Abstract
At rest the proportion between systolic and diastolic periods of the cardiac cycle is about 1/3 and 2/3 respectively. Therefore, mean blood pressure (MBP) is usually calculated with a standard formula (SF) as follows: MBP = diastolic blood pressure (DBP) + 1/3 [systolic blood pressure (SBP) – DBP]. However, during exercise this proportion is lost because of tachycardia, which shortens diastole more than systole. We analysed the difference in MBP calculation between the SF and a corrected formula (CF) which takes into account changes in the diastolic and systolic periods caused by exercise-induced tachycardia. Our hypothesis was that the SF potentially induce a systematic error in MBP assessment during recovery after exercise. Ten healthy males underwent two exercise-recovery tests on a cycle-ergometer at mild-moderate and moderate-heavy workloads. Hemodynamics and MBP were monitored for 30 minutes after exercise bouts. The main result was that the SF on average underestimated MBP by –4.1 mmHg with respect to the CF. Moreover, in the period immediately after exercise, when sustained tachycardia occurred, the difference between SF and CF was large (in the order of -20-30 mmHg). Likewise, a systematic error in systemic vascular resistance assessment was present. It was concluded that the SF introduces a substantial error in MBP estimation in the period immediately following effort. This equation should not be used in this situation.
Key points.
The standard formula often employed to calculate mean blood pressure during recovery from exercise may introduce a substantial error in the estimation of this parameter;
This equation should not be used in this situation;
Instead a corrected formula, which calculates mean blood pressure taking into account the systolic and diastolic proportion during the cardiac cycle, should be employed.
No significant hypotensive due to exercise effect was detected using this formula.
Key words: recovery, exercise, cardiac output, stroke volume, contractility
Introduction
Mean arterial blood pressure (MBP) is the average blood pressure in a subject during the cardiac cycle. Inasmuch as this parameter represents the force to sustain organ perfusion, it should be considered a fundamental cardiovascular variable. During dynamic exercise MBP usually slightly rises, depending on the mode and intensity of exercise (Crisafulli et al., 2005; 2006a; 2006b; 2007; Higginbotham et al., 1986; Lewis et al., 1983; Miles et al., 1984). Importantly, an acute hypotensive effect of dynamic exercise has been several times reported in the period immediately after exercise (Fleg and Lakatta, 1986; Kenney and Seals, 1993; McDonald et al., 2000; Nottin et al., 2002; Piepoli and Coats, 1994; Raine et al., 2001). Even though the precise mechanism of this hypotension is still to be clarified, it has been mostly ascribed to an acute vasodilation due to exercise (Halliwill et al., 2013). It is to be noticed that usually MBP has been calculated with a standard formula (SF), which considers as constant the proportion between diastolic (DBP) and systolic (SBP) blood pressure during the cardiac cycle. In detail, this formula is as follows: MBP= DBP + 1/3 (SBP – DBP) (Crisafulli et al., 2003a; Crisafulli et al., 2003b; de Almeida et al., 2010; Coats et al., 1989; Kilgour et al., 1995; Nottin et al., 2002; Raine et al., 2001). This assumption is based upon the fact that at rest the proportion between systolic and diastolic periods of the cardiac cycle is approximately 1/3 and 2/3 for the systole and the diastole respectively. However, this is only a crude estimation. When tachycardia occurs, as during exercise and recovery, this proportion is lost, as diastole shortens more than systole. It becomes that the SF may lead to a systematic error in MBP assessment.
This occurrence has been already demonstrated during exercise (Moran et al., 1995; Rogers and Oosthuyuse, 2000), where a substantial error (i.e. underestimation) due to the SF has been detected and it has been found to be linearly correlated with heart rate (HR). Authors of the quoted study proposed a corrected formula (CF), where MBP is calculated taking into consideration changes in the diastolic and systolic periods by exercise-induced tachycardia. In particular, the fraction of systole (FS) from the heart cycle was assessed and MBP was then calculated from DBP and the pulse pressure (PP) adjusted for FS as follows: DBP+FS·PP (Moran et al., 1995; Rogers and Oosthuyuse, 2000).
However, to the best of our knowledge, none has to date investigated on the difference between the SF and the CF during exercise recovery. This study was devised to compare the SF and CF in the estimation of MBP after bouts of exercise at different intensities. Given the supposed hypotensive effect and its potential role in the treatment of hypertension, this investigation would be useful in a clinical perspective in order to correctly verify and quantify this hypotensive effect.
Methods
Subjects
Ten healthy males, whose mean ± standard deviation (SD) of age, height, and mass were 29.6 ± 5.7 years, 1.73 ± 0.04 m, and 72.6 ± 8.2 kg respectively, volunteered to take part in this study. All volunteers gave written informed consent to participate in the present investigation, which was performed according to the declaration of Helsinki and approved by the local ethics committee. All subjects were physically active who trained 4 to 6 hours per week and were free of any known cardiovascular or pulmonary disease. None of them reported suffering from post-exercise intolerance or hypotension and none was assuming any kind of medication.
Experimental design
Before entering the study, each subject underwent a preliminary incremental test on an electromagnetically-braked cycle-ergometer (CUSTO Med, Ottobrunn, Germany) to assess the workload at anaerobic threshold (Wat) and the maximum workload achievable (Wmax). This test consisted of a linear increase of work load of 20W/min, starting from 20W, at a pedalling frequency of 60 rpm, up to volitional exhaustion (i.e. the point at which the subject was unable to maintain a pedalling rate of at least 50 rpm). Oxygen uptake (VO2) and carbon dioxide output (VCO2) were measured throughout this preliminary test and Wat was determined by using the V-slope method (Beaver et al., 1986). The mean ± SD values of maximum HR and Wmax reached were 195 ± 4 bpm and 248.4 ± 45.3 W respectively, while maximum values of VO2 and VCO2 were 3.13 ± 0.27 and 4.18 ± 0.31 l·min-1 respectively. Wat occurred at 179.6 ± 24.7 W (i.e. 72.2% of Wmax).
