Abstract
Optimal vascular access planning begins when the patient is in the predialysis stages of CKD. The choice of optimal vascular access for an individual patient and determining timing of access creation are dependent on a multitude of factors that can vary widely with each patient, including demographics, comorbidities, anatomy, and personal preferences. It is important to consider every patient’s ESRD life plan (hence, their overall dialysis access life plan for every vascular access creation or placement). Optimal access type and timing of access creation are also influenced by factors external to the patient, such as surgeon experience and processes of care. In this review, we will discuss the key determinants in optimal access type and timing of access creation for upper extremity arteriovenous fistulas and grafts.
Keywords: hemodialysis access, arteriovenous fistula, arteriovenous graft, timing of creation, Arteriovenous Shunt, Surgical, Fluid Therapy, Humans, renal dialysis
Introduction
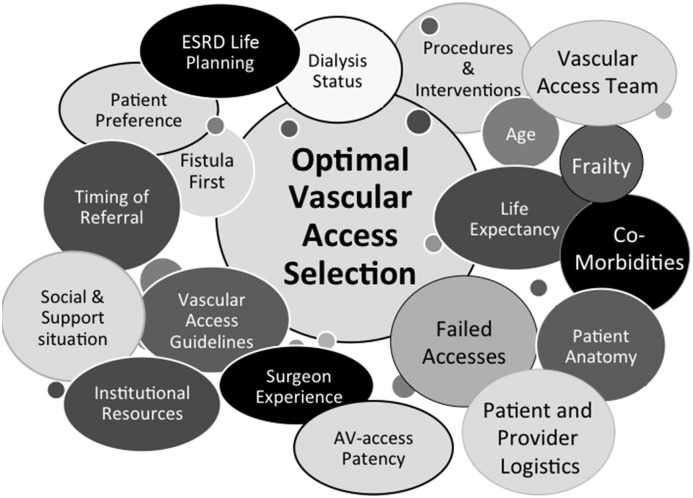
Fistula First, National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI), and other international guidelines strongly encourage the creation of autogenous arteriovenous (AV) fistulas (AVFs) for hemodialysis (HD) vascular access over prosthetic AV grafts and discourage the use of HD central venous catheters (CVCs). However, determining the optimal type and timing of dialysis vascular access creation in any individual patient can be more complex than suggested, necessitating consideration of a multitude of factors (Figure 1). In this review, we will address the most common types of AV access: upper extremity AVFs and AV grafts. This review will cover key determinants in deciding the optimal AV access for the individual patient, including patient-related factors, anatomic access circuit–related characteristics, surgical features, and process considerations, all of which affect vascular access outcomes.
Figure 1.
Considerations for achieving the right vascular access at the right time for the right patient. AV, arteriovenous.
Patient-Related Factors
Patient-related considerations include patient demographics, comorbidities, patient preference, and social and logistics variables.
Demographics
Key patient demographic factors that influence vascular access outcomes and decision making include their dialysis dependence status, sex, age, and ethnicity/race.
Dialysis Dependence.
Patients who need an HD vascular access are preparing to initiate dialysis (predialysis), transitioning from another renal replacement modality (peritoneal dialysis [PD] or failing/failed kidney transplant), or already on dialysis (dialysis dependent) with a failing AV access or CVC (Table 1). Dialysis dependence status is one of the most important factors to consider when determining the optimal AV access for a given patient. For patients who are predialysis, the urgency to initiate dialysis is a core consideration affecting AV access choice. For example, a CVC can be avoided by using a prosthetic early cannulation AV graft that can be used in as little as 24 hours after creation (1,2) in patients without an already established vascular access, who urgently need to initiate HD. In contrast, while AVFs should be considered in all appropriate patients, AVFs require a minimum of 4 weeks and up to 9 months before they are suitable for HD in North America. Should the initial attempt at an AVF fail to become functional for use, another AV access must be created. During this time, if the patient needs to initiate HD or is already dialysis dependent, a bridging CVC is required and brings with it increased risks of infection, central stenosis, hospitalizations, morbidity, and mortality (3). In fact, patients who are predialysis who have AVFs have been shown to have a significantly increased incidence of CVC dependence at dialysis initiation compared with patients who have AV grafts (4). In patients who are dialysis dependent, the benefits of an AVF must be weighed against the potentially detrimental effects of CVC use during the AVF’s maturation period.
Table 1.
