Abstract
Background and objectives
Trajectories of eGFR in patients with CKD are highly variable. Only a subset of patients with CKD experiences a steady decline in eGFR. The objective of our study was to investigate whether eGFR trajectory patterns differ by APOL1 risk status.
Design, setting, participants, & measurements
Our study was a longitudinal observational study of 622 participants in the African American Study of Kidney Disease and Hypertension with APOL1 genotyping and sufficient follow-up for estimating GFR trajectories. The predictor was APOL1 high–risk status (having two copies of the G1 or G2 risk alleles) versus low-risk status (zero or one copy of the risk alleles), and the outcome was four eGFR trajectory patterns on the basis of the joint probabilities of linearity and progression: steady decline, unsteady decline, steady stable, and unsteady stable.
Results
Over a median follow-up of 9 years, 24.0% of participants experienced steady eGFR decline, 25.9% had an unsteady decline, 25.6% were steady and stable, and 24.6% were unsteady but stable. Those experiencing steady decline had lower eGFR and higher urine protein-to-creatinine ratio at baseline than participants with the other eGFR trajectory patterns. The APOL1 high–risk group was associated with a greater odds for the steady decline pattern than the APOL1 low–risk group (unadjusted odds ratio, 2.45; 95% confidence interval, 1.62 to 3.69). This association remained significant after adjusting for demographic factors, baseline eGFR, urine protein-to-creatinine ratio, treatment assignment, and follow-up time (adjusted odds ratio, 1.59; 95% confidence interval, 1.00 to 2.52).
Conclusions
Among patients with CKD attributed to hypertension, those with the APOL1 high–risk genotype were more likely to experience a steady decline trajectory in eGFR than those without the APOL1 high–risk genotype. These findings suggest a persistent underlying pathophysiologic process in those patients with the APOL1 high–risk genotype.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), chronic kidney disease, Epidemiology and outcomes, genetic renal disease, African Americans, Alleles, Follow-Up Studies, Genotype, glomerular filtration rate, Humans, hypertension, Kidney Diseases
Introduction
The decline of kidney function in the general population and the progression of CKD to ESRD are highly variable (1–4). Some individuals may experience an unremitting decline in eGFR, whereas others maintain stable eGFR for an extended period (2,3). Patterns of kidney function trajectory in a given patient with CKD may have implications for clinical management (5). Furthermore, the risk factors associated with trajectory patterns may provide clues regarding the underlying pathophysiology of CKD progression.
Genetic factors may influence eGFR trajectory. The APOL1 high–risk genotype, consisting of two copies of the G1 or G2 alleles, is associated with approximately twofold higher risk for CKD progression (6–9). This risk genotype has a population frequency of 13% in blacks and <1% in European Americans (10). The mechanism by which APOL1 high–risk genotype affects CKD progression is still unclear, although environmental and genetic risk factors have been reported to act synergistically with the APOL1–associated renal susceptibility (11–14). Evaluating patterns of eGFR trajectory associated with APOL1 risk status in patients with CKD may yield insight on APOL1–associated renal susceptibility. A steady decline trajectory may suggest an inevitable descent because of an ongoing pathophysiologic process, whereas an unsteady decline may reflect intermittent insults, such as AKI, contributing to CKD progression.
The African American Study of Kidney Disease and Hypertension (AASK) contributed to studies that established that participants with the APOL1 high–risk genotype were two times as likely to experience CKD progression compared with those without the high-risk genotype (6,15). However, these studies did not assess the eGFR trajectory patterns associated with the APOL1 high–risk genotype, an analysis that is possible in the AASK given its long–term follow-up (median of 9 years) and the frequent assessment of kidney function. We hypothesized that the AASK participants with the APOL1 high–risk genotype were more likely to experience a pattern of steady decline in eGFR.
