Abstract
The central nervous system plays a crucial role in fatigue during endurance exercise. Branched-chain amino acids (BCAA) could reduce cerebral serotonin synthesis by competing with its precursor tryptophan for crossing the blood brain barrier. Arginine and citrulline could prevent excess hyperammonemia accompanied by BCAA supplementation. This study investigated the combination of BCAA, arginine, and citrulline on endurance performance in two consecutive days. Seven male and three female endurance runners ingested 0.17 g·kg-1 BCAA, 0.05 g·kg-1 arginine and 0.05 g·kg-1 citrulline (AA trial) or placebo (PL trial) in a randomized cross-over design. Each trial contained a 5000 m time trial on the first day, and a 10000 m time trial on the second day. The AA trial had significantly better performance in 5000 m (AA: 1065.7 ± 33.9 s; PL: 1100.5 ± 40.4 s) and 10000 m (AA: 2292.0 ± 211.3 s; PL: 2375.6 ± 244.2 s). The two trials reported similar ratings of perceived exertion. After exercise, the AA trial had significantly lower tryptophan/BCAA ratio, similar NH3, and significantly higher urea concentrations. In conclusion, the supplementation could enhance time-trial performance in two consecutive days in endurance runners, possibly through the inhibition of cerebral serotonin synthesis by BCAA and the prevention of excess hyperammonemia by increased urea genesis.
Key points.
The combined supplementation of BCAA, arginine, and citrulline could enhance performance in 5000 m and 10000 m in 2 consecutive days in competitive runners. The supplementation may be helpful in multi-day competitions.
The supplemented BCAA may alleviate central fatigue, allowing the subjects to run faster at the same degree of perceived exertion.
The hyperammonemia that is usually accompanied with BCAA supplementation may be prevented by arginine and citrulline through increased urea genesis.
Key words: Central fatigue, time trial, neurotransmitter, hyperammonemia, tryptophan
Introduction
The elevated cerebral serotonin (5-hydroxytryptamine) level is one of the mechanisms that contribute to the central nervous system fatigue during exercise (Newsholme and Blomstrand, 2006). Serotonin, a is associated with the feeling of lethargy and tiredness that may contribute to the loss of central drive and motivation (Davis and Bailey, 1997). This hypothesis is supported by several human and animal studies. The cerebral uptake of tryptophan, the precursor for serotonin synthesis, was significantly increased in humans during 3-hr cycling (Blomstrand et al., 2005). In addition, cerebral serotonin synthesis was elevated after treadmill running in rats (Chaouloff, 1997). The running time to exhaustion was significantly decreased after the administration of a serotonergic agonist, while it was significantly improved when given a serotonergic antagonist in rats (Bailey et al., 1993).
The rate of cerebral serotonin synthesis is regulated by the transport of plasma tryptophan across the blood-brain barrier (Sharp et al., 1992). The ability of branched-chain amino acids (BCAA) to compete with tryptophan for crossing the blood brain barrier through the same transporter has provoked the hypothesis that the supplementation of these amino acids could reduce cerebral serotonin synthesis and prevent central fatigue during prolonged exercise (Blomstrand et al., 1997; Fernstrom, 2005). Indeed, the administration of BCAA prevented exercise-induced serotonin release in rat hippocampus (Gomez-Merino et al., 2001). Human studies have also shown that oral supplementation of BCAA could reduce ratings of perceived exertion and mental fatigue in maximal exercise (Blomstrand et al., 1997) and improve cognitive function after a 30-km cross-country race through reduced plasma tryptophan/BCAA ratio (Hassmen et al., 1994). However, except one study undertaken in warm conditions (Mittleman et al., 1998), most studies showed that BCAA supplementation had no effect on endurance performance (Blomstrand et al., 1995; 1997; Struder et al., 1998; van Hall et al., 1995).