In separate days from this preliminary test (the interval was at least 3 days), each subject underwent the following study protocol, randomly assigned to eliminate any order effect:
Recovery after exercise performed above Wat (130%Wat test): this test consisted of a period of three minutes of rest in the upright position seated on the cycle-ergometer, in order to obtain baseline data; a warm-up of three minutes pedalling at 60 rpm against a resistance of 40W; this period was then followed by exercise, performed against a resistance equivalent to 130% of Wat for 10 minutes or till exhaustion, i.e. the point at which the subject was unable to maintain a pedalling rate of at least 50 rpm. The mean power output and duration of the 130%Wat test were 233.5 ± 52.3 W and 264.4 ± 142.4 s respectively. After the 130%Wmax test, recovery was studied for 30 minutes: three minutes were active recovery pedalling at 60 rpm against 40W, followed by 27 minutes of passive recovery during which the subject remained on the bicycle without moving the legs.
Recovery after exercise performed below Wat (70%Wat test): the same rest-warm-up-exercise-recovery protocol employed in the 130%Wat test was used, but the exercise was performed against a resistance equivalent to 70% of Wat and it always lasted 10 minutes. The mean power output of the 70%Wmax test was 124.5 ± 27.9 W.
A similar protocol was already used to study differences in cardiovascular response after tasks of various intensities (Crisafulli et al., 2006a). In particular, we chose these two exercise intensities to have mild-moderate and moderate-heavy effort intensities. All experiments were conducted between 9 A.M. and 2 P.M. in a temperature-controlled room (room temperature set at 22°C and relative humidity 50%) and were spaced from each other by at least three days. Subjects had a light meal at least two hours before exercising.
Hemodynamic measurements
Hemodynamic parameters during the recovery sessions of the 130%Wat and the 70%Wat tests were collected by means of an impedance cardiograph (NCCOM 3, BoMed Inc., Irvine, CA), a device which allows continuous non-invasive cardiodynamic measuring during rest, exercise, and recovery. This method has been employed in similar studies dealing with hemodynamics during exercise and recovery (Crisafulli et al., 2000; 2003a; 2004; 2006a; 2006b; 2007; Kilgour et al., 1995; Miles et al., 1984; Moore et al., 1992; Richard et al., 2001; Takahashi and Miyamoto, 1998). This technique assumes that, when an electrical current circulates through the thorax, the pulsatile aortic blood flow induces a proportional fluctuation in electrical conductivity. Therefore, changes in thoracic electrical impedance during systole are representative of stroke volume (SV) (Bernestein, 1986). Our group has previously used the impedance method in similar experimental setting in order to evaluate hemodynamics during recovery following submaximal, maximal, and supramaximal exercise and detailed descriptions of its rationale and application can be found in these reports (Crisafulli et al., 2003b; 2004; 2006a; 2007; 2011). Briefly, NCCOM 3 was connected to the subject by arranging eight commercially available electrodes: two pairs were thoracic and cervical sensing electrodes, while two other pairs were sensing electrodes placed above the cervical and below the thoracic pairs. By means of a digital chart recorder (ADInstruments, PowerLab 8sp, Castle Hill, Australia) NCCOM 3-derived analog traces of electrocardiogram, thorax impedance (Z0), and Z0 first derivative (dZ/dt) were stored and offline cleaned from signals affected by movement and respiratory artifacts. Traces were then analysed offline, paying particular attention to deriving hemodynamic variables only from traces not affected by impedance artifacts. This signal processing procedure has been previously employed and, although time-consuming, it allows the obtaining of reliable and reproducible cardiodynamic data estimation during various kind of exercise and recovery (Crisafulli et al., 2003a; 2003b; 2004; 2006a; 2006b; 2007; 2011). Stroke volume was assessed by using the Sramek-Bernstein equation (Bernestein et al., 1986):
| SV= (VEPT·Z0-1)·dZ/dtmax·VET |
where VEPT is the volume of electrical participating tissue and was derived using a nomogram from sex, height, and weight of the subject; Z0 was the thorax impedance measured at the end of cardiac diastole; VET was the left ventricular ejection time, measured as the interval between the beginning and the minimum of the deflection in dZ/dt trace during systole (Crisafulli et al., 2000; Crisafulli et al., 2001). HR was calculated as the reciprocal of the electrocardiogram R-R interval and cardiac output (CO) was obtained by multiplying SV·HR. Also measured were the pre-ejection period (PEP), identified as the time interval between the electrocardiogram Q wave and the beginning of the dZ/dt deflection during systole (Crisafulli et al., 2001), and diastolic time (DT), calculated subtracting the ejection period (ET, i.e. the sum of PEP and VET) from the total cardiac cycle period, assessed as 60/HR (Crisafulli et al., 2000; Marongiu et al., 2013).
Subjects were also connected to a standard manual sphygmomanometer for SBP and DBP assessment, which was made in the left arm by the same physician throughout all protocol sessions. In order to calculate MBP, both the SF (MBPSF) and the CF (MBPCF) formulas previously described were utilised. Further assessed were systemic vascular resistance (SVR), obtained by multiplying the MBP/CO ratio by 80, where 80 is a conversion factor to change units to standard resistance units. Thus, two measures of SVR were obtained: SVRSF, calculated as MBPSF/CO, and SVRCF, calculated as MBPCF/CO.