Materials and Methods
Study Population and Design
The study design of the AASK has been reported previously (16,17). Briefly, the AASK had a randomized, controlled trial phase followed by a cohort phase. The randomized trial phase (1995–2001) enrolled 1094 patients ages 18–70 years old with self-reported race of black and CKD attributed to hypertension. In a 3×2 factorial design, participants were randomly assigned to one of three initial medications (ramipril, an angiotensin–converting enzyme inhibitor; metoprolol, a sustained release β-blocker; or amlodipine, a dihydropyridine calcium channel blocker) and one of two BP control targets: intensive control (goal of mean arterial pressure ≤92 mmHg) and standard control (goal of mean arterial pressure =102–107 mmHg). After the trial phase, patients who had not received a diagnosis of ESRD were invited to enroll in the cohort phase (April of 2002 through 2007), which provided BP management according to a standardized protocol on the basis of the results of the trial. Among all patients in the trial phase, 836 patients provided written informed consent for genetic studies. This study included 622 patients with the APOL1 risk allele genotype data, ≥3 years of follow-up, and eight measures of eGFR for trajectory analysis. This work was approved by the Johns Hopkins Institutional Review Board.
Genotyping
ABI Taqman Assay was used to genotype the APOL1 risk variants, G1 and G2. G1 consists of two missense single–nucleotide polymorphisms in high linkage disequilibrium on the same chromosome (rs73885319 and rs60910145) (7). G2 (rs71785313) is a two–amino acid deletion, which is in high linkage disequilibrium with G1 on the opposite chromosome. On the basis of the reported recessive inheritance mode of the APOL1 risk variants (6), we defined the APOL1 high–risk genotype as having two copies of the G1 or G2 alleles (G1/G1, G1/G2, or G2/G2).
Categories of eGFR Trajectory Patterns
Serum creatinine was measured twice at randomization (<3 months apart), at 3 and 6 months of follow-up, and then, every 6 months for the rest of the study. eGFR was calculated using the AASK estimating equation: eGFR=329×(serum creatinine)−1.096×(age)−0.294×(0.736 for women). Probabilities of linearity and progression were estimated on the basis of the monthly slopes of eGFR trajectories approximated using a Bayesian smoothing technique (18). The details of algorithms for the estimation were reported previously (3). A brief description is available in Supplemental Material.
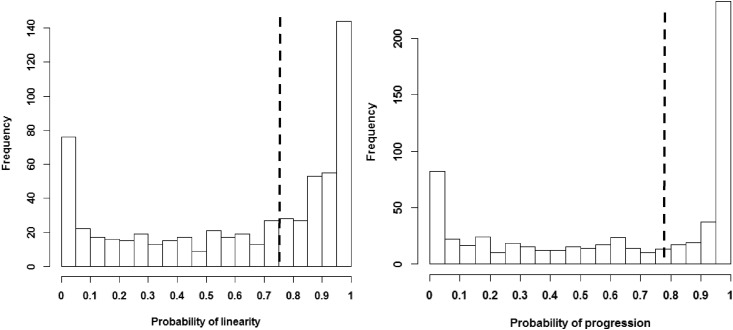
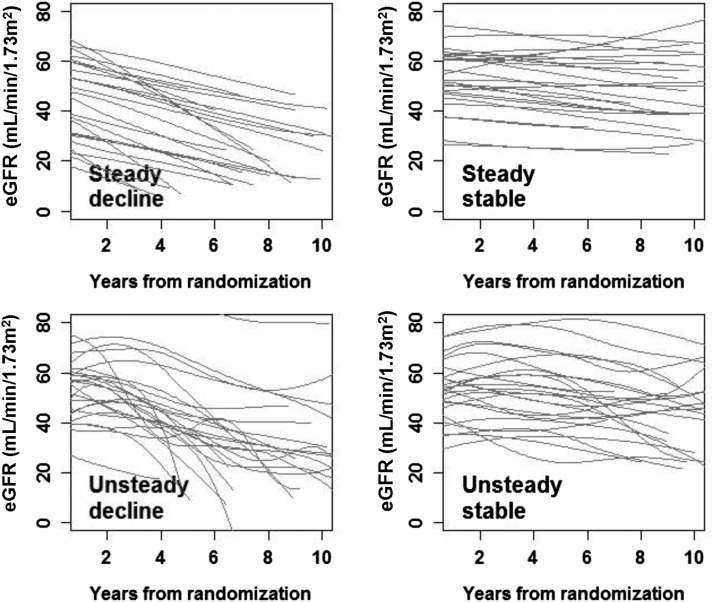
As shown in Figure 1, the probabilities of progression and linearity clustered on the two ends of the distribution. In other words, many participants had probabilities of progression or linearity close to 0% or 100%. Therefore, we dichotomized the probabilities at the median to create groups with approximately equal sizes and classified eGFR trajectory patterns into four categories: steady decline (probability of linearity ≥ median and probability of progression ≥ median), unsteady decline (probability of linearity < median and probability of progression ≥ median), steady stable (probability of linearity ≥ median and probability of progression < median), and unsteady stable (probability of linearity < median and probability of progression < median). The median was 75% for the probability of linearity and 78% for the probability of progression. Histograms of the probabilities of linearity and progression are presented in Figure 1. Random samples of the trajectories from the four categories are shown in Figure 2.