One possible explanation for the lack of ergogenic effect of BCAA supplementation is the accompanied excess hyperammonemia resulted from the oxidation of these amino acids (MacLean and Graham, 1993; MacLean et al., 1994, 1996; Meeusen et al., 2006; Struder et al., 1998). It has been shown that cerebral uptake and accumulation of ammonia (NH3) was increased in humans during prolonged exercise (Nybo et al., 2005), which could induce central fatigue by alterations of cerebral energy metabolism and neurotransmission, and signaling pathways within the neuron (Wilkinson et al., 2010). Therefore, we hypothesized that incorporating arginine and citrulline with BCAA could improve endurance exercise performance by alleviating excess NH3 production and reducing plasma tryptophan/BCAA ratio.
Both arginine and citrulline could reduce exercise-related accumulations of NH3 by increasing the urea cycle (Curis et al., 2005; Schaefer et al., 2002) and nitric oxide (NO) biosynthesis (Clarkson et al., 1996; Curis et al., 2005). Citrulline is more potent because of its high bioavailability (Rouge et al., 2007). It has been revealed that citrulline supplementation could increase plasma urea concentration and NO production (Sureda et al., 2010), while suppressing the exercise-induced hyperammonemia (Takeda et al., 2011) in prolonged exercise. Moreover, a combined supplementation of citrulline, arginine, and ornithine reduced plasma ammonia concentration after a single bout of exhaustive exercise in rats (Meneguello et al., 2003).
The purpose of this study was to investigate the combined supplementation of BCAA, arginine, and citrulline on endurance performance in two consecutive days in trained runners. The majority of previous studies investigating the alleviation of central fatigue in endurance exercise used a single bout of exercise. From our previous results, the effect of BCAA and arginine supplementation appears to be effective on the second consecutive day of intermittent high-intensity exercise, when the central fatigue is more apparent, in well-trained athletes (Chang et al., 2015). It is common for endurance athletes to participate in more than one event in a competition. The athletes would race in two or more consecutive days, which would lead to the accumulation of fatigue. However, to our best knowledge, the nutritional strategy to alleviate central fatigue and improve endurance performance in consecutive days has not been investigated. The use of race-like environment in performance measurement would make the results more applicable to real competitions. Each trial contained two consecutive days of exercise, with a 5000 meter (m) time trial on the first day, and a 10000 m time trial on the second day.
Methods
Participants
Thirteen endurance runners (10 male and 3 female) were originally recruited from the track and field team in National Taiwan University of Sport, Taichung, Taiwan. The subjects have trained and competed in events ranging from 1500 m to 10000 m. Three of them withdrew from the study because of sickness or injuries unrelated to the supplements and tests. The remaining 10 participants (7 male and 3 female) have been participating in endurance training for 7.3 ±0 .9 years and competed at the national level. The 7 male participants have the age of 20.6 ± 1.1 years, the height of 1.72 ± 0.08 m, the weight of 57.03 ± 4.92 kg, body mass index of 19.14 ± 1.35 kg·m-2, and the body fat of 12.9 ± 2.1%. The 3 female participants have the age of 22.7 ± 2.3 years, the height of 1.58 ± 0.06 m, the weight of 46.80 ± 4.57 kg, the body mass index of 18.67 ± 0.58 kg·m-2, and the body fat of 18.6 ± 0.8%. The body composition was measured by bioelectrical impedance analysis (IOI 353, Jawon Medical, Gyeongsan-si, Korea). The exclusion criteria included major cardiovascular disease risks, musculoskeletal injuries, upper respiratory infection, smoking, and consumption of any medicine or protein/amino acids supplement in the past 3 months. The participants were advised to maintain their regular training schedule and dietary routine during the study period. The participants were instructed to refrain all training activity, any strenuous physical activity, and consumption of alcohol and caffeine-containing foods on the day prior to the trial. All participants gave their written informed consent after the experimental procedure and potential risks were explained. The study protocol was approved by the Research Ethics Committee of China Medical University and Hospital, Taichung, Taiwan.