Timing of hemodialysis vascular access creation
| Organization | Country | Recommendation |
|---|---|---|
| National Kidney Foundation Kidney Disease Outcomes Quality Initiative | United States | “Patients with a glomerular filtration rate (GFR) less than 30 ml/min/1.73 m2 (CKD stage 4) should be educated on all modalities of kidney replacement therapy (KRT) options, including transplantation, so that timely referral can be made for the appropriate modality and placement of a permanent dialysis access, if necessary … A fistula should be placed at least 6 months before the anticipated start of HD treatments. This timing allows for access evaluation and additional time for revision to ensure a working fistula is available at initiation of dialysis therapy … A graft should, in most cases, be placed at least 3 to 6 weeks before the anticipated start of HD therapy. Some newer graft materials may be cannulated immediately after placement.” |
| Canadian Society of Nephrology | Canada | “Set specific targets for tasks during CKD Stages 3, 4, and 5 management according to the rate of decline of eGFR: (a) Assuming a usual rate of decline of 2–5 ml/min/yr, modality education should usually begin at eGFR=30 ml/min, modality decisions should usually be finalized by eGFR=20 ml/min, and that those who choose HD (and who are expected to survive long enough and are suitable), should usually be referred to a vascular surgeon for consideration/evaluation of AVF when eGFR=15–20 ml/min (as per 2006 CSN guideline). (b) In patients whose rate of decline of eGFR over time is greater than 5 ml/minute/year, these tasks should be undertaken earlier than proposed before … Assuming that local resources are available, we recommend that if and when patients reach eGFR=15 ml/minute and are unable or unwilling to make a modality decision, consideration should be given to refer suitable patients to the VA surgical team for assessment concerning possible dialysis access options … Note that this recommendation is about when to consider referral to the surgeon for evaluation, and does not contemplate when vascular surgery is to be scheduled.” |
| The Renal Association | United Kingdom | “Vascular access planning should commence at some point after an individual reaches CKD stage 4 … exact timing of placement of vascular access will be determined by rate of decline of renal function, co-morbidities and by the surgical pathway” |
| National Health and Medical Research Council | Australia | “No recommendations possible based on Level I or II evidence.” Suggestions for clinical care (suggestions are on the basis of levels 3 and 4 evidence): all patients, especially those with comorbid conditions, should be referred to a vascular access surgeon well in advance of the anticipated need for hemodialysis. The exact timing depends on patient-related factors and local facilities |
| Japanese Society for Dialysis Therapy | Japan | “VA construction should be considered when eGFR is less than 15 ml/min/1.73 m2 (CKD stages 4 and 5) as well as taking into account clinical conditions. In patients with diabetic nephropathy, who have a tendency to show overhydration, VA construction should be considered at a higher eGFR … Anticipating the start of hemodialysis from the results of various laboratory tests and clinical symptoms, ideally the AVF should be constructed at least 2 to 4 weeks before the initial puncture. In the case of an AVG, the time from construction to initial puncture should be 3 to 4 weeks.” |
HD, hemodialysis; AVF, arteriovenous fistula; CSN, Canadian Society of Nephrology; VA, vascular access; AVG, arteriovenous graft.
Dialysis access planning should begin early on when a patient is predialysis. For both patients who are predialysis and patients who are dialysis dependent, their ESRD life plan or life strategy (hence, their overall dialysis access life plan) should be considered. No decision about a single vascular access creation or placement should be made in isolation or independent of the patient’s overall ESRD life plan (5).
The ESRD life plan is a strategy for living with ESRD, ideally made by the patient with a coordinated CKD management team. It is a strategy that should start in the predialysis period, and it encompasses a continuum of care model for CKD and transitions to ESRD. It aims to maximize ESRD modality choices and utilization for a specific patient’s foreseeable lifespan and specifically considers the patient’s current medical situation, their current and future life goals, and their preferences, social support, functional status, and logistic and other practical feasibilities. In doing so, the dialysis access strategy is considered and reflects the ESRD life plan, whereby the appropriate access aligns with the modality for RRT (dialysis or transplant) to help each patient achieve their life goals safely and with their preferences considered. For example, a young active patient with residual renal function might best initiate PD with a PD catheter in anticipation of a living donor kidney transplant and as that transplant eventually fails, will receive an AVF in view of starting home HD and so on. In contrast, an older and more complex but active patient may start HD with a matured AVF with plans for future AVFs or AV grafts (depending on intercurrent events) as the AVF becomes problematic. Lastly, a palliative patient may best be served with an early cannulation graft or CVC for shorter-term HD. This concept relies on clear, timely, and effective communication between the patient, their family/personal supporters, and key healthcare and vascular access team members. As such, dialysis access and renal replacement modality short– and long–term plans (ESRD life plan) should be updated on a regular basis.
Sex.
Studies conflict regarding the association between women, AVF nonmaturation, and reduced patency (6–9), which is hypothesized to be related to smaller diameter veins in women compared with men. This is difficult to assess, because many studies do not perform preoperative vein mapping or report vein diameters. However, one study of 148 consecutive patients showed no association between sex and diameter of the cephalic vein (10). Similarly, another study showed inferior AVF maturation rates in women that were not explained by differences in vein diameter (11). Additionally, pediatric patients with small veins can have successful AVFs created, suggesting that surgical skill may be a contributing factor or effect modifier (below). With such speculation about the association between sex and AVF outcomes, patient sex alone should not serve as a deciding factor regarding optimal AV access type for an individual patient.
Age.
Patient age is a significant consideration for the ideal AV access type because of the complex implications associated with age: life expectancy, likelihood of associated comorbidities, and age–related personal goals. Life expectancy is reduced with increasing age and dialysis vintage (time on dialysis) (12). For example, the 12-month survival rates of patients with incident ESRD ≥75 or 45–64 years old are 62% and 86%, respectively (12). Life expectancy (death), particularly in the elderly, is a competing risk for AVF maturation time and use. There is growing literature showing inferior outcomes of AVFs in elderly patients compared with younger patients on HD. For example, patients ages ≥65 years old have been reported to have an increased risk of AVF maturation failure with a relative risk of 1.7 compared with patients <65 years old (13). A meta-analysis also showed that 12-month primary and secondary patency as well as maturation rates are significantly worse in the elderly patient (14). Until the AVF matures enough to be used, the patient cannot enjoy the benefits of an AVF over AV graft, such as a lower infection risk. Indeed, in elderly patients with limited life expectancy, AVFs confer a very modest reduction in the risk of bacteremia compared with AV grafts (15). Among patients with an estimated life expectancy in the 25th percentile, >200 AVFs would need to be placed to prevent one episode of AV graft–related bacteremia (15).