Figure 1.
Histogram of the probabilities of linearity and progression. The dashed line indicates the median.
Figure 2.
Random samples (n=25) of the trajectories from the four categories of eGFR trajectory patterns.
Analyses
We compared the baseline characteristics of participants by the four trajectory patterns using the chi-squared test for categorical variables, the Kruskal–Wallis test for skewed continuous variables, and linear regression for nonskewed continuous variables. To understand whether the inclusion criteria for this analysis differed between APOL1 genotypes, we tested for the association between the APOL1 high–risk status and the requirement criteria for eGFR trajectory pattern analysis (≥3 years of follow-up and eight measures of eGFR) using the chi-squared test. The association of APOL1 high–risk status with the four eGFR trajectory patterns was evaluated using multinomial regression controlling for age at randomization, sex, drug treatment and BP target during the trial phrase, baseline clinical characteristics (eGFR, log–transformed urinary protein-to-creatinine ratio [UPCR], and diastolic BP), and follow-up time. Covariates were selected on the basis of literature review (3,19) or significant association with the trajectory categories in univariate analysis.
Because the pattern of steady decline in eGFR is our outcome of interest, in our primary analysis, we estimated the unadjusted and adjusted odds ratios (ORs) of the APOL1 high–risk group for steady decline versus the other three trajectory patterns combined using logistic regression. We also evaluated whether the association between APOL1 high–risk status and the steady decline pattern differed by baseline eGFR and UPCR levels. The covariates in logistic regression were the same as those used in multinomial regression. To evaluate whether the association between APOL1 high–risk status and steady decline is sensitive to the cut point for categorizing the probabilities of linearity and progression, we conducted a sensitivity analysis using the 55th percentile of the two probabilities as a cut point. In addition, because participants with fewer measures of eGFR were more likely to have a linear trajectory, we also repeated the analysis including only participants with ≥12 measures of eGFR. To verify the recessive model of risk (which combines zero copies and one copy of the APOL1 risk allele into a single risk group), we evaluated the association of the number of APOL1 risk alleles with steady decline using logistic regression as well as the composite outcome of ESRD or doubling of serum creatinine using Cox regression. The covariates in this analysis were the same as those used in the primary analysis. Finally, we assessed whether the associations between the APOL1 high–risk status and steady decline differ by baseline eGFR and proteinuria. Analysis of baseline characteristics was conducted using R. Other analyses were conducted using Stata 13.1 (StataCorp., College Station, TX).
Results
Overall, the percentages of participants in the four eGFR trajectory categories were 24.0% for steady decline, 25.9% for unsteady decline, 25.6% for steady stable, and 24.6% for unsteady stable (Table 1). Those experiencing steady decline were more likely to have lower diastolic BP, lower eGFR, higher proteinuria, and been assigned to the metoprolol treatment group and less likely to have been assigned to the amlodipine treatment group. Of the 622 participants, 22.0% had the APOL1 high–risk genotype. This proportion was lower than that among the 71 participants not meeting criteria for inclusion in the eGFR trajectory pattern analysis (22.0% versus 32.4%; P=0.05) (Supplemental Table 1A). Similarly, the proportion of participants with the APOL1 high–risk genotype was lower in the cohort phase than that in the trial phase (19.7% versus 35.1%; P<0.001) (Supplemental Table 1B). Among the four trajectory patterns, the steady decline trajectory pattern had the highest proportion of participants with the APOL1 high–risk genotype (34.9% versus 25.5% in unsteady decline, 15.1% in steady stable, and 13.1% in unsteady stable). Participants with the steady decline trajectory had similar follow-up time as those with the unsteady decline trajectory and shorter follow-up time than those with the steady stable and unsteady stable trajectories.
