Abstract
Background and objectives
Infection is the most common cause of death in severe AKI, but many patients receiving continuous RRT do not reach target antibiotic concentrations in plasma. Extended infusion of β-lactams is associated with improved target attainment in critically ill patients; thus, we hypothesized that extended infusion piperacillin-tazobactam would improve piperacillin target attainment compared with short infusion in patients receiving continuous RRT.
Design, setting, participants, & measurements
We conducted an institutional review board–approved observational cohort study of piperacillin-tazobactam pharmacokinetics and pharmacodynamics in critically ill patients receiving continuous venovenous hemodialysis and hemodiafiltration at three tertiary care hospitals between 2007 and 2015. Antibiotic concentrations in blood and/or dialysate samples were measured by liquid chromatography, and one– and two–compartment pharmacokinetic models were fitted to the data using nonlinear mixed effects regression. Target attainment for piperacillin was defined as achieving four times the minimum inhibitory concentration of 16 μg/ml for >50% of the dosing cycle. The probabilities of target attainment for a range of doses, frequencies, and infusion durations were estimated using a Monte Carlo simulation method. Target attainment was also examined as a function of patient weight and continuous RRT effluent rate.
Results
Sixty-eight participants had data for analysis. Regardless of infusion duration, 6 g/d piperacillin was associated with ≤45% target attainment, whereas 12 g/d was associated with ≥95% target attainment. For 8 and 9 g/d, target attainment ranged between 68% and 85%. The probability of target attainment was lower at higher effluent rates and patient weights. For all doses, frequencies, patient weights, and continuous RRT effluent rates, extended infusion was associated with higher probability of target attainment compared with short infusion.
Conclusions
Extended infusions of piperacillin-tazobactam are associated with greater probability of target attainment in patients receiving continuous RRT.
Keywords: pharmacokinetics, Acute Kidney Injury, piperacillin, extended-infusion, Anti-Bacterial Agents, Critical Illness, Dialysis Solutions, Humans, Microbial Sensitivity Tests, Renal Replacement Therapy
Introduction
AKI is a common and devastating complication of medical and surgical illness, affecting 6% of hospital admissions, and it is an independent risk factor for prolonged inpatient length of stay and mortality (1–4). Prospective randomized clinical trials comparing intensive and conventional doses of continuous hemodiafiltration have not shown a survival benefit to intensive renal support in AKI (5,6). Because infection has been among the leading causes of death in acute renal failure and because early appropriate antibiotic therapy seems critical to treatment success (7–10), we directed our attention to adequacy of antimicrobial therapy in patients with AKI receiving continuous RRT (CRRT) (11–13). We showed that CRRT waste (effluent) can serve as a biomatrix for estimating plasma free drug concentrations in lieu of repeated phlebotomy and that interindividual variability in β-lactam pharmacokinetics (PK) undermines target attainment in critically ill patients receiving CRRT (11,12,14). Indeed, recent publications from the Kidney Disease Improving Global Outcomes and the Kidney Health Initiative groups highlight the knowledge gap and need for improved drug dosing in acute renal disease (15,16). It is now clear that one size fits all dosing using standard adjustments to account for renal failure and dialysis does not guarantee adequate plasma concentrations in all or even most patients. These concerns are present in the general intensive care unit (ICU) population as well, motivating comparisons of different antibiotic administration schedules. In particular, continuous infusion (CI) and extended infusion (EI) of time-dependent antibiotics have attracted attention as strategies to improve the probability of target attainment. Studies in critically ill patients have examined CIs and 30-minute infusions (short infusions [SIs]) of β-lactams and concluded that prolonging the infusion duration of β-lactams improves target attainment (17–20). Whether EI of piperacillin-tazobactam improves target attainment in patients receiving CRRT is unknown. In this institutional review board–approved observational study, we used measurements of piperacillin and tazobactam concentration in the blood and dialysate to characterize the variability in PK among patients treated at one of three institutions with contrasting practices: University of Alabama at Birmingham (UAB) and Cleveland Clinic (CCF), where patients received piperacillin-tazobactam as 30-minute infusions, and Vanderbilt University (VU), where patients received piperacillin-tazobactam as 240-minute infusions.
Materials and Methods
Patients admitted to the ICUs of UAB (n=25), CCF (n=29), and VU (n=14) were enrolled between February 1 2009 and July 1, 2015. The study protocol was approved by the institutional review board at each institution. All patients or their surrogate decision makers at UAB and CCF provided written informed consent to participate in the study. At VU, patients who provided blood and effluent samples (n=5) or their surrogates gave written informed consent. Patients providing samples of waste effluent only (n=9) were enrolled in a consent-waivered protocol.