The complexities of AV access planning are highlighted in a study of octogenarians, which showed that, of those who had preoperative vein mapping during the predialysis period, all had AVFs created (16); however, 32% died before needing to start dialysis (16). Furthermore, within the cohort, 57.5% of patients died within 18 months of starting dialysis (16). Of these patients who died, 55.5% died within 6 months of starting dialysis: 70% of them had AVFs created, but none of the AVFs matured to allow for cannulation before the patients died (16).
It can be challenging to find the balance between the likelihood of patient survival, access survival, and their respective potential complications. However, one might consider that, if an elderly patient is expected to have a high 1-year mortality, an AV graft may be appropriate. DeSilva et al. (17) reported that there was no difference in survival among patients ages >80 years old who had an AV graft as their initial access placed predialysis compared with those with AVFs. Other studies have also found lower AVF maturation or use, greater need for interventions, and prolonged catheter dependency compared with AV grafts in the elderly (17–20).
However, elderly age alone should not preclude a patient from undergoing AVF creation. If an elderly patient has few comorbidities and good functional status, an AVF may be appropriate. In such instances, despite KDOQI guidelines that recommend creation of a radiocephalic fistula before an upper arm fistula, consideration should be given to performing a brachiocephalic AVF, particularly if the radial artery or forearm cephalic vein is not of a robust diameter. In a meta-analysis comparing patency of brachiocephalic AVFs with that of radiocephalic AVFs in the elderly, brachiocephalic AVFs were found to have superior patency at 12 months (72.7% versus 65.1%, respectively) (21). Others have also shown increased primary functional patency of brachiocephalic AVFs at 1 year (22). Recent national position statements and other commentary may provide further guidance with respect to tailoring vascular access according to specialized circumstances pertaining to the elderly, including palliative care (15,23–25).
Race/Ethnicity.
There is a paucity of consistent data on the association of race/ethnicity with AV access outcomes. Black patients on HD have been found to be at increased risk for vascular access complications requiring intervention, regardless of the type of access (26). Similarly, non-black race has been shown to reduce the risk of failure of AVF maturation, with an odds ratio of 0.43 (27). At the same time, other authors have shown that AVF maturation and patency rates in blacks are comparable with those in other populations (28). Race and ethnicity may play a role in access to care, making it difficult to truly evaluate AV access outcomes (29,30). The limited and conflicting available evidence makes it difficult to critically consider race/ethnicity when determining ideal AV access.
Comorbidities
Diabetes.
There is significant controversy as to whether diabetes mellitus itself is associated with AVF maturation and patency. No studies have reported on the relationship between the degree of diabetes control and/or its duration (and associated effect on vessels) and AVF outcomes. Studies have shown prolonged maturation times in patients with diabetes (31) and decreased primary and secondary patency (32). However, other studies have also shown no association between diabetes and time to maturation (33), risk of nonmaturation (34,35), or patency (36). Even in a well validated prediction model (37), no association between diabetes and risk of nonmaturation was found (27). Given the disparate outcomes, it is likely that the relationship between diabetes and AVF outcomes is complex and may be related to a variety of diabetes–associated pathologic factors, such as the presence of arterial calcification, atherosclerosis, and vessel damage. Additionally, given that diabetes is the primary etiology of ESRD in North America and worldwide, using the diagnosis of diabetes to determine optimal AV access type would be limiting.
Coronary Artery Disease and Heart Failure.
For an AV access, whether AVF or AV graft, to be sustained, it must have adequate inflow and outflow. The presence of coronary artery disease (CAD) represents a surrogate for two issues that may affect the inflow of an AV access. Atherosclerosis is a systemic disease that affects all arteries, including vascular access inflow arteries. Patients with severe CAD may also have compromised cardiac output. This may be reflected by poor BPs. Low diastolic BP has been shown to significantly decrease AVF patency (38). For example, an AV access can thrombose easily when a patient's BP suddenly drops on HD (38,39). The patency of AVFs and AV grafts is likely to be negatively affected by a low cardiac ejection fraction regardless of whether it is associated with CAD. However, maturation, which is only required for AVF, is also negatively affected by CAD (27).
Peripheral Vessel Disease.
Similarly, peripheral vessel disease has been shown to be associated with failure to mature (27) and decreased patency in both AVF and AV grafts (40). When atherosclerosis affects the peripheral arteries to the extent that the ankle-brachial index is decreased, it is highly likely that atherosclerosis is also affecting the inflow arteries used for vascular access. Peripheral veins damaged by venipuncture, peripherally inserted central catheters lines, or other trauma are particularly associated with poor AVF outcomes. Central veins damaged by CVCs, pacemakers, or other interventions may prohibit both AVF and AV graft creation.
Obesity.