Table 1.
Characteristics of participants by eGFR trajectory category
| Characteristic | Steady Decline, n=149 | Unsteady Decline, n=161 | Steady Stable, n=159 | Unsteady Stable, n=153 |
|---|---|---|---|---|
| Probability of linearity | ≥Median | <Median | ≥Median | <Median |
| Probability of progression | ≥Median | ≥Median | <Median | <Median |
| Percentage (n) | 24.0 (149) | 25.9 (161) | 25.6 (159) | 24.6 (153) |
| Age at randomization, yr, mean (SD) | 54.4 (11.0) | 52.5 (10.6) | 56.0 (9.6) | 54.5 (9.7) |
| Women, % (n) | 45.0 (67) | 38.5 (62) | 45.3 (72) | 35.3 (54) |
| Treatment drug, % (n)a,b | ||||
| Ramipril (ACE inhibitor) | 44.3 (66) | 39.8 (64) | 47.2 (75) | 39.2 (60) |
| Metoprolol (β-blocker) | 47.0 (70) | 34.8 (56) | 37.1 (59) | 39.2 (60) |
| Amlopdipine (calcium channel blocker) | 8.7 (13) | 25.4 (41) | 15.7 (25) | 21.6 (33) |
| BP target = standard, % (n) | 49.0 (73) | 49.7 (80) | 52.2 (83) | 53.6 (82) |
| Systolic BP, mean (SD) | 149.7 (23.4) | 153.1 (24.4) | 148.3 (23.4) | 147.9 (26.9) |
| Diastolic BP, mean (SD)a | 93.7 (15.0) | 97.8 (14.4) | 95.2 (13.7) | 95.7 (16.1) |
| eGFR, mean (SD)a,b,c | 45.7 (14.3) | 49.9 (13.7) | 51.2 (12.8) | 50.7 (12.2) |
| UPCR, mg/g, median (first, third quartiles)a,b,c | 189 (52, 657) | 99 (36, 294) | 38 (21, 92) | 40 (22, 97) |
| European ancestry percentage, median (first, third quartiles)d | 0.12 (0.07, 0.21) | 0.13 (0.08, 0.24) | 0.12 (0.06, 0.24) | 0.13 (0.07, 0.20) |
| Zero copies of APOL1 risk alleles, % (n) | 25.5 (38) | 32.9 (53) | 39.6 (63) | 38.6 (59) |
| One copy of APOL1 risk alleles, % (n) | 39.6 (59) | 41.6 (67) | 45.3 (72) | 48.4 (74) |
| Two copies of APOL1 risk alleles, % (n) | 34.9 (52) | 25.5 (41) | 15.1 (24) | 13.1 (20) |
| Follow-up time, yr, median (first, third quartiles)b,c | 8.1 (5.6, 9.5) | 8.0 (5.7, 9.6) | 9.9 (9.0, 10.8) | 10.4 (9.0, 11.0) |
| No. of eGFR measures, median (first, third quartiles)b,c | 18 (13, 22) | 18 (14, 22) | 22 (18, 24) | 23 (19, 25) |
Median (50th percentile) of the probability of linearity: 0.75. Median (50th percentile) of the probability of progression: 0.78. ACE, angiotensin–converting enzyme inhibitor; UPCR, urine protein-to-creatinine ratio.
P value <0.05 between steady decline and unsteady decline.
P value <0.05 between steady decline and unsteady stable.
P value <0.05 between steady decline and steady stable.
The sample size for European ancestry percentage was 603.