Study design
This study used a single-blind, randomized cross-over design. Each subject completed amino acids (AA) and placebo (PL) trials in a random order, separated by a wash-out period of seven days. Each trial contained two consecutive days of exercise, with a 5000 m time trial on the first day, and a 10000 m time trial on the second day. During the two days prior to each trial, the participants were provided with the same three meals per day, purchased from local convenience stores. The meals provide approximately 2250 kcal·day-1 with 55% energy from carbohydrate, 30% from fat, and 15% from protein, according to the manufacturer’s label.
Procedures
Supplementation: On the days of the trials, the participants reported to the stadium at 0630 after an overnight fast. After blood sampling, two different supplements were consumed. In the AA trial, the participants ingested 0.17 g·kg-1 BCAA (leucine: isoleucine: valine = 10:7:3, containing vitamin E 6.67 IU/g BCAA, capsule, General Nutrition Corporation, Pittsburgh, PA, USA), 0.05 g·kg-1 arginine and 0.05 g·kg-1 citrulline (arginine: citrulline = 1:1, tablet, General Nutrition Corporation). In the PL trial, the participants consumed the identical amount of empty capsule and tablet containing starch (Chung-Yu Biotech Co LTD, Taichung, Taiwan) to the AA trial and one capsule of vitamin E (100 IU, General Nutrition Corporation). All supplements were taken with water within 10 min. The time trials started 60 min after the supplements were consumed. Our preliminary study has shown that plasma BCAA and arginine concentrations would peak after one hr of ingestion (data not shown).
Time trial: All subjects completed a vigorous warm-up that was identical to their pre-competition routine prior to the time trials. The 5000 m (day 1) and 10000 m (day 2) time trials were held in a certified polyurethane 400-m outdoor running track, using the international rules. All participants from both trials competed at the same time to encourage the best performance. The running time was recorded by stop watches. The subjects were aware of their performance and pace during the trials through their own watches. No food or fluid was provided during the time trial. The ratings of perceived exertion (RPE) were recorded immediately before and after each time trial using the Borg’s 20-point scale (Borg, 1982). This study did not require a familiarization trial because all participants were very used to the training and competition in the early morning, and the race-like time trials from their years of experience.
Measurement of blood biochemical parameters: Venous blood samples were collected before the supplementation and immediately after the time trials into tubes containing EDTA. Hemoglobin and hematocrit in whole blood were measured immediately after collection by a blood cell analyzer (Sysmex Kx-21, Diamond Diagnostics, Holliston, MA, USA). After centrifugation, the plasma samples were aliquoted and stored at -70°C
Plasma BCAA concentration was measured enzymatically (Biovision, Milpitas, CA, USA) with a microplate spectrophotometer (Benchmark Plus, Bio-Rad, Hercules, CA, USA). Plasma tryptophan concentration was analyzed with a fluorescence assay (Bridge-It, Mediomics, St. Louis, MO, USA). The fluorescence at excitation 485 nm and emission 665 nm was read by a microplate fluorescence reader (Plate Chameleon, Hidex, Turku, Finland). Plasma NOx concentrations were determined using the Griess reagent (Green et al., 1982). Plasma concentrations of urea, glucose, lactate, NH3, glycerol, and non-esterified fatty acids were measured with an automatic analyzer (Hitachi 7020, Tokyo, Japan) using commercial kits (Randox, Antrim, UK). The changes in plasma volume were corrected for all blood parameters using hemoglobin concentration and hematocrit in whole blood (Costill and Fink, 1974).
Statistical analysis
All data were expressed as mean±SD. The results were analyzed by two-way (trial x time) analysis of variance with repeated measurements. If the main effect is significant, the differences were identified by Ryan-Holm-Bonferroni post hoc analysis (Atkinson, 2002). A p < 0.05 was considered statistically significant.