Participants
Patients ages 18 years old or older who were receiving piperacillin-tazobactam with concomitant CRRT in the ICU were included. Patients were excluded if they were known to be pregnant. Age, sex, weights at admission and time of study entry, dialysis prescription, and urine output were recorded on case report forms.
Antimicrobial Therapy
Patients in the study received piperacillin-tazobactam (2 g piperacillin and 250 mg tazobactam or 3 g piperacillin and 375 mg tazobactam) administered over 30 minutes scheduled every 6, 8, or 12 hours (UAB and CCF) or 240 minutes every 8 hours (VU). The antimicrobial dose and frequency were determined by collaboration between the primary ICU service, critical care clinical pharmacists, the consulting nephrology service, and the consulting infectious disease service if involved in the patient’s case.
Dialysis Therapy
Patients received continuous venovenous hemodialysis or continuous venovenous hemodiafiltration using the Gambro Prismaflex System (Gambro Renal Products, Boulder, CO) with M-100 or HF-1500 dialyzer sets (acrylonitrile [AN69] membranes with a 0.9-m2 membrane surface area or polysulfone with a 1.5-m2 area, respectively) or the NxStage System One (NxStage Medical, Lawrence, MA) dialysis system with the Express Cartridge (polyethersulfone membrane with a 1.5-m2 membrane surface area) according to instrument availability and nephrology consulting service discretion. CRRT dose was targeted to 25 ml/kg per hour, and all convective modalities were in predilution mode. CRRT prescriptions were determined by the nephrology consulting service. Anticoagulation was achieved with an infusion of unfractionated heparin (250–500 IU/h) as tolerated or regional citrate anticoagulation (UAB). For this manuscript, we have chosen to call waste fluids (both spent dialysate and ultrafiltrate) effluent.
Sampling
Sampling consisted of blood samples and CRRT waste (effluent). Because piperacillin-tazobactam concentrations in CRRT effluent agree well with free piperacillin and tazobactam concentrations in plasma (14), some participants underwent CRRT effluent sampling alone, and effluent concentrations were used to estimate plasma concentrations for PK modeling. Sampling commenced after administration of at least two doses of piperacillin-tazobactam and 24 hours of concomitant uninterrupted CRRT. If the sampling period was interrupted by a disruption of CRRT, the sampling protocol allowed one additional collection attempt during a subsequent drug dosing period. In patients who received 30-minute infusions, paired sets of blood and CRRT effluent samples were collected immediately before the start of infusion (trough 1), 30 minutes after the end of infusion (peak), and immediately before the subsequent dose (trough 2; CCF and UAB). Participants who received 240-minute infusions were sampled at 0, 30, 60, 120, and 240 minutes after the end of the infusion (VU). Blood samples were collected in 4.5-ml plasma separator vacutainer tubes. CRRT effluent samples were collected in 7-ml vacutainer tubes containing no additive. Because the majority of participants were oligoanuric, no urine samples were collected or analyzed. All samples were stored on ice and processed within 60 minutes of collection. Blood samples were centrifuged at 3000×g for 10 minutes at 4°C. Effluent samples were centrifuged identically to ensure that samples injected on the HPLC column were free of particulates. Two aliquots from each specimen were then placed in liquid nitrogen. All samples were analyzed in the laboratory of one of the investigators.
HPLC Analyses
Piperacillin and tazobactam concentrations were quantified by reverse-phase HPLC (Agilent 1200 Series; Agilent Technologies, Santa Clara, CA). Separation was achieved using a Phenomenex XB-C18 Column (100×3 mm; 00D-4496-Y0; Phenomenex, Torrance, CA) with Phenomenex SecurityGuard ULTRA (AJ0–8775; Phenomenex). A temperature-controlled autosampler (4C) was programmed to mix samples with an internal standard and inject onto the column (10 μl). Piperacillin and tazobactam were quantified with a gradient elution of A (PBS [P4417; Sigma-Aldrich, St. Louis, MO] with methanol [2% A412; Fisher Scientific, Waltham, MA]) and B (trifluoroacetic acid in acetonitrile [0.1% vol/vol TX1277P-1; EMD Millipore, Billerica, MA]). Elution occurred over 5 minutes (0.8 ml/min; 0%–20% B) followed by 2 minutes of re-equilibration. Penicillin was used as an internal standard.