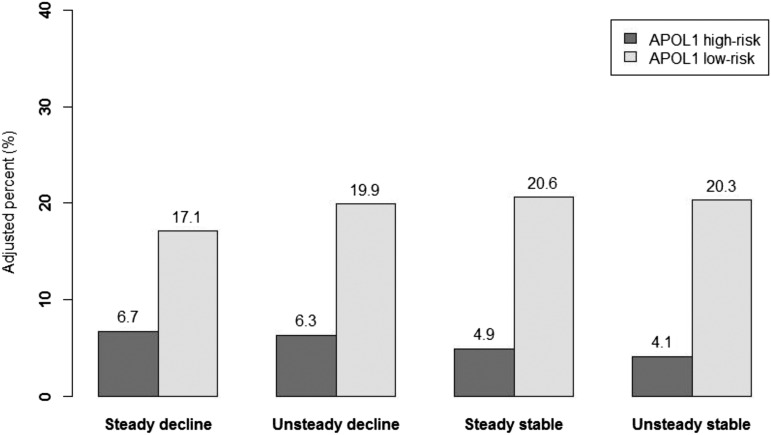
In unadjusted analysis, the proportions of participants across all eight combinations of APOL1 risk status and eGFR trajectory category were as follows: 8.4% with APOL1 high–risk genotype and steady decline, 6.6% with APOL1 high–risk genotype and unsteady decline, 3.9% with APOL1 high–risk genotype and steady stable, 3.2% with APOL1 high–risk genotype and unsteady stable, and 15.6%, 19.3%, 21.7%, and 21.4% in the corresponding trajectory groups with the APOL1 low–risk genotype, respectively (Supplemental Table 2). In adjusted multinomial analysis, the adjusted percentage for those with the APOL1 high–risk genotype and steady decline was 6.7%, 6.3% for unsteady decline, 4.9% for steady stable, and 4.1% for unsteady stable (Figure 3), whereas the adjusted percentage for those with the APOL1 low–risk status and steady decline was 17.1%, 19.9% for unsteady decline, 20.6% for steady stable, and 20.3% for unsteady stable. The overall association between APOL1 high–risk status and the four trajectory categories was statistically significant (unadjusted P value <0.001 and adjusted P value =0.05).
Figure 3.
Adjusted percentage of participants by APOL1 risk status and eGFR trajectory category. Adjusted percentage total 100% across all eight combinations of APOL1 risk status and eGFR trajectory category. Overall, 22% of the participants had the APOL1 high–risk genotype, and 78% had the APOL1 low–risk genotype. The adjusted percentages were estimated from multinomial regression using the four eGFR trajectory patterns as outcomes. Covariates included age at randomization, sex, randomized drug treatment group, randomized BP target, baseline eGFR, log(proteinuria), diastolic BP, and follow-up time for trajectory estimate.
In the analysis using the steady decline trajectory pattern as the outcome, APOL1 high–risk status was significantly associated with steady decline (unadjusted OR, 2.45; 95% confidence interval [95% CI], 1.62 to 3.69) (Table 2). This association attenuated but remained statistically significant after adjusting for demographic factors, baseline eGFR, UPCR, and treatment assignment (model 1: OR, 1.70; 95% CI, 1.08 to 2.68). This association remained similar after the addition of follow-up time as a covariate (model 2: OR, 1.59; 95% CI, 1.00 to 2.52). In the sensitivity analysis using the 55th percentile as the cut point for categorizing the eGFR trajectory patterns, the association between APOL1 risk status and the steady decline trajectory pattern was slightly stronger (model 2: OR, 1.75; 95% CI, 1.07 to 2.87) (Supplemental Table 3). Including only participants with ≥12 measures of eGFR also resulted in significant association between APOL1 high–risk status and the steady decline trajectory pattern (model 2: OR, 1.67; 95% CI, 1.01 to 2.75).
Table 2.
Association between APOL1 high–risk status and steady decline
| Model | APOL1 Low–Risk Group | APOL1 High–Risk Group |
|---|---|---|
| N | 485 | 137 |
| Steady decline, % | 20.0 | 40.0 |
| Unadjusted, OR (95% CI) | 1.00 | 2.45 (1.62 to 3.69) |
| Model 1, OR (95% CI) | 1.00 | 1.70 (1.08 to 2.68) |
| Model 2, OR (95% CI) | 1.00 | 1.59 (1.00 to 2.52) |
| Model 2 by subgroups, OR (95% CI) | ||
| eGFR<45 ml/min per 1.73 m2 | 1.00 | 2.12 (1.00 to 4.50) |
| eGFR≥45 ml/min per 1.73 m2 | 1.00 | 1.46 (0.76 to 2.78) |
| UPCR<220 mg/g | 1.00 | 1.40 (0.75 to 2.62) |
| UPCR≥220mg/g | 1.00 | 1.68 (0.80 to 3.53) |
Model 2 P for interaction between APOL1 risk status and eGFR stratified at 45 ml/min per 1.73 m2: 0.42. Model 2 P for interaction between APOL1 risk status and UPCR stratified at 0.22 g/g: 0.55. Model 1 covariates included age at randomization, sex, randomized drug treatment group, randomized BP target, baseline eGFR, log(proteinuria), and diastolic BP. Model 2 added follow-up time for trajectory estimate. OR, odds ratio; 95% CI, 95% confidence interval; UPCR, urine protein-to-creatinine ratio.