Results
The running time in 5000 m on the first day was significantly faster in the AA trial by 2.98 ± 3.24% (AA: 1065.7 ± 33.9 s; PL: 1100.5 ± 40.4 s; p = 0.019) (Figure 1A). The performance in 10000 m on the second day was also significantly better in the AA trial by 3.38±3.10% (AA: 2292.0 ± 211.3 s; PL: 2375.6 ± 244.2 s; p = 0.009) (Figure 1A). The individual running time in 5000 m and 10000 m in the AA and PL trials is presented in Fig 1B and 1C, respectively. On the first day, eight participants had better performance in the AA trial (running time reduced by 1.48-7.96%), while two others were slower in the AA trial (running time increased by 1.40 and 2.96%). On the second day, all participants ran faster in the AA trial, with running time reduced by 0.33-8.87%. The percentages of performance improvement in 5000 m and 10000 m were not significantly different. Despite the improvement in running time, the post-exercise RPE were similar between the two trials (Figure 2).
Figure 1.
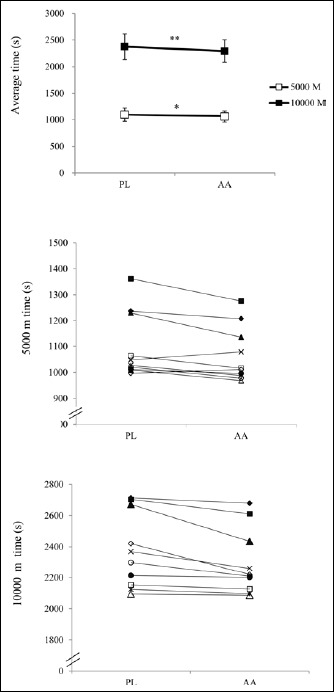
The results of 5000 m and 10000 m time trials in the AA and PL trials. (A, top) average time; (B, middle) individual 5000 m time; (C, bottom) individual 10000 m time). * p < 0.05; ** p < 0.01, significantly different between the AA and PL trials.
Figure 2.
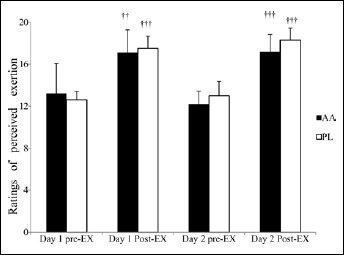
Ratings of perceived exertion before and after exercise on day 1 and day 2 in the AA and PL trials. Main effects: trial: p = 0.172; time: p < 0.001; interaction: p = 0.550. †† p < 0.01; †††p < 0.001, different from the pre-Ex at the same day in the same trial
The AA trial resulted in increases in post-exercise plasma BCAA concentrations by 71.1% and 60.4% on day 1 and 2, respectively, compared to the baseline (Figure 3A). Post-exercise plasma tryptophan levels were significantly increased from the baseline on both days in the PL trial, while it was increased only on day 2 in the AA trial (Figure 3B). The larger magnitude of BCAA increase led to the significantly lower post-exercise tryptophan/BCAA ratio in the AA trial, compared to that in the PL trial (Figure 3C).
Figure 3.
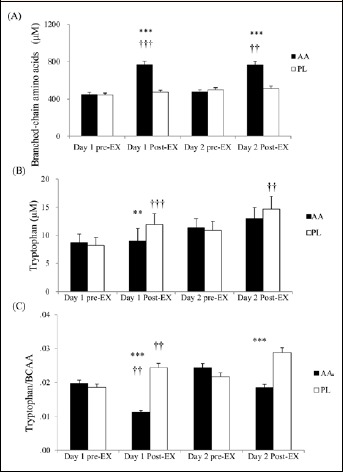
Plasma concentrations of (A) BCAA and (B) tryptophan, and (C) tryptophan/BCAA ratio in the AA and PL trials. Main effects: (A) trial: p < 0.001; time: p < 0.001; interaction: p < 0.001; (B) Main effects: trial: p = 0.005; time: p < 0.001; interaction: p = 0.003; (C) Main effects: trial: p < 0.001 ; time: p = 0.040; interaction: p < 0.001. *p < 0.05; ** p <0 .01; *** p < 0.001, AA vs PL trial at the same time point. † p < 0.05; †† p < 0.01; ††† p < 0.001, different from the pre-Ex at the same day in the same trial
The excess accumulation of NH3, commonly seen after BCAA supplementations in previous studies, was absent in the AA trial as the two trials showed similar post-exercise plasma NH3 concentrations (Figure 4A). The AA trial showed significantly higher plasma urea concentration after exercise than that in the PL trial on both days (Figure 4B). Pre- and post-exercise plasma concentrations of NOx, glucose, lactate, glycerol, and non-esterified fatty acids are presented in Table 1. These variables were not statistically different between the two trials.