Data analysis
Descriptive statistics were performed on each variable to confirm the assumption of normality by means of the Kolmogorov-Smirnov test. The alpha level was set at P<0.05. Difference in absolute hemodynamic values at the third minute of rest preceding the two exercise tests were found out by the paired t test. During post-exercise, cardiovascular parameters were averaged over one minute for the first 5 minutes of recovery (Crisafulli et al., 2003b; 2004; 2006a), and over 5 minutes for the remaining period (i.e. 25 minutes) of recovery. For statistical analysis during recovery, changes from rest values were considered. Comparisons in cardiovascular responses during the recovery phases of the two tests were performed using the two-way ANOVA (factors: intensity of exercise and time) followed by Tukey post-hoc if appropriate. The same statistics were applied to discover differences between MBPSF and MBPCF and between SVRSF and SVRCF during the recovery periods from each test (factors: mode of calculation of the variable and time). Moreover, Bland and Altman statistics (Bland and Altman, 1986) to assess agreement between two methods of measurement were carried out in order to evaluate agreement between the two modes to calculate MBP and SVR. Finally, a least-squares regression line was fitted to the mean HR versus the differences in MBP and in SVR provided by the two methods of MBP calculation. Correlation was examined using the Pearson correlation coefficient. Statistics were carried out employing commercially available software (Graph-Pad Prism). Significance was set at a P value of <0.05 in all cases.
Results
All subjects completed the study protocol and none of them experienced symptoms of hypotension after the efforts. The Kolmogorov-Smirnov test confirmed that data were normally distributed, and as such deemed appropriate for parametric analysis. Data at rest did not show any significant difference between the two protocol sessions (Table 1).
Table 1.
Mean (±SD) values of hemodynamic data during the rest periods of the 130%Wat and 70% Wat tests respectively.
| Variable | 70% Wat | 130% Wat |
|---|---|---|
| HR (bpm) | 78(7) | 79(8) |
| SV (ml) | 67.5 (6.9) | 66 (7.9) |
| CO (L·min-1) | 5.2 (.5) | 5.1 (.6) |
| DT (s) | .38 (.07) | .39 (.08) |
| SBP (mmHG) | 119.5 (10.1) | 116.0 (5.1) |
| DBP (mmHg) | 75.5 (4.9) | 75.5 (5.9) |
| MBPSF (mmHg) | 90.1 (6.4) | 89 (5.7) |
| MBPCF (mmHg) | 89.5 (6.3) | 87.8 (5.5) |
| SVRSF (dynes·s-1·cm-5) | 1380.1 (343.7) | 1398.3 (410.1) |
| SVRCF (dynes·s-1·cm-5) | 1377.9 (343.7) | 1378.2 (388.4) |
Figure 1 shows time courses of HR (panel A), SV (panel B), and CO (panel C) during the 30 min period of recovery after the two tests. Heart rate was increased with respect to baseline during the first minute after the two exercise sessions, with the highest increment reached after the 130%Wat. Then, HR returned gradually to rest level, without showing any significant difference between the 130%Wat and the 70%Wat test. This parameter remained higher than rest till the 25th minute after the 130%Wat test, while it returned to baseline at 20th minute of recovery after the 70%Wat test. SV (panel B) was increased as compared to baseline by both tests during the first two minutes of recovery. Afterward, SV decreased to baseline without any difference between tests. CO (panel C) was increased by the exercise bouts without any difference due to effort intensity. However, the increment in CO lasted longer after the 130%Wat than after the 70%Wat test (i.e. till the 15th and the 5th minute of recovery respectively).
Figure 1.
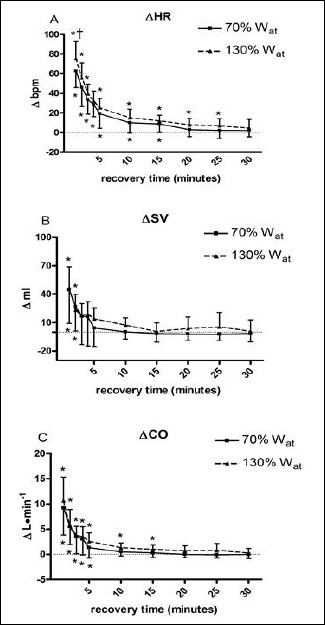
Changes from baseline in heart rate (ΔHR, panel A), stroke volume (ΔSV, panel B), and cardiac output (ΔCO, panel C) during 30 recovery minutes following the 130%Wat and the 70%Wat tests. A horizontal dotted line represents the pre-exercise level. Values are mean ± SD. * = p<0.05 vs. pre-exercise; † = p<0.05 vs. corresponding time point of 70%Wat.
Figure 2 exhibits the behaviour of DT (panel A) and ET (panel B). In detail, both DT and ET showed an almost identical time course: they decreased with respect to baseline after the two exercise bouts, with the longer decrement after the 130%Wat test (till the 25th minute of recovery) in comparison with the 70%Wat test (till the 15th minute of recovery). In addition, Figure 2 shows the DT and ET variations, expressed as percent of the total cardiac cycle duration (panels C and D for recovery after the 70%Wat and 130%Wat respectively). From panels, it can be gleaned that ET duration accounted to about 75% of the cardiac cycle immediately after the two exercise bouts, while the contribution of DT was reduced to about 25%. During the recovery period both DT and ET gradually approached the 50% of the cardiac cycle time. However, after the 130%Wat test, ET remained higher and DT lower as compared to the 70%Wat test.
Figure 2.
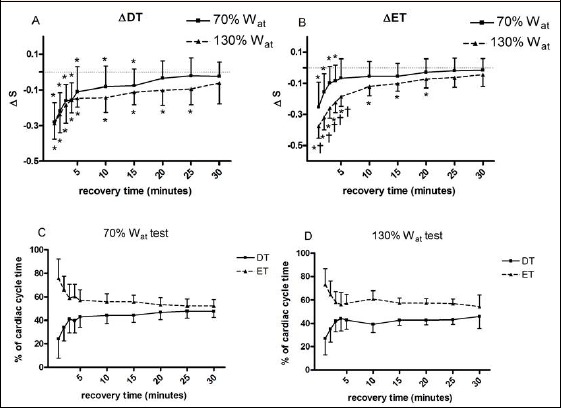
Changes from baseline in diastolic time (ΔDT, panel A) and ejection time (ΔET, panel B) during 30 recovery minutes following the 130%Wat and the 70%Wat tests. A horizontal dotted line represents the pre-exercise level. Panel C and D represent the percent of the total cardiac cycle duration of DT and ET. Values are mean ± SD. * = p < 0.05 vs. pre-exercise; † = p < 0.05 vs. corresponding time point of 70%Wat.
