Abstract
Background and objectives
Blacks have high rates of cardiovascular disease and mortality. Diabetes and CKD, risk factors for cardiovascular mortality in the general population, are common among blacks. We sought to assess their contribution to cardiovascular disease and mortality in blacks.
Design, setting, participants, & measurements
This observational cohort study was of 3211 participants in the Jackson Heart Study (enrolled 2000–2004). Rates of incident stroke, incident coronary heart disease, and cardiovascular mortality were quantified in participants with diabetes, CKD (eGFR<60 ml/min per 1.73 m2, urine albumin-to-creatinine ratio ≥30 mg/g, or both), or both through 2012, with a median follow-up of 6.99 years.
Results
Four hundred fifty-six (14.2%) participants had only diabetes, 257 (8.0%) had only CKD, 201 (6.3%) had both, and 2297 (71.5%) had neither. Diabetes without CKD was associated with excess risks of incident stroke, incident coronary heart disease, and cardiovascular mortality after adjustment for demographic and clinical covariates, including prevalent cardiovascular disease (excess incidence rates, 2.6; 95% confidence interval, 0.5 to 4.7; 2.6; 95% confidence interval, 0.3 to 4.8; and 2.4; 95% confidence interval, 0.4 to 4.3 per 1000 person-years, respectively). CKD without diabetes was associated with comparable nonsignificant excess risks for incident stroke and coronary heart disease (2.5; 95% confidence interval, −0.1 to 5.2 and 2.4; 95% confidence interval, −0.8 to 5.5 per 1000 person-years, respectively) but a larger excess risk for cardiovascular mortality (7.3; 95% confidence interval, 3.0 to 11.5 per 1000 person-years). Diabetes and CKD together were associated with greater excess risks for incident stroke (13.8; 95% confidence interval, 5.3 to 22.3 per 1000 person-years), coronary heart disease (12.8; 95% confidence interval, 4.9 to 20.8 per 1000 person-years), and cardiovascular mortality (14.8; 95% confidence interval, 7.2 to 22.3 per 1000 person-years). The excess risks associated with the combination of diabetes and CKD were larger than those associated with established risk factors, including prevalent cardiovascular disease.
Conclusions
The combination of diabetes and kidney disease is associated with substantial excess risks of cardiovascular events and mortality among blacks.
Keywords: diabetes mellitus; chronic kidney disease; stroke; cardiovascular disease; cardiovascular mortality; African Americans; Albumins; coronary artery disease; creatinine; Follow-Up Studies; Humans; Renal Insufficiency, Chronic
Introduction
Blacks experience a higher rate of cardiovascular disease (CVD) than whites (1). Blacks residing in certain regions of the United States, such as Mississippi, have even higher rates of cardiovascular (CV) mortality than those residing elsewhere in the country (2–4). Diabetes and CKD are both significant risk factors for CVD and mortality. In fact, CVD is the predominant cause of mortality among people with diabetes and kidney disease (5,6). Previous studies, mostly in white or ethnically mixed populations, have shown that CKD is a powerful predictor of the excess mortality in diabetes (7–9). Using data from the National Health and Nutrition Examination Survey (NHANES III), we previously reported that, among people with diabetes, those with kidney disease have the highest cumulative incidence of 10-year mortality. Interestingly, absent kidney disease, mortality in people with diabetes was not drastically higher than that of a reference population without diabetes or kidney disease (9).
Blacks are 1.7 times more likely than whites to be diagnosed with diabetes (10), 2.5 times more likely to develop ESRD caused by diabetes (11), and 1.7 times more likely to die as a result of diabetes (12). In this study, we asked whether kidney disease is predictive of CV outcomes among blacks. To address this, we examined the association between diabetes, kidney disease, and excess risk for incident stroke and coronary heart disease (CHD) as well as CV mortality in the Jackson Heart Study (JHS) population.