Figure 4.
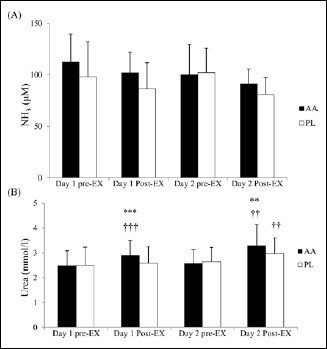
Plasma concentrations of (A) NH3 and (B) urea in the AA and PL trials. Main effects: (A) trial: p = 0.182; time: p = 0.056; interaction: p = 0.560; (B) Main effects: trial: p = 0.213 ; time: p < 0.001; interaction: p = 0.007. * p < 0.05; ** p < 0.01; *** p < 0.001, AA vs PL trial at the same time point. † p < 0.05; †† p < 0.01; ††† p < 0.001, different from the pre-Ex at the same day in the same trial
Table 1.
Plasma biochemical parameters before and after exercise in AA and PL trials. Values are means (±SD).
| Trial | Day 1 pre-EX | Day 1 Post-EX | Day 2 pre-EX | Day 2 Post-EX | |
|---|---|---|---|---|---|
| NOx (μM) | AA | 100.8 (31.6) | 134.4 (65.4) | 89.6 (36.5) | 131.1 (60.0) |
| PL | 93.3 (28.9) | 111.4 (76.6) | 100.9 (39.0) | 86.3 (34.1) | |
| Glucose (mM) | AA | 5.2 (.7) | 7.6 (1.8) * | 6.0 (1.2) | 7.9 (2.5) * |
| PL | 5.6 (1.4) | 8.0 (1.9) * | 6.3 (1.2) | 7.8 (2.5) * | |
| Lactate (mM) | AA | 1.7 (.3) | 7.7 (3.6) * | 1.6 (.3) | 6.0 (2.2) * |
| PL | 1.8 (.5) | 7.1 (3.9) * | 1.8 (.6) | 5.0 (2.3) * | |
| Glycerol (μM) | AA | 39.3 (17.7) | 127.3 (60.9) * | 38.9 (26.5) | 166.7 (64.2) * |
| PL | 39.9 (14.9) | 122.8 (64.9) * | 36.9 (31.0) | 177.9 (96.4) * | |
| NEFA (mM)a | AA | .66 (.33) | .54 (.30) | .72 (.50) | .93 (.61) |
| PL | .74 (.26) | .63 (.39) | .64 (.45) | 1.15 (.77) |
a non-esterified fatty acid.
* p < 0.05, significantly different from pre-exercise on the same day in the same trial.
Discussion
The results of this study suggested that the combined supplementation of BCAA, arginine, and citrulline could improve endurance performance on both consecutive days of exercise. The participants in the AA trial could run faster at the same degree of perceived exertion, possibly resulting from the reduced plasma tryptophan/BCAA ratio. In addition, the elevated urea synthesis, conceivably from arginine and citrulline supplementation, prevented the excess hyperammonemia in the AA trial.