Antibiotic concentrations were measured by ultraviolet absorption with an Agilent Diode Array Detector (G1315C; Agilent Technologies). Piperacillin and tazobactam were quantified by ultraviolet absorption at 214 nm. Five concentration standards were used to calibrate the detector and mixed with the same stocks that were used to make the test concentrations above. The within– and between–day coefficients of variation for piperacillin at a concentration of 25 μg/ml were 1.9% and 10.2%, respectively, and at a concentration of 100 μg/ml were 0.7% and 2.0%, respectively. The lower limit of quantitation for piperacillin is 0.5 μg/ml. We have not specifically investigated lower limit of detection of the assay, because the lower limit of quantitation is far lower than any clinically relevant plasma concentration.
PK and Pharmacodynamic Calculations
Both one– and two–compartment linear differential equation models were used to describe first–order drug elimination. Separate solutions to the homogeneous (between antibiotic infusions) and nonhomogeneous equations (during antibiotic infusions) were combined to describe the time course of drug concentration throughout the dosing interval. Plasma concentrations were modeled as the sum of effects from the most recent dose and prior doses. Steady–state trough values were approximated as the concentration of drug at the end of five consecutive dosing intervals. The fraction of the dosing interval in which the free plasma concentration was greater than four times the minimum inhibitory concentration (MIC; denoted fT>4×MIC) was calculated using the compartment model solution for drug concentration. The primary pharmacodynamic target was >0.5 fT>4×MIC or 50% of the dosing interval, where the MIC was 16 μg/ml for piperacillin and 0.25 μg/ml for tazobactam. For piperacillin, we evaluated a range of alternative MIC values (4, 8, 32, and 64 μg/ml) for both interval fraction targets (50% and 100%) (21–24).
Statistical Analyses and Monte Carlo Simulation
Nonlinear mixed effects (NLME) regression was used to analyze the concentration-time data using the one- or two-compartment model as described above. The model parameters were expressed using fixed and random effects to account for between-subject heterogeneity, where the latter was assumed to be normally distributed with mean of zero and unstructured covariance. The random effects were estimated from this marginal model (i.e., not conditional on demographic or clinical factors) using the empirical Bayes method and plotted against subject–level covariates weight (at study enrollment), age, sex, and CRRT effluent rate. Simple linear regression was used to quantify the pairwise associations. The adjusted R2 and chi-squared test were used to evaluate statistical significance. To further examine these associations, the fixed effects were multiply regressed onto the subject-level covariates. The statistical significance of covariate effects in this conditional model was assessed using the Wald test. Covariates that did not show statistically significant association with any parameter were omitted from the final conditional model. For both the marginal and conditional models, the antibiotic dose, frequency, and infusion duration were adjusted implicitly, because those details are necessary to evaluate the compartment model solution for each participant. We did not adjust for center or CRRT modality because of confounding with antibiotic and CRRT prescriptions and participant weight, age, and sex. The estimated random effects from the marginal model were used to compute the predicted concentrations for each subject and drug. The quality of the marginal model fit was assessed by plotting the predicted versus observed concentrations for each subject (Supplemental Appendix).
For a given dosing regimen (amount, frequency, and infusion duration), the median fT>4×MIC and probability of target attainment were computed using a Monte Carlo simulation method by sampling from the marginal NLME–estimated population distribution of volume of distribution (V) and the elimination rate (k; marginal model fixed effects and random effects distribution). A sample of size 10,000 was used for all calculations. Samples were reused where possible to eliminate the effect of Monte Carlo error in comparing dosing regimens. Probability of target attainment was approximated as the sample proportion that achieved the pharmacodynamic target. SI versus EI regimens were compared by computing the probability ratio. The SEM for the log probability ratio was computed by propagating sampling uncertainty from the fixed effect estimates. Specifically, the SEM was approximated by integrating over the NLME–estimated sampling distribution of the fixed effects estimates, which is approximately multivariate normal. Gauss–Hermite quadrature with 100 sample points was used to approximate the integral. The estimated SEM was used to compute a Wald–type 95% confidence interval (95% CI) and test. Except for the regimen of 2 g piperacillin and 0.25 g tazobactam every 12 hours, all combinations of doses (piperacillin at 2, 3, and 4 g; tazobactam at 0.25, 0.375, and 0.500 g) and frequencies (every 6, 8, and 12 hours) were evaluated. In addition, for each dose and frequency combination, both SI and EI (30 and 240 minutes, respectively) were considered.
A second series of Monte Carlo simulations was implemented to study the effects of patient weight and CRRT effluent rate on the probability of target attainment among patients who receive 3 g piperacillin every 8 hours (the most frequently administered regime in these data). For these analyses, a sample of size 10,000 was drawn from the conditional (on weight and CRRT effluent rate) NLME–estimated distribution of V and k. Probability of target attainment was approximated for EI and SI regimens over a range of representative patient weights and CRRT effluent rates. These results are presented graphically.