Materials and Methods
Study Population
The JHS is a single–site, community–based, prospective cohort study of risk factors and course of CVD in noninstitutionalized adult blacks (13,14). Between 2000 and 2004, the study enrolled 5301 blacks ages 21–94 years old residing in three counties within the Mississippi Metropolitan Area (Hinds, Madison, and Rankin). During this interval, the baseline data were collected using a self-administered questionnaire, an in-home interview, and a clinic visit. This study used data from 3211 JHS participants, excluding participants with missing data on baseline diabetes status (n=61), serum creatinine (n=34), serum cystatin C (n=81), and urine albumin and creatinine (n=1914). Follow-up clinical examinations were conducted in 4-year intervals, with examination 2 conducted between 2005 and 2008 and examination 3 conducted between 2009 and 2012. Median follow-up duration was 6.99 years. In addition, interim medical events and vital statistics were also collected using annual telephone interviews. The study was approved by the institutional review boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center. All of the participants gave written informed consent. The use of deidentified data for this study was considered nonhuman subjects research by the Human Subjects Division of the University of Washington.
Diabetes Definition
Diabetes was defined as self-reported diabetes, hemoglobin A1c ≥6.5% (15), fasting glucose >126 mg/dl as per the 2010 American Diabetes Association guidelines (16), or use of glucose-lowering medications at baseline examination. Glycated hemoglobin A1c concentration was measured using an HPLC system (Tosoh Corporation, Tokyo, Japan) in blood samples collected after an overnight fast (17).
CKD Definition
CKD was defined as albuminuria, reduced eGFR, or both using data from the baseline visit. Albuminuria was defined as a urine albumin-to-creatinine ratio ≥30 mg/g. Reduced eGFR was defined as an eGFR≤60 ml/min per 1.73 m2. More details on the definition of CKD and other covariates are in Supplemental Material.
Outcomes
The outcomes were incident CHD, incident stroke, and mortality from CV causes. These outcomes were captured during the annual telephone interviews with participants and their family members as well as the JHS examinations 2 and 3. During the interviews and examinations, trained staff identified interim medical events, including new health events, diagnostic tests, hospitalizations, new diagnoses, and death. These were subsequently confirmed by review of medical records, including discharge summaries, International Classification of Diseases, Ninth Revision codes, and procedure codes. Cohort deaths were additionally identified from the monthly printout of the Mississippi State Department of Health, systematic review of death certificates, hospital chart review, use of obituary notices, and linkage to the National Death Index. Final classification of all CV events required medical chart review and adjudication by trained physicians. Incident CHD was defined as myocardial infarction or need for coronary revascularization on the basis of data abstracted from medical records, which included presenting symptoms, relevant clinical data (cardiac biomarkers, electrocardiogram, etc.), and diagnostic and therapeutic procedures. A detailed description of other CV outcomes and statistical analysis is in Supplemental Material.
Statistical Methods
We categorized participants by presence or absence of diabetes and CKD and compared the distribution of demographics and covariates in these four mutually exclusive groups. Participants were considered at risk for each of the outcomes from their baseline visit until the first occurrence of the outcomes or censoring because of loss to follow-up, the end of available follow-up, or death from non-CV causes. Participants with a prior stroke were excluded from the analyses where the outcome was incident stroke. Participants with prior CHD were excluded from the analyses where the outcome was incident CHD. For the outcome of CV mortality, participants with prior stroke or CHD were included in the analyses. Incidence rates (per 1000 person-years with 95% confidence intervals [95% CIs]) for incident stroke, incident CHD, or CV mortality were calculated using Poisson regression in four groups: participants with no diabetes or CKD, participants with diabetes but no CKD, participants with CKD but no diabetes, and participants with both diabetes and CKD. Risk differences were estimated by comparing incidence rates in each group with those in the reference group (participants with no diabetes or CKD) from the Poisson regression. The 95% CIs for the risk differences were computed from a bootstrap analysis of 1000 samples. Cox proportional hazards regression was used to estimate the relative hazard of each outcome, adjusting for relevant covariates, in the same four groups. In each analysis, models were first adjusted for age, age2, sex, and income (model 1). Given the large age range, adjustment for age was done using both simple and quadratic terms. Income was dichotomized by collapsing the four categories into two categories of low (combination of poor and lower middle) and high (combination of upper middle and affluent). Education was not included in the final model, because it did not remain significant in the presence of income. The final model was additionally adjusted for smoking status, hypertension, hyperlipidemia, and prevalent CVD (model 2).