In the AA trial, the supplementation led to the significantly decreased plasma tryptophan/BCAA ratio, resulting from the elevation in BCAA concentration. The lower tryptophan/BCAA ratio would reduce cerebral uptake of tryptophan, hence decreases cerebral serotonin synthesis and alleviates central fatigue (Gomez-Merino et al., 2001). This is evidenced by the fact that the participants could run faster while feeling the same magnitude of effort. The results were similar to our previous study in which the supplementation of BCAA and arginine allowed the participants to perform better in the intermittent sprints under the same RPE (Chang et al., 2015). Although it has been suggested that BCAA could alleviate the feeling of fatigue during the exercise with fixed intensities in general populations (Blomstrand et al., 1997), this may not be the case in this study. The time-trial and race-like protocol used in this study would drive the athletes to complete the trial with their maximal effort. Thus, it is conceivable that the participants reported similar RPE in both trials.
The AA and PL trials produced similar post-exercise plasma NH3 concentrations, indicating the absence of excess hyperammonemia from BCAA oxidation. Arginine and citrulline appeared to enhance NH3 removal by increasing urea synthesis in the AA trial (Meneguello et al., 2003; Schaefer et al., 2002; Takeda et al., 2011). It is noteworthy that in the one study that showed ergogenic effect of BCAA, the supplemented trial had similar post-exercise plasma NH3 concentration to that in the control trial (Mittleman et al., 1998).
In our previous study that also used a two-day protocol, the ergogenic effect of BCAA and arginine was only present on the second day, presumably with accumulated central and/or peripheral fatigue (Chang et al., 2015). However, post-exercise excess hyperammonemia from BCAA oxidation was not completely prevented. By alleviating excess NH3 accumulation with arginine and citrulline in the present study, the endurance performance was improved on the first and second day. It indicated that although most studies failed to show the ergogenic effect of BCAA on a single bout of endurance exercise, it was probably due to the concomitantly elevated plasma NH3 concentrations that nullified the potential benefit of BCAA on alleviation of central fatigue (MacLean and Graham, 1993; MacLean et al., 1994; 1996; Meeusen et al., 2006; Struder et al., 1998). In the AA trial in this study, all but two participants had better performance in the 5000 m event on the first day, and all participants ran faster in the 10000 m event on the second day, compared to the PL trial. Although the degree of improvement varied among the participants, the general agreement among this group of trained runners indicated that the supplementation could be applicable to real endurance running events.
Conclusion
In conclusion, the combined supplementation of BCAA, arginine, and citrulline could enhance endurance performance in two consecutive days in college runners. This supplementation could be used in multi-day competitions that are common for endurance athletes. The potential mechanisms responsible for the ergogenic effect include the alleviation of central fatigue by BCAA and the prevention of hyperammonemia through increased urea genesis by arginine and citrulline. Future studies could examine the role of other neurotransmitters such as dopamine and epinephrine. In addition, the effect of this supplementation on fed state requires further investigation.
Acknowledgements
This study is supported by the Ministry of Science and Technology, Taiwan (102-2410-H-028-002-MY3). The authors thank Ms Yu-Fang Huang for her technical assistance. The experiments performed in this study comply with the current laws of Taiwan. The authors declare that they have no conflicts of interest concerning this article.
Biographies
I-Shiung CHENG
Employment
Professor, Department of Physical Education, National Taichung University of Education, Taiwan.
Degree
PhD
Research interests
Sport nutrition, nutritional biochemistry, fitness
E-mail: ischeng1965@mail.ntcu.edu.tw
Yi-Wen WANG
Employment
Master student, Department of Physical Education, National Taichung University of Education, Taiwan.
Degree
MS
Research interests
Sport nutrition, yoga, fitness
E-mail: zellin1009@yahoo.com.tw
I-Fan CHEN
Employment
PhD student, Graduate Institute of Sport Coaching Science, Chinese Culture University, Taipei, Taiwan.
Degree
PhD
Research interests
Sport nutrition, nutritional biochemistry, nutritional intervention in cognitive function in athletes
ifchen16@gmail.com
Gi-Sheng HSU
Employment
Assistant professor, Department of Sport Performance, National Taiwan University of Sport, Taichung, Taiwan.
Degree
MS
Research interests
Physiology in endurance running, coaching in endurance running, fitness assessment and training
E-mail: gshsu@ntupes.edu.tw
Chun-Fang HSUEH
Employment
PhD student, Graduate Institute of Sport Coaching Science, Chinese Culture University, Taipei, Taiwan.