Panel A of Figure 3 illustrates SBP time course. There was no difference in SBP values between the 130%Wat and the 70%Wat test, even though this variable rose with respect to baseline during the first four minutes of recovery after the 130%Wat test, while this increment was present only till the second minute of recovery following the 70%Wat test. Statistics did not highlight any variation due to exercise or time in DBP (panel B), although after the 70%Wat test this parameter appeared to decrease. Figure 3 also depict MBP time course calculated by the standard formula (panel C) and the corrected formula (panel D). In both cases MBP was increased by exercise bouts with respect to baseline. However, this increment was more evident if the corrected formula was applied. In detail, an increment in the order of 25 mmHg with respect to baseline was detected at the first minute of recovery for MBPCF, while this increment was only of about 10 mmHg for the MBPSF. Moreover, the MBP increment lasted longer for MBPCF than for MBPSF. When the two MBP measures were directly compared (panels E and F), statistics discovered that the SF systematically underestimated this variable in the two first minutes of recovery as compared to the CF, irrespectively from the exercise workload.
Figure 3.
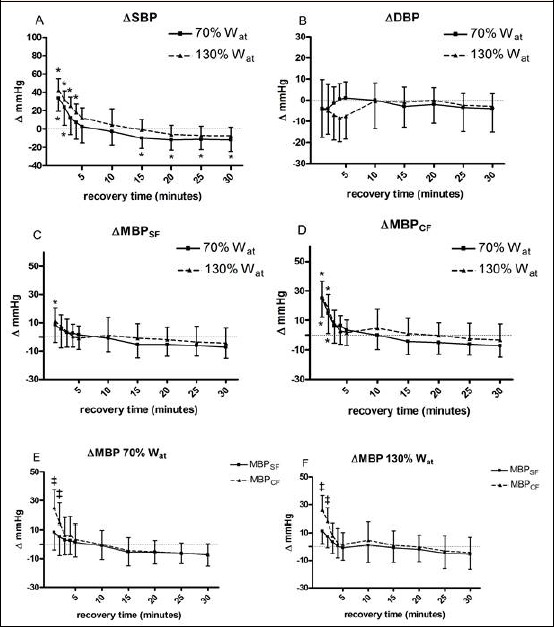
Changes from baseline in systolic blood pressure (ΔSBP, panel A), diastolic blood pressure (ΔDBP, panel B), mean blood pressure calculated with the standard formula (ΔMBPSF, panel C), and mean blood pressure calculated with the corrected formula (ΔMBPCF, panel D) during 30 recovery minutes following the 130%Wat and the 70%Wat tests. A horizontal dotted line represents the pre-exercise level. Panels E and F illustrate the comparison of the two formulas applied at the same workload. Values are mean ± SD. * = p < 0.05 vs. pre-exercise; ‡ = p < 0.05 vs. MBPSF.
Resulting from the difference in MBP calculation, the reduction in SVR was found more pronounced when the SF was employed in comparison with the CF (Figure 4, panel A and B respectively). This fact became more evident when results from the two formulas were directlycompared (panels C and D for the 70%Wat and the 30%Wat respectively). In detail, immediately after exercise bouts (i.e. in the first two minutes) SVR was underestimated by the SF.
Figure 4.
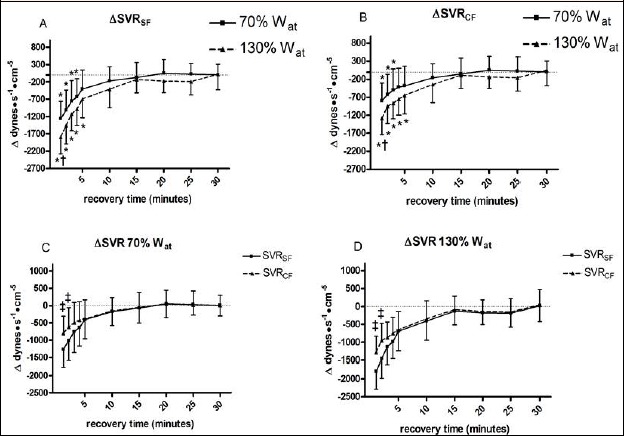
Changes from baseline in systemic vascular resistance calculated with the standard formula (ΔSVRSF, panel A) and systemic vascular resistance calculated with the corrected formula (ΔSVRCF, panel B) during 30 recovery minutes following the 130%Wat and the 70%Wat tests. A horizontal dotted line represents the pre-exercise level. Panels C and D illustrate the comparison of the two formulas applied at the same workload. Values are mean ± SD. * = p < 0.05 vs. pre-exercise; † = p < 0.05 vs. corresponding time point of 70%Wat; ‡ = p < 0.05 vs. SVRSF.
Upper panels of Figure 5 show results from the Bland-Altman analysis. Panel A demonstrates that the difference between MBPSF and MBPCF was on average –4.1 mmHg, with limits of agreement between +10.8 and –19.2 mmHg. Panel B shows that the mean difference between SVRSF and SVRCF was –34.9 dynes·s-1·cm-5, with limits of agreement between +80.8 and –151 dynes·s-1·cm-5. Finally, panels C and D illustrate results from the linear regression analysis. The differences in MBP (panel C) and SVR (panel D) between values yielded by the two calculation methods were significantly and inversely related to HR, with the more pronounced difference found when HR was at its highest level.