The association between APOL1 risk status and the steady decline pattern did not differ by baseline eGFR (P for interaction =0.42 between APOL1 risk status and eGFR stratified at 45 ml/min per 1.73 m2) and UPCR (P for interaction =0.55 between APOL1 risk status and UPCR stratified at 0.22 g/g) (Table 2).
In sensitivity analysis that separated the low-risk status into zero or one APOL1 risk allele, the unadjusted ORs for steady decline in those with two copies of the risk allele were significantly higher than in those with zero or one copy of the risk allele (two versus one copy: unadjusted OR, 2.82; 95% CI, 1.72 to 4.61; two versus one copy: OR, 2.21; 95% CI, 1.41 to 3.46) (Supplemental Table 4), whereas the ORs for steady decline were not significantly different between those with zero copies and those one copy of risk allele (one versus zero copies: unadjusted OR, 1.28; 95% CI, 0.81 to 2.01). In adjusted analysis, the ORs for steady decline in those with two copies of risk allele remained significantly higher than those with zero copies of the risk allele (two versus zero copies; model 2: OR, 1.97; 95% CI, 1.14 to 3.39) but were not significantly higher than those with one copy of the risk allele (two versus one copy; OR, 1.34; 95% CI, 0.81 to 2.23). Using ESRD or doubling of serum creatinine as outcome, the risk associated with two copies of the risk allele was significantly higher than that with zero or one copy of the risk allele in both unadjusted and adjusted analyses in this analyzed sample (Supplemental Table 5).
Discussion
Main Finding
Among patients with CKD attributed to hypertension, those with the APOL1 high–risk genotype were more likely to experience a steady decline in eGFR than those without the APOL1 high–risk genotype.
In the Context of the Literature
Our study extends previous research that examines the relationship of APOL1 risk variants with eGFR trajectory. The APOL1 high–risk genotype has been associated with twofold higher risk for CKD progression (6,20), but eGFR trajectory pattern was not examined in these studies. In contrast, Li et al. (3) estimated the probability of linearity and progression of eGFR trajectories in the AASK and found that participants with lower eGFR and higher UPCR at baseline had higher probability of progression in univariate analysis; however, this study did not examine associations with APOL1. This study combined the estimated probabilities of progression and linearity to characterize the eGFR trajectory pattern associated with APOL1 risk status. We found that the APOL1 high–risk genotype was associated with a greater risk of steady decline, independent of baseline risk factors, compared with the APOL1 low–risk variants. On the basis of previous findings, our primary analysis used the recessive genetic model, which combined participants with zero copies and participants with one copy of the APOL1 risk variant into the low-risk group (6). This assumption of recessive genetic model was examined in sensitivity analysis using the number of risk variants as the predictor. The recessive genetic model held in unadjusted analysis using steady decline as the outcome and unadjusted and adjusted analyses using the composite outcome of ESRD or doubling of serum creatinine. Our sense is that the most appropriate model for APOL1 risk variants is the recessive genetic model.
Although the mechanisms by which the APOL1 risk variants influence kidney function remain unclear, these risk variants were reported to act synergistically with some environmental and genetic risk factors, such as higher levels of HIV viral load, hemostatic factors, and the glutathione-S-transferase-μ1 null allele, and independent of other risk factors of CKD progression, including smoking and net endogenous acid production (11–13,21). Our study found that, among patients with CKD attributed to hypertension, those with the APOL1 high–risk genotype were more likely to experience a steady decline in eGFR. Importantly, this finding suggests that an ongoing, relentless CKD progression process may be more common among those patients with CKD with the APOL1 high–risk genotype than those with the low–risk APOL1 genotype. Additional work is warranted to unravel the pathophysiologic factors underlying this steady decline pattern.