Degree
MS
Research interests
Sport nutrition, physiology in swimming, coaching in swimming and triathlon
E-mail: swim_speedo@hotmail.com
Chen-Kang CHANG
Employment
Professor, Sport Science Research Center, National Taiwan University of Sport, Taichung, Taiwan.
Degree
PhD
Research interests
Sport nutrition, nutritional biochemistry, nutrition intervention in cognitive function in athletes
E-mail: wspahn@seed.net.tw
References
- Atkinson G. (2002) Analysis of repeated measurements in physical therapy research: multiple comparisons amongst level means and multi-factorial designs. Physical Therapy in Sport 3(4), 191-203. [Google Scholar]
- Bailey S.P., Davis J.M., Ahlborn E.N. (1993) Serotonergic agonists and antagonists affect endurance performance in the rat. International Journal of Sports Medicine 14(6), 330-333. [DOI] [PubMed] [Google Scholar]
- Blomstrand E., Andersson S., Hassmen P., Ekblom B., Newsholme E. A. (1995). Effect of branched-chain amino acid and carbohydrate supplementation on the exercise-induced change in plasma and muscle concentration of amino acids in human subjects. Acta Physiologica Scandinavia 153(2), 87-96. [DOI] [PubMed] [Google Scholar]
- Blomstrand E., Hassmen P., Ek S., Ekblom B., Newsholme E.A. (1997). Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiologica Scandinavia 159(1), 41-49. [DOI] [PubMed] [Google Scholar]
- Blomstrand E., Moller K., Secher N. H., Nybo L. (2005) Effect of carbohydrate ingestion on brain exchange of amino acids during sustained exercise in human subjects. Acta Physiologica Scandinavia 185(3), 203-209. [DOI] [PubMed] [Google Scholar]
- Borg G.A. (1982) Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise 14(5), 377-381. [PubMed] [Google Scholar]
- Chang C.K., Chang Chien K.M., Chang J.H., Huang M.H., Liang Y.C., Liu T.H. (2015) Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: a randomized trial. PLoS One 10(3), e0121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. (1997) Effects of acute physical exercise on central serotonergic systems. Medicine and Science in Sports and Exercise 29(1), 58-62. [DOI] [PubMed] [Google Scholar]
- Clarkson P., Adams M.R., Powe A.J., Donald A.E., McCredie R., Robinson J., McCarthy S.N., Keech A., Celermajer D.S., Deanfield J.E. (1996) Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. Journal of Clinical Investigation 97(8), 1989-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costill D.L., Fink W.J. (1974) Plasma volume changes following exercise and thermal dehydration. Journal of Applied Physiology 37(4), 521-525. [DOI] [PubMed] [Google Scholar]
- Curis E., Nicolis I., Moinard C., Osowska S., Zerrouk N., Benazeth S., Cynober L. (2005) Almost all about citrulline in mammals. Amino Acids 29(3), 177-205. [DOI] [PubMed] [Google Scholar]
- Davis J.M., Bailey S.P. (1997). Possible mechanisms of central nervous system fatigue during exercise. Medicine and Science in Sports and Exercise 29(1), 45-57. [DOI] [PubMed] [Google Scholar]
- Fernstrom J.D. (2005) Branched-chain amino acids and brain function. Journal of Nutrition 135(6 Suppl), 1539S-1546S. [DOI] [PubMed] [Google Scholar]
- Gomez-Merino D., Bequet F., Berthelot M., Riverain S., Chennaoui M., Guezennec C.Y. (2001) Evidence that the branched-chain amino acid L-valine prevents exercise-induced release of 5-HT in rat hippocampus. International Journal of Sports Medicine 22(5), 317-322. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry 126(1), 131-138. [DOI] [PubMed] [Google Scholar]
- Hassmen P., Blomstrand E., Ekblom B., Newsholme E.A. (1994) Branched-chain amino acid supplementation during 30-km competitive run: mood and cognitive performance. Nutrition 10(5), 405-410. [PubMed] [Google Scholar]