Figure 5.
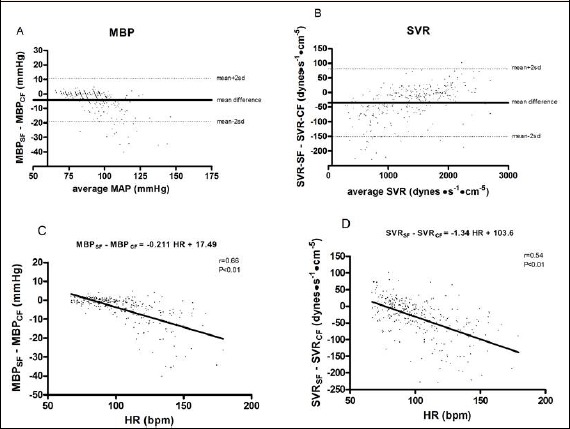
Bland and Altman plots of the difference between mean blood pressure (MBP, panel A) and systemic vascular resistance (SVR, panel B) calculated with the standard and the corrected formulas. Panels C and D show results of the linear regression statistics applied to HR versus the differences in MBP and in SVR provided by the two methods of MBP calculation.
Discussion
The main finding of the present study is that the SF, often employed to calculate mean blood pressure during the recovery from dynamic exercise (Coats et al., 1989; Crisafulli et al., 2003b; de Almeida et al., 2010; Guidry et al., 2006; Kilgour et al., 1995; Nottin et al., 2002; Raine et al., 2001), potentially induces a substantial error in MBP estimation in the period immediately following exercise. Indeed, MBPSF was lower in comparison with MBPCF during the first two minutes of recovery. This difference was in the order of 15-20 mmHg and it was unrelated with the exercise intensity, as demonstrated by the fact that there was no difference in MBP level between the 130%Wat and the 70%Wat tests. However, after the first few minutes of recovery, the two methods to calculate MBP yielded similar blood pressure estimation, thereby suggesting that they can be used interchangeably. This result seems to indicate that only when substantial tachycardia occurs there is the necessity to take into account the fact that DT shortens more than ET in the cardiac cycle.
In the present investigation, during the first two minutes of recovery ET was about 70-75% of the cardiac cycle total duration (see Figure 2, panel C and D). In this situation HR was on average in the order of 140-150 bpm. Only when ET was at this level MBP was systematically underestimated by the SF. Then, the contribution of ET in the total cardiac cycle duration gradually decreased to a level close to 50% and the two equations produced similar MBP results. Likewise MBP, SVR were lower if calculated with the SF as compared to the CF in the first minutes immediately after exercise bouts, when ET was at its highest level.
The fact that the higher the HR the wider the difference between methods for MBP calculation also appears looking at results of the linear regression graph (Figure 5, panel C), where differences between the two methods for MBP calculation were plotted with HR.
The Bland-Altman plot reveals that on average the SF underestimated MBP by 4.1 mmHg in comparison with the CF, with limits of agreements between methods laying between +10.8 and – 19.1 mmHg. Furthermore, from the Bland and Altman plot it can further gleaned that the higher the MBP the larger the difference between the two methods, thereby reinforcing what previously described and strengthening the concept that results from the SF should be taken with caution in the presence of high blood pressure levels, as immediately after exercise.
As concerns central hemodynamics, the behaviours of SV and CO found in the present investigation were similar to what reported in previous studies dealing with recovery from exercise at various workloads (Crisafulli et al., 2003b; 2004; 2006a). In detail, it is of note that SV did not decrease with respect to baseline after exercise. This fact is in agreement with the concept that SV is generally well maintained despite the reduction in central venous pressure and cardiac pre-load which normally takes place after exercise when subjects are seated upright (Crisafulli et al., 2003b; 2004; 2006a; Halliwill et al., 2000; 2013; 2014; Kilgour et al., 1995). This phenomenon suggests that mechanisms controlling the cardiovascular apparatus can successfully manage to maintain SV in the face of an impaired pre-load and thanks to the modulation of cardiac inotropism (Crisafulli et al., 2003b; 2004; 2006a; Kilgour et al., 1995). It should be also considered that the reduction in afterload could probably take part in this phenomenon. Another aspect deserving attention is that, while there was no difference in the DT values between the 130%Wat and the 70%Wat tests, during the first five minutes of recovery ET following the 130%Wat was lower than following the 70%Wat test. This fact is probably to be ascribed to a more pronounced enhancement in cardiac contractility after the 130%Wat test which shortened ET more in this setting in comparison with the 70%Wat test. Actually, it is well known that ET is sensible to enhancement in contractility, which shortens this time interval when cardiac pre-load and after-load are kept constant (Crisafulli et al., 2001; Lewis et al., 1977).
Finally, it should be underscored that present data do not highlight any MBP drop after exercise bouts, independently from the equation employed for its calculation. Rather, this parameter was increased immediately after the two exercise tests, to return close to baseline already during the first recovery period. Thus, present findings suggest that exercise bouts did not lead to any significant hypotensive effect in our study population, i.e. physically active healthy males. Nevertheless, this parameter showed the tendency to slightly and gradually decrease (although without any statistical significance) with respect to pre-exercise level throughout recovery from both exercise tests. This apparent reduction in blood pressure was mainly the effect of the SBP behaviour, which tended to decrease with respect to baseline throughout recovery, reaching significance after the 70%Wat test, whereas DBP appeared to be well maintained. Hence, it is conceivable to hypothesise that the hypotensive effect (i.e. MBP reduction) would have been detected if the period of observation was longer. Moreover, the number of subjects enrolled was not large enough to have a sufficient statistical power to discover a blood pressure reduction in the order of 5-10 mmHg, a quantity often reported in the scientific literature dealing with the potential hypotensive effect of exercise (Eicher et al., 2010; Guidry et al., 2006; MacDonald et al., 1999; Piepoli et al., 1993; Raine et al., 2001). However, the present study was not set to investigate on the potential effect of exercise on lowering blood pressure. Rather, our target was to investigate on the difference between two methods of blood pressure calculation. Nevertheless, it should be mentioned that some authors failed to detect any drop in blood pressure below pre-exercise level during recovery after exercise performed at various intensities ranging from submaximal to supramaximal workloads (Carter et al., 1999; Crisafulli et al., 2003b; 2004; 2006a; Johnson et al., 1990; Kilgour et al., 1995; Nottin et al., 2002). Further research is warranted to better clarify this point.
