post-hoc Newman-Keuls test shows a significant difference in the DA level at ZT2 between the control and diabetic groups, whereas no significant difference was observed at ZT14. The DOPAC content and the DOPAC/DA ratio are similar between the two groups at the two ZTs. Data are represented as mean ± SEM, * p≤0.05.
Discussion
Diabetes alters the molecular machinery of the retinal clock
Dysregulation of clock gene expression has been identified as a key factor in several disease pathogenesis, including diabetes. Diabetes is associated with circadian disruption of the central and peripheral circadian clocks, such as in the heart and the liver [20,33,34]. Db/db mice, a model of type 2 diabetes, show the hallmark features of diabetic retinopathy associated with dampened oscillations of clock genes in the vasculature [21]. Inversely, Clock and Bmal1 conditional mice develop type 1 diabetes [35-37], and Per2 mutant mice present retinal vascular damage and neuronal loss in the bone marrow similar to that found in type 2 diabetes [38]. In rat models of type 1 and type 2 diabetes, the expression of several clock genes, including Clock, Bmal1, Per1, Per2, Cry1, and Cry2, are altered in the retina, whereas few effects are observed in the SCN [22,23,34]. In agreement with these studies, we found variable effects of diabetes on the expression of clock genes in the retina and only slight differences in phase and/or amplitude in the SCN. The main differences observed in the SCN include an increase in the amplitude of Bmal1 expression and a phase delay in Cry1 in STZ-diabetic animals compared to the control animals. In the retina of the STZ-diabetic animals, the amplitude of Per1 and Cry1 gene expression increased leading to a significant circadian rhythm of their expression, whereas Per3 lost rhythmicity. However, our results disagree with the study by Wang et al.; they found a decrease in Per1 expression and an increase in Bmal1 expression in the retina of STZ-diabetic rats [34]. The discrepancy could be related to species or to experimental design. We euthanized the mice at an advanced stage of diabetes (12 weeks post-injection of STZ) in circadian conditions, whereas Wang et al. [34] analyzed the diurnal rhythm of clock genes at an early stage of the pathology (6 weeks post-injection).
In general, a circadian rhythm of only a few clock genes is observed in the wild-type retina in comparison to the SCN [39]. This has been related to the existence of several clocks in the retina that oscillate with different periods and phases, resulting in low amplitude rhythmicity or masking of coherent clock gene expression [32,40] and cellular coupling among clock cells/layers is not uniform throughout the retina [40]. In the SCN, in contrast, the oscillators are strongly coupled [41]. Intercellular coupling confers robustness against mutations in the SCN circadian clock network, and this may confer robustness of the SCN clock versus the retinal clock against clock gene mutations [42] or pathological conditions.
Clock gene response to light is altered in the retina during diabetes
Light entrains the circadian clock in the retina and the SCN and induces c-fos, Per1, and Per2 expression [32,43-45]. Photic entrainment of the mammalian SCN requires cones, rods, and ipRGCs [2-6], but their role in entrainment of the retinal clock is under debate. Two recent studies by the same group suggested that none of these photoreceptors are necessary for light entrainment of the retinal clock [46]. However, melanopsin has previously been shown to be involved [47-51]. In particular, ipRGCs provide excitatory sustained light responses to dopaminergic neurons with peak sensitivity near 480 nm, the maximum sensitivity of melanopsin [50]. In the absence of melanopsin, this sustained melanopsin-driven response is eliminated [51], and 480 nm light fails to induce Per1 and Per2 mRNA expression in the retinal photoreceptor layer [32].
We found that c-fos and Per1 were induced with a 480 nm light pulse only in the control group at 12 weeks post-induction of diabetes, whereas this photic induction was abolished in the STZ-diabetic animals. The Per2 gene was not induced by light in either group. This suggests a deficit in light-induced neuronal activation in the retina. Several studies have shown that ipRGCs are altered in diabetes [52-54]. Diabetes induced with STZ treatment gives rise to late neural cell death in the retina [55,56]. We also found in the STZ-diabetic mice an atrophy of ipRGCs associated with decreased photic signaling to the SCN at 12 weeks post-onset of diabetes [24]. Because the diminished light input in the retina of the STZ-diabetic mice was not likely due to the development of cataract or changes in opsin levels [24], our results suggest that the altered photic response of the retinal clock may result from impaired ipRGCs during diabetes.