To evaluate for additive interactions between diabetes and CKD (i.e., to determine whether diabetes and CKD increased the risk difference of incidence rates of an event in an additive fashion), the risk difference for an event was calculated for each group (diabetes or CKD), and the effect modification between diabetes and CKD was evaluated by using the relative excess risk because of interaction (RERI) (18). The RERI is the difference of the observed effect of the joint exposure with the sum of the effects of each factor acting separately: 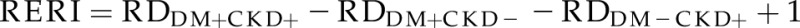 , where DM is diabetes mellitus and RDDM+CKD+ indicates risk difference of incidence rates for both diabetes and CKD. If diabetes is present but CKD is not, then the RD is RDDM+CKD−; similarly, if diabetes is absent and CKD is present, the RD is RDDM−CKD+. A value of zero implies exact additivity, a value greater than zero indicates a positive interaction between the two variables (or more additivity), and a value less than zero implies less than additivity.
, where DM is diabetes mellitus and RDDM+CKD+ indicates risk difference of incidence rates for both diabetes and CKD. If diabetes is present but CKD is not, then the RD is RDDM+CKD−; similarly, if diabetes is absent and CKD is present, the RD is RDDM−CKD+. A value of zero implies exact additivity, a value greater than zero indicates a positive interaction between the two variables (or more additivity), and a value less than zero implies less than additivity.
All analyses were conducted in STATA, version 13 (StataCorp., College Station, TX) and SPSS, version 22 (IBM SPSS, Chicago, IL), and P values <0.05 were considered statistically significant.
Results
Characteristics of Study Participants
Of the 5301 JHS participants, we excluded those with missing measures of diabetes (n=61), serum creatinine (n=34), cystatin (n=81), and urine albumin-to-creatinine ratio (n=1914), leaving 3211 who were included in this study (Supplemental Figure 1). The JHS participants excluded from this analysis (n=2090) were less affluent, were more likely to smoke, had lower mean GFR, and had higher percentages of albuminuria and prevalent CVD (Supplemental Table 1). Among the study population, 456 (14.2%) had only diabetes, 257 (8.0%) had only CKD, 201 (6.3%) had both, and 2297 (71.5%) had neither at study entry (Table 1). The majority of the participants with CKD had albuminuria (Supplemental Figure 2). Participants with diabetes and/or CKD were older and more obese. Presence of CKD and diabetes was associated with higher prevalence of hypertension and use of antihypertensive medications. Despite higher prevalence of antihypertensive use, the mean systolic BPs were higher in subgroups with CKD. More participants with diabetes were using hydroxymethyl glutaryl–CoA reductase inhibitors. However, total cholesterol as well as LDL cholesterol concentrations were comparable with or without CKD or diabetes. Participants with diabetes and/or CKD included more people with lower incomes. In addition, diabetes and CKD were more common in people with lower income: CKD was present in 16% of people in the combined poor and lower–middle categories versus 9% in the combined upper–middle and affluent categories. Diabetes was present in 25% of people in the combined poor and lower–middle categories versus 14% in the combined upper–middle and affluent categories.
Table 1.
Baseline characteristics of the Jackson Heart Study participants by diabetes and CKD status
| Variables | All | No Diabetes | Diabetes | ||
|---|---|---|---|---|---|
| No CKD | CKD | No CKD | CKD | ||
| N, % | 3211 | 2297 (71.5) | 257 (8.0) | 456 (14.2) | 201 (6.3) |
| Age, yr | 54 (13) | 52 (13) | 59 (14) | 59 (11) | 62 (11) |
| Men | 1218 (38%) | 912 (40%) | 88 (34%) | 137 (30%) | 81 (40%) |
| Income | |||||