Obesity, defined as a body mass index ≥30, is often accompanied by increased depth of the superficial veins, making AVF creation challenging and access of the AVF for HD difficult, even if it has matured in terms of diameter and flow (41). For this reason, AV grafts are sometimes preferred over AVF in obese patients because of the ease with which AV grafts can be placed superficially. Many techniques have been described to decrease the distance between a vein and the skin. The most common technique is surgical elevation of the AVF (42), but other techniques include open lipectomy and liposuction (43,44).
Obesity alone should not rule a patient out for AVF creation. In a study of 1486 patients, 340 of whom were obese, only the morbidly obese (body mass index ≥35) had an increased risk of AVF failure to mature (45); there was no association between obesity and AVF patency. However, the increased surgical complexity of an AVF in the obese patient and whether the patient can tolerate the more extensive operation, the potential longer healing time, and the challenging cannulation should be considered.
Frailty and Functional Status.
Unlike the prior specific conditions discussed, frailty and functional status represent the cumulative interactions of chronologic age and disease processes affecting a patient with CKD. The components of frailty encompass functional status (46) and include weight loss, poor endurance, low energy, and weakness, and may manifest as slow walking speed and low physical activity (47). Up to 75% of patients on dialysis ages ≥60 years old meet the criteria for frailty (48). Frailty increases the risk of mortality with a hazard ratio of 2.24 (48). In patients with poor functional status and/or frailty, consideration should be given to whether their life expectancy is such that it will allow for AVF maturation and the patient to enjoy the benefits of having an AVF versus an AV graft (above). Having a catheter with a maturing AVF has been reported to be associated with a greater risk of mortality than AV graft alone in patients with limited functional status (49).
Patient Choice.
Many times, clinicians are not diligent in taking into account patient preference for vascular access type; however, patient preference should warrant significant consideration in the decisions to create AV access and to use a specific type. Depending on whether the patients are predialysis or on dialysis, they would have had differing exposures and experiences with AV access—either their own or shared by other patients in waiting rooms or on dialysis. As clinicians, we are concerned about the AV access type and their associated complications; however, patients may have far differing concerns (50,51). Patients are much more worried about daily quality of life issues with their vascular access, such as pain with cannulation, and influenced by their prior experiences with a failed AV access that required painful interventions. Fistulas may require two to three interventions until they are suitable/mature enough for dialysis. However, AV grafts are more likely to be suitable for HD earlier than AVFs but often require multiple procedures to maintain functional patency (52).
Often, the decision regarding AV access type depends on the patient’s prior experience, how frail they are, their desired quality of life, and life goals. A recent Dialysis Outcomes and Practice Patterns Study (DOPPS) survey of 1400 United States patients on dialysis asked about their preference for an AVF/AV graft versus a CVC (53). Overall, only 24% of patients had no preference; 12% preferred a CVC (nonblack women had the highest CVC preference at 17%). The most common reasons for preferring a CVC were no puncture and no bleeding, avoidance of disfigurement, and better cosmesis (51,54). The DOPPS survey revealed that up to 20% of patients received little or no vascular access–related education, suggesting that they did not fully understand the risks and benefits of various vascular access types. These data emphasize the need for better education and counseling from clinicians. However, although some patients’ vascular access concerns may be associated with deficiencies in patient education (55), others are not easily managed by education alone, especially quality of life and aesthetic concerns (51). Under valid circumstances, strong patient preference for a specific access type should be respected, especially when the competent patient has been properly informed and consented as to their vascular access choice, including dialyzing through a CVC and refusing AVF or AV graft creation.
Anatomic Access Circuit–Related Factors
The complete AV access circuit begins with the heart and includes the complete feeding artery from its central origin, the AV access, and the complete venous drainage up to the superior vena cava-right atrial junction.
Vein Size/Quality
Vein size is nonlinearly correlated with AVF maturation and patency (35,56). Results from the prospective multi–institution Hemodialysis Fistula Maturation Study show that a draining vein diameter of 2–3 mm is associated with increased risk of early thrombosis (defined as thrombosis within 18 days of AVF creation) (57). Upper arm AVFs have larger vein diameters than forearm AVFs and are at lower risk for AVF maturation failure (58) and early thrombosis (57), with increased long–term patency (58). What is unclear is the diameter cutoff between success and failure. Diameters of 1.6–4.0 mm have been used as cutoffs (9,34,35). The varying definitions of maturation, patency, and techniques of measuring vein diameter likely contribute to the wide range of threshold diameters cited. To further complicate the situation, some authors have shown that there can be substantial overlap in the vein diameters of those whose AVF fail and those that succeed (7). Vein quality (e.g., distensibility) has not been studied extensively (59–61); however, the great majority of surgeons would not use a vein that appears sclerotic on either preoperative duplex ultrasound or direct examination. In such patients, re-evaluation is required to decide on an AV graft in the same location or an AVF with alternate vessels.
Arterial Size/Quality
Similarly, smaller inflow artery diameter associates with AVF failure, with brachial artery–based AVF being more likely to mature and have superior patency compared with radial artery–based AVF (22,57). There seems to be more consensus on the required minimum artery diameter of 2 mm (10,34). Calcification of arterial inflow is often anecdotally regarded by clinicians as a negative prognostic factor. However, data from the Hemodialysis Fistula Maturation Study reveal that noncompliant feeding arteries are associated with a lower incidence of early thrombosis (57). Arterial microcalcification itself may not be associated with maturation failure (62,63) if the artery is of adequate size and overall quality to allow for surgical proximal and distal control and suture placement.