To assess the sufficiency of the single-compartment model, all of the above analyses were reimplemented using a two-compartment model with one additional fixed effect parameter, which represented the rate of intercompartmental clearance. There was insufficient information to model the additional parameter using a random effect or regress the fixed effect onto subject-level covariates. The open source R Statistical Package and nlme add–on package were used to implement all analyses and graphics (25–27). P values <0.01 were considered statistically significant.
Results
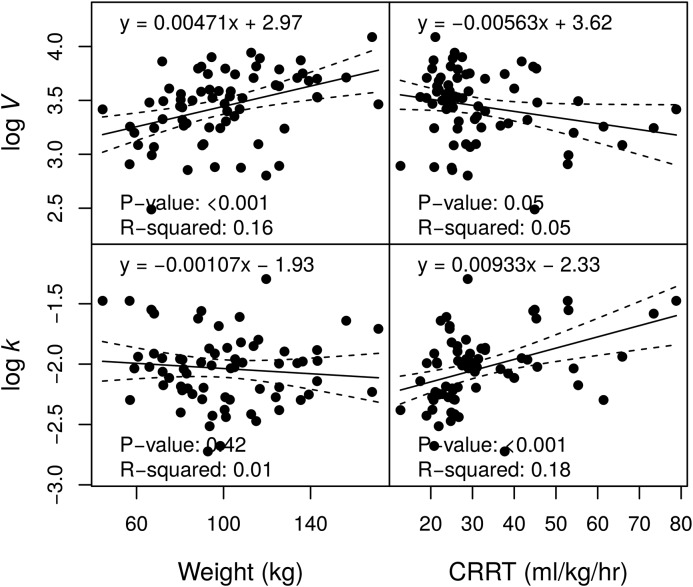
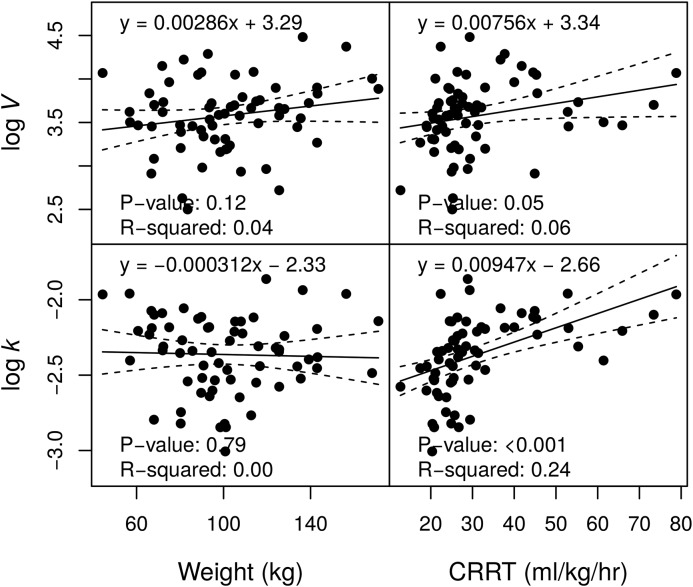
Sixty-eight participants were enrolled and had data for analysis (Table 1). In total, there were 214 piperacillin and 203 tazobactam concentration measurements. Participants were predominantly men and heavier than the participants in prior studies. Overall mortality was 46%, similar to mortality rates observed in recent large studies of dialysis dose in AKI (5,6). For piperacillin, V was significantly correlated with body weight, and k was significantly associated with CRRT effluent rate (Figure 1). For tazobactam, k was significantly associated with CRRT effluent rate (Figure 2). These findings were confirmed by the conditional NLME regression analyses. No other pairwise associations between PK parameters and subject-level factors were statistically significant (at P<0.01). There was no evidence in this analysis that CRRT modality (continuous venovenous hemodiafiltration versus continuous venovenous hemodialysis) had an effect on piperacillin or tazobactam PK (P>0.14). This result corroborates an earlier study (11).
Table 1.