It is also possible that in our investigation we did not observe any hypotensive response because our subjects were healthy and physically active persons who were accustomed to strain and had developed specific adaptations to cardiovascular recovery. This concept is in accordance with reports demonstrating that single or repeated bouts of supramaximal exercise are well tolerated by a population specifically trained for this kind of effort (Crisafulli et al., 2004; 2006a). It is also possible that in our setting the exercise duration was too short to sufficiently stress the cardiovascular system.
As previously stated, our finding of a significant difference in MBP between the SF and the CF during the recovery that immediately followed exercise was most probably to be ascribed to the exercise-induced tachycardia, which shortened diastolic more than systolic time. Hence, a study that systematically compares MBP during exercise employing the two methods is warranted. Actually, given that during exercise the degree of tachycardia is even higher than during recovery, the application of the CF during exercise may be much more important.
Limitations of the study
So far, the Fick and the dye-dilution methods have been considered the “gold standard” for hemodynamic assessment at rest, during exercise and recovery (Warburton et al., 1999a). However, both techniques are invasive and potentially dangerous, so that they are not suitable for hemodynamic investigations involving healthy subjects who do not need invasive procedures for clinical purposes. Among non-invasive methods the choice is restricted to re-breathing, Doppler echocardiography, pulsed contour analysis, and impedance cardiography, but none of them has unanimously been considered accurate and reproducible yet. For instance, hemodynamics estimated by the re-breathing method requires the steady-state condition and is affected by errors arising from the difficulty in quantifying the venous-arterial difference in CO2 blood contents. This fact limits its usefulness during maximal exercise (Warburton et al., 1999a). On its side, hemodynamic measurements using Doppler Echocardiography is not very easily performed and requires a lot of practice and skilled operators (Warburton et al., 1999b). Further, this method tends to underestimate CO at maximal exercise workloads compared with invasive measures (Warburton et al., 1999b). In the present study we used the impedance method, which likewise the others, suffers from some limitations for exercise application (Warburton et al., 1999b). Probably, the major source of errors in hemodynamic assessment with this technique is that heavy efforts affect impedance traces by generating artefacts due to legs and chest movements. To avoid this problem we have developed a method of impedance traces processing which is shown in detail in previous papers from our Laboratory (Crisafulli et al., 2000; 2003b; 2004). Briefly, visual inspection of recorded signals was performed by a skilled physician, so as to calculate hemodynamic parameters only in the traces in which at least 20% of impedance waveforms were reliable. This method is time-consuming in processing signals, but the results during rest, exercise, and recovery have been demonstrated to be reliable and highly reproducible (Crisafulli et al. 2000, 2003b; 2004). Thus, in our opinion the impedance method can be utilised in study such as the present one paying particular attention to avoid/eliminate artefacts from impedance traces.
Another potential limitation of the present paper is that arterial blood pressure was measured manually by using a standard manual sphygmomanometer. This technique may involve a systemic error compared to automatic devices as it suffers from deficiencies due to poor observer technique, and problems due to poor maintenance of the devices (Pickering, 2003). However, manual manometer is still accepted as the “gold standard” for routine clinical measurement as there is no generally accepted alternative. The most widely advocated candidate are oscillometric devices, which have the advantages of eliminating observer error and mechanical drift. However, the oscillometric method suffers from inherent limitations too, and it cannot become the “gold standard” for clinical measurement (Pickering, 2003). Thus, the standard manual sphygmomanometry is still the “gold standard”, providing that attention is paid in its use and employing a standardised approach with trained operators (Sharman and LaGerche, 2015).
Conclusion
In conclusion, the present investigation conducted on healthy, physically active subjects demonstrates that the standard formula, often employed to calculate MBP during the recovery from dynamic exercise, may introduce a substantial error in the MBP estimation in the period immediately following the effort. This equation should not be used in this situation. Instead, the corrected formula, which calculates MBP taking into account the proportion between DBP and SBP during the cardiac cycle, should be employed. A further finding was that, in our population, no significant hypotensive effect was induced by exercise, even though a slight reduction in SBP was detected.