| Poor | 354 (13%) | 235 (12%) | 31 (15%) | 62 (17%) | 26 (17%) |
| Lower middle | 611 (23%) | 402 (21%) | 66 (31%) | 92 (25%) | 51 (33%) |
| Upper middle | 828 (31%) | 593 (31%) | 63 (30%) | 120 (32%) | 52 (33%) |
| Affluent | 882 (33%) | 703 (36%) | 52 (25%) | 100 (27%) | 27 (17%) |
| Smoking | |||||
| Never | 2254 (71%) | 1645 (72%) | 170 (66%) | 300 (66%) | 139 (70%) |
| Former | 555 (17%) | 354 (16%) | 50 (20%) | 113 (25%) | 38 (19%) |
| Current | 375 (12%) | 277 (12%) | 36 (14%) | 41 (9%) | 21 (11%) |
| SBP, mmHg | 126 (18) | 124 (17) | 136 (21) | 127 (17) | 138 (22) |
| DBP, mmHg | 79 (10) | 80 (10) | 82 (13) | 77 (11) | 78 (11) |
| Use of antihypertensives | 1578 (60%) | 898 (50%) | 164 (74%) | 342 (79%) | 174 (92%) |
| Hypertension | 1940 (60%) | 1180 (51%) | 202 (79%) | 371 (81%) | 187 (93%) |
| Cholesterol, mg/dl | 198 (39) | 198 (39) | 200 (42) | 197 (38) | 202 (44) |
| LDL, mg/dl | 127 (36) | 127 (36) | 129 (37) | 123 (33) | 126 (41) |
| Use of HMG-CoA reductase inhibitors | 377 (12%) | 176 (8%) | 24 (9%) | 122 (27%) | 55 (27%) |
| Hyperlipidemia | 850 (27%) | 537 (23%) | 72 (28%) | 166 (36%) | 75 (37%) |
| Prevalent cardiovascular disease | 317 (10%) | 159 (7%) | 46 (18%) | 66 (15%) | 46 (23%) |
| Creatinine, mg/dl | 0.92 (0.32) | 0.89 (0.18) | 1.10 (0.71) | 0.85 (0.19) | 1.18 (0.61) |
| Cystatin C, mg/L | 0.74 (0.25) | 0.69 (0.12) | 0.96 (0.58) | 0.73 (0.15) | 1.00 (0.46) |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 104 (21) | 108 (17) | 88 (30) | 103 (18) | 82 (32) |
| ACR, mg/g | 6 [4–13] | 5 [4–8] | 63 [35–169] | 8 [5–12] | 81 [41–359] |
| ACR≥30 | 399 (12%) | 0 (0%) | 220 (86%) | 0 (0%) | 179 (89%) |
Data are presented as numbers (%), means (SDs), or medians [interquartile ranges]. Hypertension was defined as SBP≥140 mmHg, DBP≥90 mmHg, or use of antihypertensive medications. Hyperlipidemia was defined as LDL≥160 or use of HMG-CoA reductase inhibitors. eGFR was calculated using serum concentrations of creatinine and cystatin C measured at baseline using the 2012 CKD-EPI equation. To convert GFR in milliliters per minute to milliliters per second, multiply by 0.01667. To convert cholesterol in milligrams per deciliter to millimoles per liter, multiply by 0.0259. SBP, systolic BP; DBP, diastolic BP; HMG-CoA, hepatic hydroxymethyl glutaryl–CoA; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACR, albumin-to-creatinine ratio.
CV Outcomes by Diabetes Status
The incidence rate of stroke was higher in JHS participants with diabetes than in those without diabetes: 6.5 (95% CI, 3.8 to 9.1) versus 1.3 (95% CI, 0.6 to 1.9) per 1000 person-years after adjustment for sociodemographic and clinical covariates (Table 2). Similarly, incident CHD and CV mortality were more frequent in those with diabetes than in those without diabetes: 6.9 (95% CI, 4.1 to 9.6) versus 1.9 (95% CI, 1.1 to 2.7) and 6.0 (95% CI, 3.7 to 8.3) versus 1.3 (95% CI, 0.6 to 1.9) per 1000 person-years, respectively.
Table 2.
Adjusted incidence rates of cardiovascular outcomes per 1000 person-years in people with and without diabetes in the Jackson Heart Study
| Outcome | N | Events | Adjusted Incidence Rate (95% CI) |
|---|---|---|---|
| Incident stroke | |||
| No diabetes | 2473 | 35 | 1.3 (0.6 to 1.9) |
| Diabetes | 615 | 36 | 6.5 (3.8 to 9.1) |
| Incident CHD | |||
| No diabetes | 2401 | 48 | 1.9 (1.1 to 2.7) |
| Diabetes | 571 | 33 | 6.9 (4.1 to 9.6) |
| Cardiovascular mortality | |||
| No diabetes | 2554 | 52 | 1.3 (0.6 to 1.9) |
| Diabetes | 657 | 46 | 6.0 (3.7 to 8.3) |
Incidence rates were calculated using Poisson regression and adjusted for age, age2, sex, income, hypertension, hyperlipidemia, current smoking, and prevalent cardiovascular disease. Participants with a prior stroke (or CHD) were excluded from the analyses where the outcome was incident stroke (or CHD). 95% CI, 95% confidence interval; CHD, coronary heart disease.