- MacLean D.A., Graham T.E. (1993) Branched-chain amino acid supplementation augments plasma ammonia responses during exercise in humans. Journal of Applied Physiology 74(6), 2711-2717. [DOI] [PubMed] [Google Scholar]
- MacLean D.A., Graham T.E., Saltin B. (1994) Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. American Journal of Physiology 267(6 Pt 1), E1010-1022. [DOI] [PubMed] [Google Scholar]
- MacLean D.A., Graham T.E., Saltin B. (1996) Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. Journal of Physiology, 493(Pt 3), 909-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R., Watson P., Dvorak J. (2006). The brain and fatigue: new opportunities for nutritional interventions? Journal of Sports Sciences 24(7), 773-782. [DOI] [PubMed] [Google Scholar]
- Meneguello M.O., Mendonca J.R., Lancha A.H., Jr., Costa Rosa L.F. (2003) Effect of arginine, ornithine and citrulline supplementation upon performance and metabolism of trained rats. Cellular and Biochemical Functions 21(1), 85-91. [DOI] [PubMed] [Google Scholar]
- Mittleman K.D., Ricci M.R., Bailey S.P. (1998). Branched-chain amino acids prolong exercise during heat stress in men and women. Medicine and Science in Sports and Exercise 30(1), 83-91. [DOI] [PubMed] [Google Scholar]
- Newsholme E.A., Blomstrand E. (2006). Branched-chain amino acids and central fatigue. Journal of Nutrition 136(1 Suppl), 274S-276S. [DOI] [PubMed] [Google Scholar]
- Nybo L., Dalsgaard M.K., Steensberg A., Moller K., Secher N. H. (2005) Cerebral ammonia uptake and accumulation during prolonged exercise in humans. Journal of Physiology 563(Pt 1), 285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge C., Des Robert C., Robins A., Le Bacquer O., Volteau C., De La Cochetiere M. F., Darmaun D. (2007) Manipulation of citrulline availability in humans. American Journal of Physiol Gastrointestinal and Liver Physiology 293(5), G1061-1067. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Piquard F., Geny B., Doutreleau S., Lampert E., Mettauer B., Lonsdorfer J. (2002) L-Arginine reduces exercise-induced increase in plasma lactate and ammonia. International Journal of Sports Medicine 23, 403-407. [DOI] [PubMed] [Google Scholar]
- Sharp T., Bramwell S.R., Grahame-Smith D.G. (1992) Effect of acute administration of L-tryptophan on the release of 5-HT in rat hippocampus in relation to serotoninergic neuronal activity: an in vivo microdialysis study. Life Science 50(17), 1215-1223. [DOI] [PubMed] [Google Scholar]
- Struder H. K., Hollmann W., Platen P., Donike M., Gotzmann A., Weber K. (1998) Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Hormone and Metabolic Research 30(4), 188-194. [DOI] [PubMed] [Google Scholar]
- Sureda A., Cordova A., Ferrer M.D., Perez G., Tur J.A., Pons A. (2010) L-citrulline-malate influence over branched chain amino acid utilization during exercise. European Journal of Applied Physiology 110(2), 341-351. [DOI] [PubMed] [Google Scholar]
- Takeda K., Machida M., Kohara A., Omi N., Takemasa T. (2011) Effects of citrulline supplementation on fatigue and exercise performance in mice. Journal of Nutritional Science and Vitaminology (Tokyo) 57(3), 246-250. [DOI] [PubMed] [Google Scholar]
- van Hall G., van der Vusse G. J., Soderlund K., Wagenmakers A. J. (1995) Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. Journal of Physiology 489(Pt 1), 251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.J., Smeeton N.J., Watt P.W. (2010) Ammonia metabolism, the brain and fatigue; revisiting the link. Progress in Neurobiology 91(3), 200-219. [DOI] [PubMed] [Google Scholar]