Strengths of our study include the long duration of follow-up (median of 9 years) and the longitudinal eGFR measures that were obtained at frequent intervals (at least every 6 months).To our knowledge, no other study has such frequent measurement of eGFR over the long term. Furthermore, the association between APOL1 risk status and the steady decline pattern was robust after excluding participants with <12 measures of eGFR. Our study also has limitations. The eGFR trajectory categories were on the basis of estimated probabilities, which included some level of uncertainty. However, the estimated trajectories smoothed out physiologic fluctuation in GFR and measurement errors in serum creatinine, the biomarker for GFR estimation. Therefore, the estimated probability of trajectory patterns reflect the sustained pattern of CKD progression. The percentage of participants with the APOL1 high–risk genotype in this analyzed sample (22%) was higher than the frequency estimated from population-based cohorts (approximately 13%) (20,22). However, the participants excluded from the analysis of eGFR trajectory pattern because of the follow-up requirement (≥3 years of follow-up and eight measures of eGFR) had even higher proportions with the APOL1 high–risk genotype (32%). This is likely because of the fact that those with the APOL1 high–risk genotype had faster CKD progression to ESRD and thus, shorter follow-up. This exclusion might have led to underestimation of the association between the APOL1 high–risk genotype and steady decline.
Among patients with CKD attributed to hypertension, those in the APOL1 high–risk group were more likely to experience unremitting steady decline in eGFR than those in the low-risk group. These findings suggest that patients with the APOL1 high–risk variants have an ongoing pathophysiologic process that perpetuates CKD progression.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the African American Study of Kidney Disease and Hypertension for important contributions.
A.T. was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant T32 DK007732. M.E.G. is supported by NIH/NIDDK grant K08DK092287. T.H.G. is supported by NIH/NIDDK grant 5R01DK090046. L.L. is supported by NIH/NIDDK grant 5R01DK090046 and NIH/National Cancer Institute grant P30CA016672.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12221115/-/DCSupplemental.
References
- 1.Zhong Y, Muñoz A, Schwartz GJ, Warady BA, Furth SL, Abraham AG: Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol 25: 913–917, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg J, Maynard C, Rodriguez RA, Murtagh FE, Larson EB, Williams DE: Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 59: 513–522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, Toto RD, Wang X, Wright JT Jr., Greene TH: Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59: 504–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J: Race, APOL1 risk, and eGFR decline in the general population [published online ahead of print March 10, 2016]. J Am Soc Nephrol doi:ASN.2015070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosansky SJ: Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol 36: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed]
- 10.O’Seaghdha CM, Parekh RS, Hwang SJ, Li M, Köttgen A, Coresh J, Yang Q, Fox CS, Kao WH: The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet 20: 2450–2456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrella MM, Li M, Tin A, Abraham AG, Shlipak MG, Penugonda S, Hussain SK, Palella FJ Jr., Wolinsky SM, Martinson JJ, Parekh RS, Kao WH: The association between APOL1 risk alleles and longitudinal kidney function differs by HIV viral suppression status. Clin Infect Dis 60: 646–652, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, Fornage M, Parekh RS, Coresh J, Kao WH: Hemostatic factors, APOL1 risk variants, and the risk of end-stage renal disease in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol 10: 784–790, 2015 [DOI] [PMC free article] [PubMed]
- 13.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodonyi-Kovacs G, Ma JZ, Lipkowitz MS, Kopp JB, Winkler CA, Le TH: Combined effects of GSTM1 null allele and APOL1 renal risk alleles in CKD progression in the African American Study of Kidney Disease and Hypertension Trial [published online ahead of print March 3, 2016]. J Am Soc Nephrol doi:ASN.2015050487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JBCINKIJ; SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gassman JJ, Greene T, Wright JT Jr., Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crainiceanu CM, Goldsmith AJ: Bayesian functional data analysis using WinBUGS. J Stat Softw 32: i11, 2010 [PMC free article] [PubMed] [Google Scholar]
- 19.Appel LJ, Wright JT Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TK, Choi MJ, Kao WH, Astor BC, Scialla JJ, Appel LJ, Li L, Lipkowitz MS, Wolf M, Parekh RS, Winkler CA, Estrella MM, Crews DC: Examination of potential modifiers of the association of APOL1 alleles with CKD progression. Clin J Am Soc Nephrol 10: 2128–2135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR: Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 22: 2098–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.