CV Outcomes by CKD and Diabetes Status
In the absence of diabetes and CKD, the unadjusted rate of incident stroke was 1.7/1000 person-years (95% CI, 1.1 to 2.4) (Table 3). In the presence of diabetes alone, CKD alone, or diabetes and CKD together, this rate was higher at 5.7 (95% CI, 2.1 to 9.8), 5.9 (95% CI, 2.1 to 9.8), and 17.0 (95% CI, 9.4 to 24.7) per 1000 person-years, respectively. Compared with the reference group without either diabetes or CKD, presence of diabetes only was associated with an excess risk for incident stroke of 2.6 (95% CI, 0.5 to 4.7) per 1000 person-years after adjustment for sociodemographic and clinical variables. CKD alone was associated with an excess risk of 2.5 (95% CI, −0.1 to 5.2) per 1000 person-years for stroke, which lost significance after adjustment for sociodemographic variables. The combination of diabetes and CKD was associated with an excess risk for incident stroke of 13.8 (95% CI, 5.3 to 22.3) per 1000 person-years in fully adjusted analyses.
Table 3.
Rates of incident stroke, coronary heart disease, and cardiovascular mortality (per 1000 person-years) by diabetes and CKD status in the Jackson Heart Study
| Outcome | N | Events | Unadjusted Incidence Rate (95% Confidence Interval) | Risk Difference Per 1000 person-yr (95% Confidence Interval) | |
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| Incident stroke | |||||
| No diabetes or CKD | 2235 | 26 | 1.7 (1.1 to 2.4) | Reference | Reference |
| Diabetes but no CKD | 431 | 17 | 5.7 (2.1 to 9.8) | 2.8 (0.5 to 5.1); P=0.02 | 2.6 (0.5 to 4.7); P=0.02 |
| CKD but no diabetes | 238 | 9 | 5.9 (2.1 to 9.8) | 2.9 (−0.1 to 6.1); P=0.08 | 2.5 (−0.1 to 5.2); P=0.06 |
| Diabetes and CKD | 184 | 19 | 17.0 (9.4 to 24.7) | 14.8 (6.0 to 23.7); P=0.001 | 13.8 (5.3 to 22.3); P=0.001 |
| Incident coronary heart disease | |||||
| No diabetes or CKD | 2186 | 39 | 2.7 (1.8 to 3.5) | Reference | Reference |
| Diabetes but no CKD | 409 | 17 | 6.1 (3.2 to 9.0) | 2.4 (−0.1 to 5.0); P=0.07 | 2.6 (0.3 to 4.8); P=0.03 |
| CKD but no diabetes | 215 | 9 | 6.4 (2.2 to 10.7) | 2.4 (−1.2 to 5.9); P=0.20 | 2.4 (−0.8 to 5.5); P=0.15 |
| Diabetes and CKD | 162 | 16 | 15.7 (8.0 to 23.3) | 12.4 (4.4 to 20.3); P=0.002 | 12.8 (4.9 to 20.8); P=0.002 |
| Cardiovascular mortality | |||||
| No diabetes or CKD | 2297 | 30 | 2.0 (1.3 to 2.7) | Reference | Reference |
| Diabetes but no CKD | 456 | 16 | 5.1 (2.6 to 7.5) | 2.4 (0.4 to 4.4); P=0.02 | 2.4 (0.4 to 4.3); P=0.02 |
| CKD but no diabetes | 257 | 22 | 13.6 (7.9 to 19.3) | 7.7 (3.0 to 12.4); P=0.001 | 7.3 (3.0 to 11.5); P=0.001 |
| Diabetes and CKD | 201 | 30 | 23.5 (15.1 to 31.9) | 14.8 (6.8 to 22.8); P<0.001 | 14.8 (7.2 to 22.3); P<0.001 |
Incidence rates were calculated using Poisson regression. Absolute risk differences were estimated by comparing the incidence rates in each group with those in the reference group (participants with no diabetes or CKD) using Poisson regression and adjusted for age, age2, sex, and income (model 1) or additionally adjusted for hypertension, hyperlipidemia, current smoking, and prevalent cardiovascular disease (model 2). Participants with a prior stroke were excluded from the analyses where the outcome was incident stroke. Participants with prior coronary heart disease were excluded from the analyses where the outcome was incident coronary heart disease. However, participants with prior cardiovascular disease (stroke or coronary heart disease) were included in the analyses where the outcome was cardiovascular mortality.