Descriptive statistics by institution
| Characteristic | VU, n=14 | CCF, n=29 | UAB, n=25 | Combined, n=68 |
|---|---|---|---|---|
| Antibiotic dose, g | ||||
| 2 | 0% (0) | 0% (0) | 84% (21) | 31% (21) |
| 3 | 100% (14) | 100% (29) | 16% (4) | 69% (47) |
| Antibiotic infusion duration, min | ||||
| 30 | 0% (0) | 100% (29) | 100% (25) | 79% (54) |
| 240 | 100% (14) | 0% (0) | 0% (0) | 21% (14) |
| Antibiotic frequency, h | ||||
| 6 | 0% (0) | 3% (1) | 96% (24) | 37% (25) |
| 8 | 79% (11) | 76% (22) | 4% (1) | 50% (34) |
| 12 | 21% (3) | 21% (6) | 0% (0) | 13% (9) |
| CRRT effluent rate, ml/kg per h | 30.9 (28.5–33.0) | 22.8 (20.8–25.3) | 40.0 (28.5–45.5) | 26.6 (23.7–37.0) |
| CRRT mode | ||||
| CVVH | 36% (5) | 0% (0) | 0% (0) | 7.4% (5) |
| CVVHD | 0% (0) | 100% (29) | 0% (0) | 42.6% (29) |
| CVVHDF | 64% (9) | 0% (0) | 100% (25) | 50% (34) |
| Weight, kg | 99.8 (80.8–118.7) | 100.8 (90.2–125.5) | 88.3 (68.1–105.3) | 96.9 (80.8–116.2) |
| Age, yr | 53.5 (47.0–60.5) | 64.0 (55.0–72.0) | 51.0 (40.0–63.0) | 56.5 (50.0–70.0) |
| Sex | ||||
| Women | 29% (4) | 21% (6) | 56% (14) | 35% (24) |
| Men | 71% (10) | 79% (23) | 44% (11) | 65% (44) |
| Mortality | 43% (6) | 41% (12) | 52% (13) | 46% (31) |
| Piperacillin PK | ||||
| V | 27.3 (22.7–31.0) | 34.4 (30.5–44.1) | 33.8 (26.2–40.9) | 33.0 (25.7–40.5) |
| k | 0.151 (0.131–0.177) | 0.108 (0.093–0.134) | 0.140 (0.130–0.194) | 0.132 (0.106–0.153) |
| Tazobactam PK | ||||
| V | 34.5 (30.8–39.1) | 31.4 (24.7–36.6) | 47.8 (39.5–59.0) | 36.6 (28.2–46.1) |
| k | 0.102 (0.094–0.112) | 0.079 (0.071–0.091) | 0.115 (0.105–0.124) | 0.096 (0.080–0.113) |
Quantitative values are summarized using medians (interquartile ranges). Categorical variables are summarized using percentages (counts). VU, Vanderbilt University; CCF, Cleveland Clinic Foundation; UAB, University of Alabama at Birmingham; CRRT, continuous RRT; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; PK, pharmacokinetics; V, volume of distribution; k, elimination rate.
Figure 1.
Associations between piperacillin pharmacokinetic parameter estimates and demographics. The volume of distribution (V) and the elimination rate (k) are displayed on a natural logarithm scale. CRRT, continuous RRT.
Figure 2.
Associations between tazobactam pharmacokinetic parameter estimates and demographics. The volume of distribution (V) and the elimination rate (k) are displayed on a natural logarithm scale. CRRT, continuous RRT.
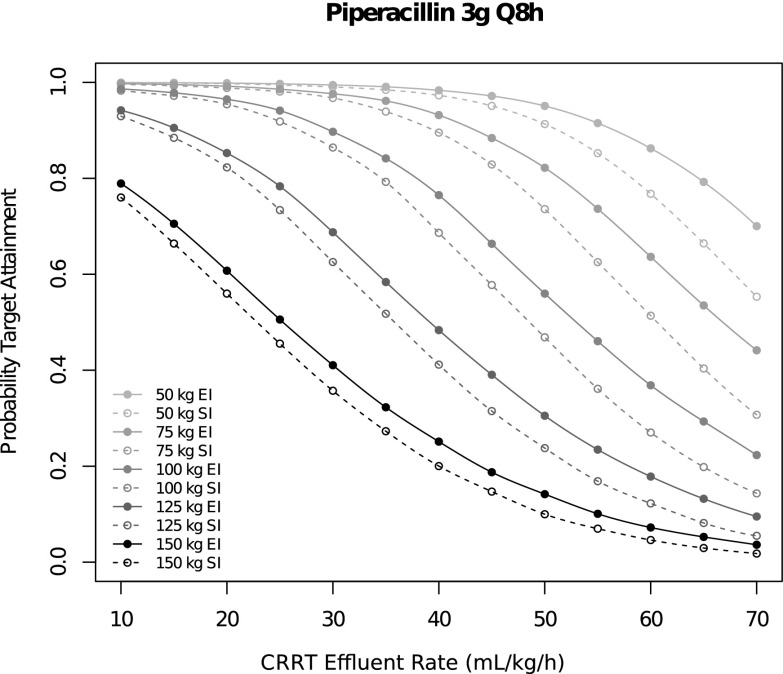
Regardless of infusion duration, 6 g/d piperacillin was associated with ≤45% target attainment, whereas 12 g/d was associated ≥95% target attainment. For 8 and 9 g/d, target attainment ranged between 68% and 85%. For each dose amount and frequency, the probability of target attainment (fT>416 μg/ml for 50% of interval) was significantly greater for EI versus SI (Table 2). The greatest differences were observed in the 4 g/12 h, 3 g/12 h, and 2 g/8 h regimens, where the probability of target attainment was greater by 14% (95% CI, 12% to 17%), 19% (95% CI, 13% to 25%), and 12% (95% CI, 9% to 16%), respectively. These findings were concordant but different in magnitude under alternative MIC and interval fraction targets (Supplemental Appendix). The probability of target attainment was lower in participants with greater weight and greater CRRT effluent rate. The probability of target attainment was greater for EI versus SI (Figure 3). The target attainment probabilities estimated using the two-compartment model were very similar in magnitude and qualitatively equivalent.