Acknowledgements
This study was supported by the University of Cagliari and the Italian Ministry of Scientific Research. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Biographies

Gianmarco SAINAS
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD
Research interests
Exercise, cardiovascular regulation, training
E-mail: gianmarcosainas@gmail.com

Raffaele MILIA
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD, PhD
Research interests
Exercise, blood pressure, cardiovascular regulation, sport medicine
E-mail: miliaraffaele@gmail.com

Girolamo PALAZZOLO
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
PhD
Research interests
Exercise, cardiovascular regulation, physical training
E-mail: girolamo.palazzolo@gmail.com
Gianfranco IBBA
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
PhD
Research interests
Exercise, physical training, strength training
E-mail: gianfranco.ibba@hotmail.it
Elisabetta MARONGIU
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD
Research interests
Exercise, sport medicine, cardiovascular regulation
E-mail: elisamar84@gmail.com

Silvana ROBERTO
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD
Research interests
Exercise, sport medicine, cardiovascular regulation
E-mail: silvy_rob@yahoo.it
Virginia PINNA
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD
Research interests
Exercise, sport medicine, cardiovascular regulation
E-mail: virginia.pinna@gmail.com

Giovanna GHIANI
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
PhD
Research interests
Exercise, physical training, diet, cardiovascular reflexes
E-mail: giovanna.ghiani@tiscali.it

Filippo TOCCO
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD, PhD
Research interests
Exercise, physical training, diet, cardiovascular reflexes
E-mail: filippo.tocco@tiscali.it

Antonio CRISAFULLI
Employment
Department of Medical Sciences, School of Sport Medicine, Sport Physiology Lab., University of Cagliari, Italy
Degree
MD, PhD
Research interests
Exercise, cardiovascular regulation, blood pressure
E-mail: crisafulli@tiscali.it
References
- Beaver W.L., Wasserman K., Whipp B.J. (1986) A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology 60, 2020-2027. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P. (1986) A new stroke volume equation for Thoracic Electrical Bioimpedance: Theory and rationale. Critical Care Medicine 14, 904-909. [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.J. (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307-310. [PubMed] [Google Scholar]
- Carter R., III, Watenpaugh D.E., Wasmund W.L., Wasmund S.L., Smith M.L. (1999) Muscle pump and central command during recovery from exercise in humans. Journal of Applied Physiology 87, 1463-1469. [DOI] [PubMed] [Google Scholar]
- Coats A.J.S., Conway J. Isea J.E. Pannarale G. Sleight P. Somers V.K. (1989) Systemic and forearm vascular resistance changes after upright bicycle exercise in man. Journal of Physiology 413, 289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli A., Melis F., Orrù V., Lener R., Lai C., Concu A. (2000) Hemodynamics during a postexertional asystolia in a healthy athlete: a case study. Medicine and Science in Sports & Exercise 32, 4-9. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Melis F., Orrù V., Lener R., Lai C., Concu A. (2001) Impedance cardiography for non invasive assessment of systolic time intervals during exercise. Sports Medicine Training and Rehabilitation 10, 13-27. [Google Scholar]
- Crisafulli A., Melis F., Lai A.C., Orrù V., Lai C., Concu A. (2002) Haemodynamics during a complete exercise-induced atrioventricular block. British Journal of Sports Medicine 36, 69-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli A., Scott A.C., Wensel R., Davos C.H., Francis D.P., Pagliaro P., Coats A.J.S., Concu A., Piepoli M.F. (2003a) Muscle metaboreflex-induced increases in stroke volume. Medicine and Science in Sports & Exercise 35, 221-228. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Orrù V., Melis F., Tocco F., Concu A. (2003b) Hemodynamics during active and passive recovery from a single bout of supramaximal exercise. European Journal of Applied Physiologt 89, 209-216. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Carta C., Melis F., Tocco F., Frongia F., Santoboni U.M., Pagliaro P., Concu A. (2004) Hemodynamic responses following intermittent supramaximal exercise in athletes. Experimental Physiology 89, 665-674. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Melis F., Tocco F., Santoboni U.M., Frongia F., Carta C., Caddeo M., Concu A. (2005) Anaerobic Threshold and the oxygen consumption/cardiac output relationship during exercise. Sport Science For Health 2, 75-80. [Google Scholar]
- Crisafulli A., Tocco F., Pittau G., Lorrai L., Porru C., Salis E., Pagliaro P., Melis F., Concu A. (2006a) Effect of differences in post-exercise lactate accumulation in athletes’ hemodynamics. Applied Physiology Nutrition and Metabolism 31: 423-431. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Tocco F., Pittau G., Caria M., Lorrai L., Melis F., Concu A. (2006b) Detection of lactate threshold by including haemodynamic and oxygen extraction data. Physiological Measurements 27, 85-97. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Piras F., Chiappori P., Vitelli S., Caria M.A., Lobina A., Milia R., Tocco F., Concu A., Melis F. (2007) Estimating stroke volume from oxygen pulse during exercise. Physiological Measurments 28, 1201-1212. [DOI] [PubMed] [Google Scholar]
- Crisafulli A., Piras F., Filippi M., Piredda C., Chiappori P., Melis F., Milia R., Tocco F., Concu A. (2011) Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. Journal of Physiological Science 61, 385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida W.S., de Jesus Lima L.C., da Cunha R.R., Simões H.G., Nakamura F.Y., Grubert F., Campbell C.S. (2010) Post-exercise blood pressure responses to cycle and arm-cranking. Science in Sports 25, 74-80. [Google Scholar]
- Eicher J.D., Maresh C.M., Tsongalis G.J., Thompson P.D., Pescatello L.S. (2010) The additive blood pressure lowering effects of exercise intensity on post-exercise hypotension. American Heart Journal 160, 513-520. [DOI] [PubMed] [Google Scholar]