Diabetes induced a reduction in TH-positive cells and retinal dopamine level
DA, synthesized and released by a sparse population of amacrine cells [57,58], is implicated in many aspects of retinal neuromodulation and plays a central role in the adaptation to light and clock gene regulation [28-30,43]. In the present study, we demonstrated a reduction in the DA content at ZT2 in mice at 12 weeks post-diabetes compared to controls, whereas no significant difference was observed at ZT14. The DOPAC/DA ratio, which is an indicator of the DA metabolism, was similar between the control and diabetic animals at both ZT times. This result is in accordance with previous studies that show a dysfunction of the dopaminergic system in the early or late stages of development of diabetes in mouse and rat models [25,27,52,59,60]. However, the underlying mechanisms are unclear. The decline in DA content may be related to a reduced number of retinal dopaminergic neurons, low levels of DA synthesis, or an impairment of DA release from the cells. In the present study, we reported a 56% reduction in the total number of TH-positive cells in STZ-diabetic mice compared to age-matched controls with no difference in DA metabolism that may account for the reduced DA level.
In addition, the retinal DA content and metabolism are not circadian in C57BL/6 mice, and DA release is strictly light-driven [61,62]. The reduction of the DA content only at ZT2 may also be induced by the decrease in light induction of the c-fos and Per1 genes in the STZ-diabetic retina. According to the anatomic and functional relations between ipRGCs and DAergic amacrine cells [50,51,63], ipRGCs may be involved as we have shown that light induction of TH mRNA, DA, and clock genes requires melanopsin [32]. In addition, in dystrophic retinas with degenerated rod and cone photoreceptors, light is still capable of increasing retinal DA release [64,65].
In conclusion, the effect of STZ-induced diabetes differs between the SCN and the retina and leads to a dysregulation of the retinal clock organization and, in particular, the response to light. In addition, disturbances in the dopaminergic system, the main output of the retinal clock, have been observed expressed as a decrease in the number of TH-positive amacrine cells and DA content.
Acknowledgments
HL and CC performed the experiments. HL and ODB analyze the data. HL, MB and ODB conceived the study and designed the experimental plan. HL, HMC, MB and ODB wrote the manuscript. All authors took part in the revision of the manuscript and approved the final version. The authors do not have any potential conflict of interest. This research was supported by Rhône-Alpes CMIRA, GDRI-Neuro (CNRST/INSERM/CNRS), CNRST-INSERM PA/RN/2013, NRJ-Institut de France, ARC Handicap-Vieillissement-Neurosciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–7. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 4.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–7. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 5.Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–87. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dollet A, Albrecht U, Cooper HM, Dkhissi-Benyahya O. Cones are required for normal temporal responses to light of phase shifts and clock gene expression. Chronobiol Int. 2010;27:768–81. doi: 10.3109/07420521003695704. [DOI] [PubMed] [Google Scholar]
- 7.Teirstein PS, Goldman AI, O'Brien PJ. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest Ophthalmol Vis Sci. 1980;19:1268–73. [PubMed] [Google Scholar]
- 8.Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N- acetyltransferase. Nature. 1983;305:133–5. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- 9.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–21. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 10.Tosini G, Menaker M. The tau mutation affects temperature compensation of hamster retinal circadian oscillators. Neuroreport. 1998;9:1001–5. doi: 10.1097/00001756-199804200-00009. [DOI] [PubMed] [Google Scholar]
- 11.Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in dystrophic retinas of homozygous and heterozygous retinal degeneration slow (rds) mice. Brain Res. 2000;884:13–22. doi: 10.1016/s0006-8993(00)02855-9. [DOI] [PubMed] [Google Scholar]
- 12.Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002;83:211–9. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 13.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 14.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–7. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 15.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 16.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 17.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications. 2012;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34:223–31. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 20.Herichova I, Zeman M, Stebelova K, Ravingerova T. Effect of streptozotocin-induced diabetes on daily expression of Per2 and Dbp in the heart and liver and melatonin rhythm in the pineal gland of Wistar rat. Mol Cell Biochem. 2005;270:223–9. doi: 10.1007/s11010-005-5323-y. [DOI] [PubMed] [Google Scholar]
- 21.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–41. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busi J, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S. LiCalzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuriyama K, Sasahara K, Kudo T, Shibata S. Daily injection of insulin attenuated impairment of liver circadian clock oscillation in the streptozotocin-treated diabetic mouse. FEBS Lett. 2004;572:206–10. doi: 10.1016/j.febslet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Lahouaoui H. Coutanson C2, Cooper HM2, Bennis M3, Dkhissi-Benyahya O2. Clock genes and behavioral responses to light are altered in a mouse model of diabetic retinopathy. PLoS One. 2014;9:e101584. doi: 10.1371/journal.pone.0101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki M, Tanaka T, Nawa H, Usui T, Fukuchi T, Ikeda K, Abe H, Takei N. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neuroptrophic factor for dopaminergic amacrine cells. Diabetes. 2004;53:2412–9. doi: 10.2337/diabetes.53.9.2412. [DOI] [PubMed] [Google Scholar]