Table 2.
Probability of target attainment for minimum inhibitory concentration =16 μg/ml
| Dose, g | Freq., h | Prob. SI | Prob. EI | Prob. Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| 2 | 6 | 0.71 | 0.74 | 1.05 (1.01 to 1.09) | <0.01 |
| 2 | 8 | 0.39 | 0.44 | 1.12 (1.09 to 1.16) | <0.001 |
| 3 | 6 | 0.96 | 0.97 | 1.01 (1.00 to 1.02) | <0.01 |
| 3 | 8 | 0.80 | 0.84 | 1.05 (1.01 to 1.09) | <0.01 |
| 3 | 12 | 0.35 | 0.42 | 1.19 (1.13 to 1.25) | <0.001 |
| 4 | 6 | 0.99 | 0.99 | 1.01 (1.00 to 1.01) | <0.001 |
| 4 | 8 | 0.95 | 0.97 | 1.03 (1.01 to 1.04) | 0.001 |
| 4 | 12 | 0.63 | 0.72 | 1.14 (1.12 to 1.17) | <0.001 |
Freq., dose frequency; Prob. SI, probability of target attainment associated with short (30-minute) infusion; Prob. EI, probability of target attainment (four times minimum inhibitory concentration >50%) associated with extended (240-minute) infusion; Prob. Ratio, ratio of extended infusion versus short infusion target attainment probabilities; 95% CI, 95% confidence interval.
Figure 3.
Probability of target attainment (four times minimum inhibitory concentration >50%; minimum inhibitory concentration =16 μg/ml) as a function of patient weight, continuous RRT (CRRT) effluent rate, and infusion duration (extended infusion [EI] versus short infusion [SI]) for the 3 g/8 h regimen.
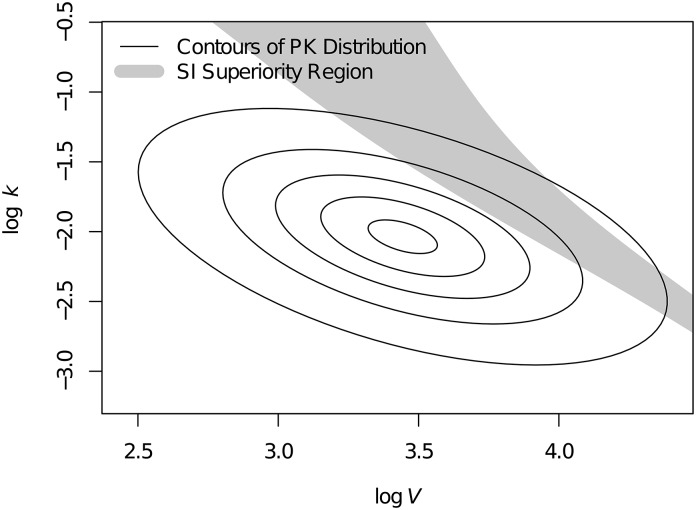
Contrary to the average trend, we discovered that, for some patients, SI may result in greater target attainment relative to EI. To investigate this further, we identified the PK parameter values for which SI was associated with greater target attainment (fT>4×MIC) relative to EI. Figure 4 illustrates this region of SI superiority for the 3 g/8 h regimen overlaid against the distribution of PK parameter values in this population. Similar regions are present for other dosing regimens but different in shape and location. Hence, for each dosing regimen, there may be a small subset of patients for which SI is associated with greater pharmacodynamic target attainment relative to EI. However, by averaging over the entire population, the likelihood of target attainment was uniformly greater for EI relative to SI, regardless of dosing regimen. For tazobactam, target attainment was 100% for every dose amount, frequency, and infusion duration. Because of the lack of variability in target attainment, no additional analysis of tazobactam concentrations was implemented.