Central Venous Stenosis
Central venous stenosis is associated with HD catheter use. Although its true incidence is unknown (64), it is an important consideration, because the majority of patients on HD initiate dialysis with a CVC. The presence of central venous stenosis/occlusion may prohibit the creation of an AV access (65); an ipsilateral access will likely result in upper extremity edema and/or early AV access failure (66). Although central venous stenosis/occlusion can be treated with stenting and/or angioplasty, the incidence of recurrence is high. Consideration should be given to using the patient’s contralateral arm. The problems of central stenosis apply to both AVF and AV grafts and therefore, may not affect decision making.
Surgical Skills and Training
Although it is intuitive that successful AV access outcomes are linked with surgical experience and technique, studies to date are limited and conflicting, necessitating more study to evaluate this association. For example, one study from the DOPPS showed that the risk of primary AVF failure was 34% lower if the surgeon created ≥25 fistulas during training (67). Another study of radiocephalic AVFs performed in the United Kingdom found that AVFs constructed by more experienced surgeons had higher primary success rates and higher primary and secondary patencies (68). In contrast, a prospective study found no difference in outcomes of primary AVFs created by qualified surgeons versus surgical trainees (69). This is supported by the recent Hemodialysis Fistula Maturation Study that found no association between subspecialty training of the attending surgeon and early thrombosis or whether the attending surgeon or the trainee performed the anastomosis (57). However, as with trainees, surgeons with limited exposure to more complex vascular access operations (e.g., requiring superficialization, extensive dissection, or revision) should likely refer to and/or obtain guidance from experienced surgeons proven successful with such complex patients.
Aside from technical experience, the advantage of surgeons with broad and deep vascular access experience is their ability to plan beyond the immediate access. Although it is unclear if training affects the immediate success of a single AV access, it is the ability of the surgeon to plan ahead to the subsequent vascular accesses along the life plan of the patient with ESRD that is critical. This foresight comes with experience, not training. The decision-making process for each AV access creation should consider “fistula first if possible, but if not possible and/or when the AV access fails, what is the backup plan and the next access?” instead of simply “which surgeon can provide fistula first.” The experienced vascular access surgeon has a plausible exit strategy for each AV access contemplated and created.
Process Factors
Timing
Table 1 lists different national recommendations on the timing of vascular access creation. However, the likelihood and timeframe of starting dialysis can be very difficult to predict, especially because GFR decline is nonlinear and varies with age and underlying conditions. Also, despite careful CKD care follow-up, patients encounter intercurrent events that lead to dialysis initiation. This is evidenced by the approximately 20%–40% of patients who initiate dialysis urgently (12,70) and are unlikely to have an established and useable vascular access. On the contrary, some patients do not progress to start dialysis. In a study by Oliver et al. (71) of 1929 patients who were predialysis and had AVF creation, 10.6% did not start dialysis 2 years later. At 6 months post-AVF creation, only 48.8% of patients required dialysis. A larger, retrospective US Renal Data System (USRDS) –based study of 17, 511 elderly patients by Hod et al. (72) found that placing an AVF >6–9 months before HD initiation may not associate with better AVF outcomes; however, this depends on knowing when a patient will start dialysis. When seeing a patient in the clinic, clinicians do not have the advantage of hindsight afforded to retrospective studies. In the study by Hod et al. (72) and other studies, increased interventions were required to maintain AVF patency before HD start. The need for such interventions must be balanced by the fact that some patients do not start dialysis at all because of competing events, such as transplantation, modality switch to PD, or death. The ratio of unnecessary to necessary procedures 2 years after AVF creation is modified by age and estimated to be 5:1 for patients ages 85–100 years old but only 0.5:1 for those ages 18–44 years old (73).
Until we can better determine and balance the timing of vascular access creation in the mix of competing risks of harms (e.g., unnecessary interventions), death, and dialysis start, perhaps simply establishing an ESRD life plan that includes AV access assessment and planning will help, even with urgent starts. Seeing a surgeon for AV access assessment only may help achieve the appropriate access. If the patient is not eligible for an AVF, an AV graft can be placed within 72 hours of dialysis initiation depending on the AV graft material. If an AVF is possible, closer follow-up may be required to better determine the rate of GFR decline to be able to estimate the 6–9 months when dialysis is anticipated. The risk of AVF failure may be estimated (27,52) to plan and coordinate the creation and potential necessary interventions required within that time, so that the AVF will be mature and suitable for cannulation when needed (below).
Infrastructure
Process of care issues have been identified as barriers to improving AVF rates and outcomes (74–76). A multidisciplinary approach involving surgeons, nephrologists, interventional radiologists, dialysis nurses, vascular access coordinators, and ultrasound technicians has been shown to result in greater numbers of functioning AVFs without increasing catheters (75) and higher primary and secondary AV access patency (76). After AVF creation, a comprehensive follow-up program can result in a high rate of AVF salvage and maturation (77). After AV graft creation, a multidisciplinary monitoring and surveillance program may reduce complications and improve outcomes. Use of a prospective database to capture clinical activity and vascular access outcomes (below) and engagement in continuous quality improvement will allow teams to analyze practices and help inform AV access decision making.