- 26.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–42. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- 27.Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34:726-36. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type1 diabetes. J Neurosci. 2014;34:726–36. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–9. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci USA. 2006;103:6386–91. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32:9359–68. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–6. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 32.Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, Bennis M, Cooper HM. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70:3435–47. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oishi K, Ohkura N, Kasamatsu M, Fukushima N, Shirai H, Matsuda J, Horie S, Ishida N. Tissue- specific augmentation of circadian PAI-1 expression in mice with streptozotocin- induced diabetes. Thromb Res. 2004;114:129–35. doi: 10.1016/j.thromres.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Tikhonenko M, Bozack SN, Lydic TA, Yan L, Panchy NL, Mcsorley KM, Faber MS, Yan Y, Boulton ME, Grant MB, Busik JV. Changes in the Daily Rhythm of Lipid Metabolism in the Diabetic Retina. PLoS One. 2014;9:e95028. doi: 10.1371/journal.pone.0095028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD. JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatwadekar AD, Yan Y, Qi X, Thinschmidt JS, Neu MB, Li Calzi S, Shaw LC, Dominiguez JM, Busik JV, Lee C, Boulton ME, Grant MB. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–82. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun. 2006;351:800–7. doi: 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger C, Sandu C, Malan A, Mellac K, Hicks D, Felder-Schmittbuhl MP. Circadian organisation of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015;29:1493–504. doi: 10.1096/fj.14-261214. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1a integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–81. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 42.Ruan GX, Gamble KL, Risner ML, Young LA, McMahon DG. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS One. 2012;7:e38985. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An antonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Namihira M, Honma S, Abe H, Masubuchi S, Ikeda M, Honmaca K. Circadian pattern, light responsiveness and localization of rPer1 and rPer2 gene expression in the rat reina. Neuroreport. 2001;12:471–5. doi: 10.1097/00001756-200103050-00010. [DOI] [PubMed] [Google Scholar]
- 45.Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J Neurosci. 2000;20:7790–7. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buhr ED. Van Gelder RN2. Local photoc entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanospin. Proc Natl Acad Sci USA. 2014;111:8625–30. doi: 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–36. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 48.Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci. 2012;35:507–18. doi: 10.1111/j.1460-9568.2011.07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkinson CL, Feng J, Zhang DQ. Functional integrity and modification of retinal dopaminergic neurons in the rd1 mutant mouse: roles of melanopsin and GABA. J Neurophysiol. 2013;109:1589–99. doi: 10.1152/jn.00786.2012. [DOI] [PubMed] [Google Scholar]
- 50.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–6. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanospin mediates retrograde visual signalling in the retina. PLoS One. 2012;7:e42647. doi: 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gastinger MJ, Singh RSJ, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–50. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S, Zhuo L. Quantitative analysis of pupillary light reflex by real- time autofluorescent imaging in a diabetic mouse model. Exp Eye Res. 2011;92:164–72. doi: 10.1016/j.exer.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez DC, Sande PH, de Zavalía N, Belforte N, Dorfman D, Casiraghi LP, Golombek D, Rosenstein RE. Effect of experimental diabetic retinopathy on the non-image-forming visual system. Chronobiol Int. 2013;30:583–97. doi: 10.3109/07420528.2012.754453. [DOI] [PubMed] [Google Scholar]
- 55.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aizu Y, Oyanagi K, Hu J, Nakagawa H. Degeneration of retinal neuronal processes and pigment epithelium in the early stage of the streptozotocin-diabetic rats. Neuropathology. 2002;22:161–70. doi: 10.1046/j.1440-1789.2002.00439.x. [DOI] [PubMed] [Google Scholar]
- 57.Björklund A, Dunnett SB. Dopamine neurons systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–47S. doi: 10.1093/jn/137.6.1539S. [DOI] [PubMed] [Google Scholar]
- 59.Nishimura C, Kuriyama K. Alterations in the retinal dopaminergic neuronal system in rats with streptozotocin-induced diabetes. J Neurochem. 1985;45:448–55. doi: 10.1111/j.1471-4159.1985.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 60.Szabadfi K, Szabo A, Kiss P, Reglodi D, Setalo G, Jr, Kovacs K, Tamas A, Toth G, Gabriel R. PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem Int. 2014;64:84–91. doi: 10.1016/j.neuint.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–2. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 62.Proll MA, Kamp CW, Morgan WW. Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sci. 1982;30:11–9. doi: 10.1016/0024-3205(82)90630-0. [DOI] [PubMed] [Google Scholar]
- 63.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion cell photoreceptors. J Physiol. 2007;582:279–96. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 65.Vugler AA, Redgrave P, Hewson-Stoate NJ, Greenwood J, Coffey PJ. Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. J Chem Neuroanat. 2007;33:9–22. doi: 10.1016/j.jchemneu.2006.10.004. [DOI] [PubMed] [Google Scholar]