Figure 4.
Region of pharmacokinetic (PK) parameter space in which short infusion (SI) is associated with greater than four times minimum inhibitory concentration relative to extended infusion for the 3 g/8 h regimen. Concentric ellipses represent the distribution of PK parameter values in the studied population, where values near the center occur more frequently than values farther from center. Thus, in contrast with the prevailing trend, some patients in this population may benefit more from SI versus extended infusion. The volume of distribution (V) and the elimination rate (k) are displayed on a natural logarithm scale.
Discussion
To our knowledge, this manuscript presents the first data on comparing standard infusion and extended infusion piperacillin in CRRT in patients at American tertiary care hospitals. A few features of our analysis have immediate clinical implications and merit discussion here.
First, as previously suggested for other critically ill populations, patients receiving extended infusion piperacillin-tazobactam are more likely to attain plasma piperacillin levels >64 μg/ml for one half of the dosing interval than those receiving standard 30-minute infusions. However, for each dose amount and frequency, the probability of target attainment was significantly greater for EI versus SI. This finding was robust under alternative MIC and interval fraction targets. The major barrier to uniform adoption of this technique is availability of vascular access.
Jamal et al. (28) reported that CI of piperacillin in patients receiving continuous venovenous hemofiltration was associated with greater pharmacodynamic target attainment relative to intermittent bolus infusions as has been described in other critically ill patients. The larger issues that have yet to be addressed in detail are whether pharmacodynamic targets in plasma map to target attainment at the site of infection (29) and whether, as raised in the work in ref. 30, the best pharmacodynamic target is known or patient specific.
Second, another finding is that EI may not be uniformly superior to SI. In simulated and actual subject–level data, large V values prevented slow infusions from attaining high concentrations in plasma. In practice, this reflects the need for larger loading doses in patients with large V values, and therefore, for patients in excess of 100 kg, 4 g piperacillin-tazobactam every 8 hours will have a greater probability of target attainment and may be a better choice for the patient.
This study has several limitations worthy of consideration. This study is observational and consists of cohorts enrolled at three separate academic medical centers. Center effects in addition to infusion interval and secular trends in patient demographics cannot be excluded. We compared one- and two-compartment models in all participants and found little difference in predicted target attainment. The similarity between one- and two-compartment models likely stems from sparse sampling that is not sensitive to the distribution phase and the continuous equilibration during EI. Additional investigation may be warranted to determine the need for multicompartment modeling in this patient population.
The finding originally reported by Bauer et al. (11) of very high interindividual variability in V and k is, of course, repeated here, because 42 of the participants are the same individuals reported previously. However, with the addition of 26 more participants and NLME modeling in lieu of the less sophisticated two–stage approach adopted by Bauer et al. (11), the interquartile range is consistently between one third and one half of the median value. This reflects the intrinsic heterogeneity of critical illness. Our ICUs admit candidates for bariatric surgery as well as patients with advanced cancers, otherwise healthy adults with pneumonia, and patients with advanced liver failure and hepatorenal syndrome. The goal of the nephrologist remains to support the patient with extracorporeal therapy and evidence-based guidance regarding drug dosage adjustments. It seems that extended infusion piperacillin-tazobactam may be more likely to attain therapeutic targets than 30-minute infusions over a wide range of body habitus, comorbidity, and PK. That said, even after accounting for these patient factors, considerable interindividual variability remains unexplained. This unexplained variability is a barrier to accurate personalization of dosing unless personalization is on the basis of therapeutic drug monitoring or a much more detailed population PK model.
Disclosures
W.H.F. recently received a Clinical Evidence Council grant from Baxter International, now the manufacturer of the dialysis system described in Materials and Methods. This grant was announced after the manuscript was submitted and has no bearing on this manuscript.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Michelle Garcia, Rita Brienza, Lydia Sweeney, and Tracy Seifert for their assistance in study execution.