Vascular Access Outcomes
Vascular access outcomes include access patency (primary, functional, and cumulative patency), interventions required to attain and maintain patency, type–specific AV access outcomes, and their associated costs. These, in turn, are influenced by the factors discussed above. Although these outcomes are key considerations in choosing the optimal vascular access for an individual patient, these discussions are beyond the scope of this review. However, it is important to emphasize the need to continually study and re-evaluate these key outcomes. For example, although past and current data do suggest superior outcomes of AVFs versus AV grafts, recent data also show equivalent or superior primary and cumulative patency of AV grafts (78,79). Complications may also change over time with improved management. For example, AV grafts have traditionally been associated with increased risk of infection. However, in a recent large USRDS–based study with prospective monthly collection of infection data of 177,875 patients on prevalent HD and 11,290 patients on incident HD, the vascular access infection rates were identical in patients with AVFs and AV grafts (80), highlighting the need to continually re-evaluate the data on vascular access outcomes for clinicians to make the best decisions in choosing the optimal vascular access.
Conclusion
Choosing the optimal AV access for a patient is complex and requires consideration of individual patient–related factors, anatomic access circuit–related characteristics, surgical features, and process and timing variables. However, central to these considerations for a single optimal AV access are how and where this optimal vascular access fits in the patient’s overall ESRD life plan. Each dialysis access along the patient’s ESRD life plan should be an optimal access—placed at the right time under the right circumstances in the right patient for the right reasons. To do so is challenging and requires a coordinated multidisciplinary team approach that educates the patient, considers patient preference, tracks and evaluates their own outcomes, and makes modifications on the basis of re-evaluation and new evidence.
Disclosures
None.
Acknowledgment
No financial support was received for work related to this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Al Shakarchi J, Houston G, Inston N: Early cannulation grafts for haemodialysis: A systematic review. J Vasc Access 16: 493–497, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Maytham GG, Sran HK, Chemla ES: The use of the early cannulation prosthetic graft (Acuseal™) for angioaccess for haemodialysis. J Vasc Access 16: 467–471, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Rehman R, Schmidt RJ, Moss AH: Ethical and legal obligation to avoid long-term tunneled catheter access. Clin J Am Soc Nephrol 4: 456–460, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Lee T, Thamer M, Zhang Y, Zhang Q, Allon M: Outcomes of elderly patients after predialysis vascular access creation. J Am Soc Nephrol 26: 3133–3140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok CE, Davidson I: Optimal choice of dialysis access for chronic kidney disease patients: Developing a life plan for dialysis access. Semin Nephrol 32: 530–537, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Ernandez T, Saudan P, Berney T, Merminod T, Bednarkiewicz M, Martin PY: Risk factors for early failure of native arteriovenous fistulas. Nephron Clin Pract 101: c39–c44, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Maya ID, O’Neal JC, Young CJ, Barker-Finkel J, Allon M: Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin J Am Soc Nephrol 4: 86–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel ST, Hughes J, Mills JL Sr.: Failure of arteriovenous fistula maturation: An unintended consequence of exceeding dialysis outcome quality initiative guidelines for hemodialysis access. J Vasc Surg 38: 439–445, 2003 [DOI] [PubMed]
- 9.Huang SG, Rowe VL, Weaver FA, Hwang F, Woo K: Compliance with surgical follow-up does not influence fistula maturation in a county hospital population. Ann Vasc Surg 28: 1847–1852, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Korten E, Toonder IM, Schrama YC, Hop WCJ, van der Ham AC, Wittens CHA: Dialysis fistulae patency and preoperative diameter ultrasound measurements. Eur J Vasc Endovasc Surg 33: 467–471, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 12.US Renal Data System: Incidence, prevalence, patient characteristics, and treatment modalities. In: USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2015, p 222 [Google Scholar]
- 13.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN: A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg 45: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Tamura MK, Tan JC, O’Hare AM: Optimizing renal replacement therapy in older adults: A framework for making individualized decisions. Kidney Int 82: 261–269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vachharajani TJ, Moossavi S, Jordan JR, Vachharajani V, Freedman BI, Burkart JM: Re-evaluating the Fistula First Initiative in octogenarians on hemodialysis. Clin J Am Soc Nephrol 6: 1663–1667, 2011 [DOI] [PubMed] [Google Scholar]
- 17.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J, Steele D, Wenger J, Kawai T, Liu F, Elias N, Watkins MT, Irani Z: Hemodialysis arteriovenous fistula as first option not necessary in elderly patients. J Vasc Surg 63: 1326–1332, 2016 [DOI] [PubMed]