- Fleg J.L., Lakatta E.G. (1986) Prevalence and significance of postexercise hypotension in apparently healthy subjects. American Journal of Cardiology 57, 1380-1384. [DOI] [PubMed] [Google Scholar]
- Guidry M.A., Blanchard B.E., Thompson P.D., Maresh C.M., Seip R.L., Taylor A.L., Pescatello L.S. (2006) The influence of short and long duration on the blood pressure response to an acute bout of dynamic exercise. American Heart Journal 151, e5-12. [DOI] [PubMed] [Google Scholar]
- Halliwill J.R., Minson C.T., Joyner M.J. (2000) Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. Journal of Applied Physiology 89, 1830-1836. [DOI] [PubMed] [Google Scholar]
- Halliwill J.R., Buck T.M., Lacewell A.N., Romero S.A. (2013) Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Experimental Physiology 98, 7-18. [DOI] [PubMed] [Google Scholar]
- Halliwill J.R., Sieck D.C., Romero S.A., Buck T.M., Ely M.R. (2014) Blood pressure regulation X: what happens when the muscle pump is lost? Post-exercise hypotension and syncope. European Journal of Applied Physiology 114, 561-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham M.B., Morris K.G., Williams R.S., McHale P.A., Coleman R.E., Cobb F.R. (1986) Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circulation Research 58, 281-291. [DOI] [PubMed] [Google Scholar]
- Kenney M.J., Seals D.R.Postexercise hypotension. (1993) Key features, mechanism, and clinical significance. Hypertension 22, 653-664. [DOI] [PubMed] [Google Scholar]
- Kilgour R.D., Mansi J.A., Williams P.A. (1995) Cardiodynamic responses during seated and supine recovery from supramaximal exercise. Canadian Journal of Applied Physiology 20, 52-64. [DOI] [PubMed] [Google Scholar]
- Johnson E.C., Hudson T.L., Greene E.R. (1990) Left ventricular hemodynamics during exercise recovery. Journal of Applied Physiology 69, 104-111. [DOI] [PubMed] [Google Scholar]
- Lewis R.P., Rittgers S.E., Forester W.F., Boudoulas H. (1977) A critical review of the systolic time intervals. Circulation 56, 146-158. [DOI] [PubMed] [Google Scholar]
- Lewis S.F., Taylor W.F., Graham R.M., Pettinger W.A., Schutte J.E., Blomqvist C.G. (1983) Cardiovascular responses to exercise as functions of absolute and relative work load. Journal of Applied Physiology 54, 1314-1323. [DOI] [PubMed] [Google Scholar]
- MacDonald J., Mac Dougall J., Hogben C. (1999) The effects of exercise intensity on post exercise hypotension. Journal of Human Hypertension 13, 527-531. [DOI] [PubMed] [Google Scholar]
- MacDonald J., Mac Dougall J., Hogben C. (2000) The effects of exercise duration on post exercise hypotension. Journal of Human Hypertension 14, 125-129. [DOI] [PubMed] [Google Scholar]
- Marongiu E., Piepoli M., Milia R., Angius L., Pinna M., Bassareo P., Roberto S., Tocco F., Concu A., Crisafulli A. (2013) Effects of acute vasodilation on the hemodynamic response to muscle metaboreflex. American Journal of Physiology (heart and circulatory physiology) 305, H1387-1396. [DOI] [PubMed] [Google Scholar]
- Miles D.S., Sawka M.N., Hanpeter D.E., Foster J.E., Doerr B.M., Basset Frey M.A., (1984) Central hemodynamics during progressive upper and lower body exercise and recovery. Journal of Applied Physiology 57, 366-370. [DOI] [PubMed] [Google Scholar]
- Moore R., Sansores R., Guimond V., Abboud R. (1992) Evaluation of cardiac output by thoracic electrical bioimpedance during exercise in normal subjects. Chest 102, 448-455. [DOI] [PubMed] [Google Scholar]
- Moran D., Epstein Y., Keren G., Laor A., Sherez J., Shapiro Y. (1995) Calculation of mean arterial pressure during exercise as a function of heart rate. Applied Human Science 14, 293-295. [DOI] [PubMed] [Google Scholar]
- Nottin S., Vinet A., Mandigout S., Nguyen L., Stecken F., Ounissi F., Lecoq A., Obert P. (2002) Left ventricular dynamics during early recovery from maximal exercise in boys and men. Medicine and Science in Sports & Exercise 34, 1951-1957. [DOI] [PubMed] [Google Scholar]
- Piepoli M., Coats A.J.S., Adamopoulos S., Bernardi L., Feng Y.H., Conway J., Sleight P. (1993) Persistent peripheral vasodilatation and sympathetic activity in hypotension after maximal exercise. Journal of Applied Physiology 75, 1807-1814. [DOI] [PubMed] [Google Scholar]
- Piepoli M., Coats A.J.S. (1994) Postexercise hypotension. Hypertension 23, 677-678. [DOI] [PubMed] [Google Scholar]
- Raine N.M., Cable T., George K.P., Campbell I.G. (2001) The influence of recovery posture on post-exercise hypotension in normotensive men. Medicine and Science in Sports & Exercise 33, 404-412. [DOI] [PubMed] [Google Scholar]
- Pickering T.G. (2003). What will replace the mercury sphygmomanometer? Blood Press Monitor 8, 23-5. [DOI] [PubMed] [Google Scholar]
- Richard R., Lonsdorfer-Wolf E., Charloux A., Doutreleau S., Buchheit M., Oswald-Mammosser M., Lampert E., Mettauer B., Geny B., Lonsdorfer J. (2001) Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. European Journal of Applied Physiology 85, 202-207. [DOI] [PubMed] [Google Scholar]
- Rogers G., Oosthuyuse T. (2000) A comparison of the indirect estimate of mean arterial pressure calculated by the conventional equation and calculated to compensate for a change in heart rate. International Journal of Sports Medicine 21, 90-95. [DOI] [PubMed] [Google Scholar]
- Sharman J.E., LaGerche A. (2015) Exercise blood pressure: clinical relevance and correct measurement. Journal of Human Hypertension 29, 351-358. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Miyamoto Y. (1998) Influence of light physical activity on cardiac responses during recovery from exercise in humans. European Journal of Applied Physiology 77, 305-311. [DOI] [PubMed] [Google Scholar]
- Warburton D.E., Haykowsky M.J., Quinney H.A., Humen D.P., Teo K.K. (1999a) Reliability and validity of measures of cardiac output during incremental to maximal aerobic exercise. Part I: Conventional techniques. Sports Medicine 27, 23-41. [DOI] [PubMed] [Google Scholar]
- Warburton D.E., Haykowsky M.J., Quinney H.A., Humen D.P., Teo K.K. (1999b) Reliability and validity of measures of cardiac output during incremental to maximal aerobic exercise. Part II: Novel techniques and new advances. Sports Medicine 27, 241-260. [DOI] [PubMed] [Google Scholar]