Funding was generously provided by the Cleveland Clinic, National Institutes of Health grant 1R21 DK088045, Gambro Renal Products (A.J.T.), and Vanderbilt University (W.H.F.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10260915/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Kellum JA, Morimatsu H, Morgera S, Schetz MR, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-Van Straaten HM, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators Writing Committee : Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs 30: 281–292, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY: Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S; RENAL Replacement Therapy Study Investigators : Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361: 1627–1638, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Woodrow G, Turney JH: Cause of death in acute renal failure. Nephrol Dial Transplant 7: 230–234, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero J, Aldabo-Pallas T, Garnacho-Montero C, Cayuela A, Jiménez R, Barroso S, Ortiz-Leyba C: Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Crit Care 10: R111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C: Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med 31: 2742–2751, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, Aldabó-Pallás T, Cayuela-Dominguez A, Marquez-Vacaro JA, Carbajal-Guerrero J, Garcia-Garmendia JL: Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: A matched cohort study. J Antimicrob Chemother 61: 436–441, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bauer SR, Salem C, Connor MJ Jr., Groszek J, Taylor ME, Wei P, Tolwani AJ, Fissell WH: Pharmacokinetics and pharmacodynamics of piperacillin-tazobactam in 42 patients treated with concomitant CRRT. Clin J Am Soc Nephrol 7: 452–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshartous D, Bauer SR, Connor MJ, Aduroja OA, Amde M, Salem C, Groszek JJ, Fissell WH: Pharmacokinetics and pharmacodynamics of imipenem and meropenem in critically ill patients treated with continuous venovenous hemodialysis. Am J Kidney Dis 63: 170–171, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shotwell MS, Madonia PN, Connor MJ, Amde M, Salem C, Aduroja OA, Bauer SR, Groszek JJ, Fissell WH: Ciprofloxacin pharmacokinetics in critically ill patients receiving concomitant continuous venovenous hemodialysis. Am J Kidney Dis 66: 173–175, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Connor MJ Jr., Salem C, Bauer SR, Hofmann CL, Groszek J, Butler R, Rehm SJ, Fissell WH: Therapeutic drug monitoring of piperacillin-tazobactam using spent dialysate effluent in patients receiving continuous venovenous hemodialysis. Antimicrob Agents Chemother 55: 557–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matzke GR, Aronoff GR, Atkinson AJ Jr., Bennett WM, Decker BS, Eckardt KU, Golper T, Grabe DW, Kasiske B, Keller F, Kielstein JT, Mehta R, Mueller BA, Pasko DA, Schaefer F, Sica DA, Inker LA, Umans JG, Murray P: Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 1122–1137, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Nolin TD, Aronoff GR, Fissell WH, Jain L, Madabushi R, Reynolds K, Zhang L, Huang SM, Mehrotra R, Flessner MF, Leypoldt JK, Witcher JW, Zineh I, Archdeacon P, Roy-Chaudhury P, Goldstein SL; Kidney Health Initiative : Pharmacokinetic assessment in patients receiving continuous RRT: Perspectives from the Kidney Health Initiative. Clin J Am Soc Nephrol 10: 159–164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafati MR, Rouini MR, Mojtahedzadeh M, Najafi A, Tavakoli H, Gholami K, Fazeli MR: Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents 28: 122–127, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Sakka SG, Glauner AK, Bulitta JB, Kinzig-Schippers M, Pfister W, Drusano GL, Sörgel F: Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 51: 3304–3310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J: Piperacillin penetration into tissue of critically ill patients with sepsis--bolus versus continuous administration? Crit Care Med 37: 926–933, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Roberts JA, Lipman J: Tissue distribution of beta-lactam antibiotics: Continuous versus bolus dosing. J Pharm Pract Res 39: 219–222, 2009 [Google Scholar]
- 21.Drusano GL: Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat Rev Microbiol 2: 289–300, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute : Clinical Laboratory Standards I. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement, Wayne, PA, Clinical and Laboratory Standards Institute, 2011 [Google Scholar]
- 23.European Committee on Antimicrobial Susceptibility T : Breakpoint Tables for Interpretation of MICs and Zone Diameters, European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Basel, Switzerland, European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2012 [Google Scholar]
- 24.VanScoy B, Mendes RE, Nicasio AM, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG: Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob Agents Chemother 57: 2809–2814, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2013 [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team: nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-109, 2013
- 27.Pinheiro J, Bates D, DebRoy S, Sarkar D; Team RDC: nlme: Linear and Nonlinear Mixed Effects Models, 2012
- 28.Jamal JA, Roberts DM, Udy AA, Mat-Nor MB, Mohamad-Nor FS, Wallis SC, Lipman J, Roberts JA: Pharmacokinetics of piperacillin in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents 46: 39–44, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Dalla Costa T, Nolting A, Kovar A, Derendorf H: Determination of free interstitial concentrations of piperacillin-tazobactam combinations by microdialysis. J Antimicrob Chemother 42: 769–778, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL; International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases : Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect Dis 14: 498–509, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.