- 19.Jadlowiec CC, Mannion EM, Lavallee M, Brown MG: Hemodialysis access in the elderly: Outcomes among patients older than seventy. Ann Vasc Surg 31: 77–84, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Woo K, Goldman DP, Romley JA: Early failure of dialysis access among the elderly in the era of fistula first. Clin J Am Soc Nephrol 10: 1791–1798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrogan D, Al Shakarchi J, Khawaja A, Nath J, Hodson J, Maxwell AP, Inston NG: Arteriovenous fistula outcomes in the elderly. J Vasc Surg 62: 1652–1657, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Gifford E, Nguyen V, Kaji AH, Chisum P, Zeng A, Dukkipati R, de Virgilio C: Increased use of brachiocephalic arteriovenous fistulas improves functional primary patency. J Vasc Surg 62: 442–447, 2015 [DOI] [PubMed] [Google Scholar]
- 23.O’Hare AM: Vascular access for hemodialysis in older adults: A “patient first” approach. J Am Soc Nephrol 24: 1187–1190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masengu A, Hanko JB, Maxwell AP: Optimizing outcomes in the elderly with end-stage renal disease--live long and prosper. J Vasc Access 16: 439–445, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Lomonte C, Forneris G, Gallieni M, Tazza L, Meola M, Lodi M, Senatore M, Morale W, Spina M, Napoli M, Bonucchi D, Galli F: The vascular access in the elderly: A position statement of the Vascular Access Working Group of the Italian Society of Nephrology. J Nephrol 29: 175–184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astor BC, Eustace JA, Klag MJ, Powe NR, Longenecker JC, Fink NE, Marcovina SM, Coresh J; CHOICE Study: Race-specific association of lipoprotein(a) with vascular access interventions in hemodialysis patients: The CHOICE Study. Kidney Int 61: 1115–1123, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Obialo CI, Tagoe AT, Martin PC, Asche-Crowe PE: Adequacy and survival of autogenous arteriovenous fistula in African American hemodialysis patients. ASAIO J 49: 435–439, 2003 [PubMed] [Google Scholar]
- 29.Arce CM, Mitani AA, Goldstein BA, Winkelmayer WC: Hispanic ethnicity and vascular access use in patients initiating hemodialysis in the United States. Clin J Am Soc Nephrol 7: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarkowsky DS, Arhuidese IJ, Hicks CW, Canner JK, Qazi U, Obeid T, Schneider E, Abularrage CJ, Freischlag JA, Malas MB: Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg 150: 529–536, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald JT, Schanzer A, Chin AI, McVicar JP, Perez RV, Troppmann C: Outcomes of upper arm arteriovenous fistulas for maintenance hemodialysis access. Arch Surg 139: 201–208, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Diehm N, van den Berg JC, Schnyder V, Bühler J, Willenberg T, Widmer M, Mohaupt MG, Baumgartner I: Determinants of haemodialysis access survival. Vasa 39: 133–139, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Dunn J, Herscu G, Woo K: Factors influencing maturation time of native arteriovenous fistulas. Ann Vasc Surg 29: 704–707, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lauvao LS, Ihnat DM, Goshima KR, Chavez L, Gruessner AC, Mills JL Sr.: Vein diameter is the major predictor of fistula maturation. J Vasc Surg 49: 1499–1504, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voormolen EH, Jahrome AK, Bartels LW, Moll FL, Mali WP, Blankestijn PJ: Nonmaturation of arm arteriovenous fistulas for hemodialysis access: A systematic review of risk factors and results of early treatment. J Vasc Surg 49: 1325–1336, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Puskar D, Pasini J, Savić I, Bedalov G, Sonicki Z: Survival of primary arteriovenous fistula in 463 patients on chronic hemodialysis. Croat Med J 43: 306–311, 2002 [PubMed] [Google Scholar]
- 39.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM: Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 22: 1526–1533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SC, Chang JM, Hwang SJ, Tsai JC, Wang CS, Mai HC, Lin FH, Su HM, Chen HC: Significant correlation between ankle-brachial index and vascular access failure in hemodialysis patients. Clin J Am Soc Nephrol 4: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Cull DL, Taylor SM, Carsten CG, Youkey JR, Snyder BA, Sullivan TM, Langan EM: The fistula elevation procedure: A valuable technique for maximizing arteriovenous fistula utilization. Ann Vasc Surg 16: 84–88, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Causey MW, Quan R, Hamawy A, Singh N: Superficialization of arteriovenous fistulae employing minimally invasive liposuction. J Vasc Surg 52: 1397–1400, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Barnard KJ, Taubman KE, Jennings WC: Accessible autogenous vascular access for hemodialysis in obese individuals using lipectomy. Am J Surg 200: 798–802, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Chan MR, Young HN, Becker YT, Yevzlin AS: Obesity as a predictor of vascular access outcomes: Analysis of the USRDS DMMS Wave II study. Semin Dial 21: 274–279, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Johansen KL, Dalrymple LS, Glidden D, Delgado C, Kaysen GA, Grimes B, Chertow GM: Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 11: 626–632, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB: The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 43: 861–867, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Grubbs V, Wasse H, Vittinghoff E, Grimes BA, Johansen KL: Health status as a potential mediator of the association between hemodialysis vascular access and mortality. Nephrol Dial Transplant 29: 892–898, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kosa SD, Bhola C, Lok CE: Hemodialysis patients’ satisfaction and perspectives on complications associated with vascular access related interventions: Are we listening? [published online ahead of print June 1, 2016]. J Vasc Access doi:10.5301/jva.5000560 [DOI] [PubMed] [Google Scholar]
- 51.Chaudhry M, Bhola C, Joarder M, Zimmerman D, Quinan P, Mendelssohn D, Lok CE: Seeing eye to eye: The key to reducing catheter use. J Vasc Access 12: 120–126, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Pisoni RL, Zepel L, Port FK, Robinson BM: Trends in US vascular access use, patient preferences, and related practices: An update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis 65: 905–915, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Xi W, Harwood L, Diamant MJ, Brown JB, Gallo K, Sontrop JM, MacNab JJ, Moist LM: Patient attitudes towards the arteriovenous fistula: A qualitative study on vascular access decision making. Nephrol Dial Transplant 26: 3302–3308, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Cavanaugh KL, Wingard RL, Hakim RM, Elasy TA, Ikizler TA: Patient dialysis knowledge is associated with permanent arteriovenous access use in chronic hemodialysis. Clin J Am Soc Nephrol 4: 950–956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khavanin Zadeh M, Gholipour F, Naderpour Z, Porfakharan M: Relationship between vessel diameter and time to maturation of arteriovenous fistula for hemodialysis access. Int J Nephrol 2012: 942950, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, Li L, Feldman HI; HFM Study Group: Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg 63: 163–170, 2016 [DOI] [PMC free article] [PubMed]
- 58.Dageforde LA, Harms KA, Feurer ID, Shaffer D: Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. J Vasc Surg 61: 170–176, 2015 [DOI] [PubMed] [Google Scholar]
- 59.van der Linden J, Lameris TW, van den Meiracker AH, de Smet AA, Blankestijn PJ, van den Dorpel MA: Forearm venous distensibility predicts successful arteriovenous fistula. Am J Kidney Dis 47: 1013–1019, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Korten E, Spronk S, Hoedt MT, de Jong GM, Tutein Nolthenius RP: Distensibility of forearm veins in haemodialysis patients on duplex ultrasound testing using three provocation methods. Eur J Vasc Endovasc Surg 38: 375–380, 2009 [DOI] [PubMed]
- 61.Kim MH, Kim YK, Jun KW, Hwang JK, Kim SD, Kim JY, Park SC, Kim YS, Moon IS, Kim JI: Clinical importance of intraoperative cephalic vein distensibility as a predictor of radiocephalic arteriovenous fistula maturation. Semin Dial 28: E64–E70, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allon M, Robbin ML, Umphrey HR, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Barker-Finkel J, Litovsky S: Preoperative arterial microcalcification and clinical outcomes of arteriovenous fistulas for hemodialysis. Am J Kidney Dis 66: 84–90, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toomay S, Rectenwald J, Vazquez MA: How can the complications of central vein catheters be reduced?: Central venous stenosis in hemodialysis patients. Semin Dial 29: 201–203, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Forauer AR, Theoharis C: Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol 14: 1163–1168, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Shingarev R, Barker-Finkel J, Allon M: Association of hemodialysis central venous catheter use with ipsilateral arteriovenous vascular access survival. Am J Kidney Dis 60: 983–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saran R, Elder SJ, Goodkin DA, Akiba T, Ethier J, Rayner HC, Saito A, Young EW, Gillespie BW, Merion RM, Pisoni RL: Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg 247: 885–891, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Fassiadis N, Morsy M, Siva M, Marsh JE, Makanjuola AD, Chemla ES: Does the surgeon’s experience impact on radiocephalic fistula patency rates? Semin Dial 20: 455–457, 2007 [DOI] [PubMed] [Google Scholar]
- 69.McGrogan DG, Maxwell AP, Inston NG, Krishnan H, Field M: Preserving arteriovenous fistula outcomes during surgical training. J Vasc Access 15: 474–480, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Lok CE: Urgent peritoneal dialysis or hemodialysis catheter dialysis. J Vasc Access 17[Suppl 1]: 56–59, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM: Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 7: 466–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hod T, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Arteriovenous fistula placement in the elderly: When is the optimal time? J Am Soc Nephrol 26: 448–456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Vargas PA, Craig JC, Gallagher MP, Walker RG, Snelling PL, Pedagogos E, Gray NA, Divi MD, Gillies AH, Suranyi MG, Thein H, McDonald SP, Russell C, Polkinghorne KR: Barriers to timely arteriovenous fistula creation: A study of providers and patients. Am J Kidney Dis 57: 873–882, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Kiaii M, MacRae JM: A dedicated vascular access program can improve arteriovenous fistula rates without increasing catheters. J Vasc Access 9: 254–259, 2008 [PubMed] [Google Scholar]
- 76.Flu H, Breslau PJ, Krol-van Straaten JM, Hamming JF, Lardenoye JW: The effect of implementation of an optimized care protocol on the outcome of arteriovenous hemodialysis access surgery. J Vasc Surg 48: 659–668, 2008 [DOI] [PubMed] [Google Scholar]
- 77.McLafferty RB, Pryor RW 3rd, Johnson CM, Ramsey DE, Hodgson KJ: Outcome of a comprehensive follow-up program to enhance maturation of autogenous arteriovenous hemodialysis access. J Vasc Surg 45: 981–985, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allemang MT, Schmotzer B, Wong VL, Lakin RO, Woodside KJ, Schulak JA, Wang J, Kashyap VS: Arteriovenous grafts have higher secondary patency in the short term compared with autologous fistulae. Am J Surg 208: 800–805, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Solid C, Foley R: Vascular access and infections from dialysis claims. Presented at ASN Kidney Week, San Diego, CA, October 30–November 4, 2012 [Google Scholar]