Compared with the reference group, diabetes alone was associated with an excess risk for incident CHD of 2.6 (95% CI, 0.3 to 4.8) per 1000 person-years after full adjustment. The combination of diabetes and CKD was associated with an excess risk for incident CHD of 12.6 (95% CI, 4.9 to 20.8) per 1000 person-years after full adjustment. Presence of diabetes alone, CKD alone, or the combination of diabetes and CKD was associated with excess risks for CV mortality of 2.4% (95% CI, 0.4% to 4.3%), 7.3% (95% CI, 3.0% to 11.5%), and 14.8% (95% CI, 7.2% to 22.3%) per year after full adjustment. Modeling time to the first event using the Cox proportional hazard showed similar results: the combination of diabetes and CKD was associated with 3.3-fold (95% CI, 1.79 to 6.20) higher rates of incident CHD, 6.23-fold (95% CI, 3.22 to 12.09) higher rates of incident stroke, and 6.4-fold (95% CI, 3.53 to 11.78) higher rates of CV mortality (Table 4). There was a trend toward additive interaction between the risk differences associated with diabetes and CKD for incident stroke, incident CHD, and CV mortality. The RERIs and 95% CIs for incident stroke, incident CHD, and CV mortality were 0.87 (95% CI, 0.01 to 1.73), 0.78 (95% CI, −0.07 to 1.65), and 0.52 (95% CI, −0.27 to 1.30), respectively. However, only the interaction for incident stroke attained statistical significance (P value =0.004).
Table 4.
Relative hazards of incident stroke, coronary heart disease, and cardiovascular mortality by diabetes and CKD status in the Jackson Heart Study
| Outcome | N | Events | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|---|---|
| Incident stroke | |||||
| No diabetes or CKD | 2235 | 26 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Diabetes but no CKD | 431 | 17 | 3.23 (1.75 to 5.97) | 1.94 (0.96 to 3.93) | 1.83 (0.90 to 3.74) |
| CKD but no diabetes | 238 | 9 | 3.65 (1.70 to 7.82) | 2.43 (1.02 to 5.74) | 1.91 (0.80 to 4.55) |
| Diabetes and CKD | 184 | 19 | 10.33 (5.70 to 18.73) | 7.41 (3.87 to 14.18) | 6.23 (3.22 to 12.09) |
| Incident coronary heart disease | |||||
| No diabetes or CKD | 2186 | 39 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Diabetes but no CKD | 409 | 17 | 2.23 (1.26 to 3.95) | 1.59 (0.84 to 3.03) | 1.36 (0.74 to 2.60) |
| CKD but no diabetes | 215 | 9 | 2.44 (1.18 to 5.05) | 1.65 (0.73 to 3.75) | 1.32 (0.58 to 3.00) |
| Diabetes and CKD | 162 | 16 | 5.90 (3.30 to 10.56) | 4.32 (2.33 to 8.04) | 3.33 (1.79 to 6.20) |
| Cardiovascular mortality | |||||
| No diabetes or CKD | 2297 | 30 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Diabetes but no CKD | 456 | 16 | 2.52 (1.37 to 4.62) | 2.06 (1.03 to 4.20) | 1.90 (0.94 to 3.84) |
| CKD but no diabetes | 257 | 22 | 7.00 (4.04 to 12.13) | 5.04 (2.70 to 9.40) | 4.22 (2.24 to 7.97) |
| Diabetes and CKD | 201 | 30 | 13.38 (7.45 to 20.56) | 7.46 (4.15 to 13.43) | 6.44 (3.53 to 11.78) |
Relative hazard for each outcome was estimated using the Cox regression and adjusted for age, age2, sex, and income (model 1) or further adjusted for hypertension, dyslipidemia, current smoking, and prevalent cardiovascular disease (model 2). Prevalent cardiovascular disease for each outcome was defined as in Table 3. HR, hazard ratio; 95% CI, 95% confidence interval.
The Excess Risk of CV Outcomes Associated with CKD and Diabetes Compared with Other Clinical Risk Factors
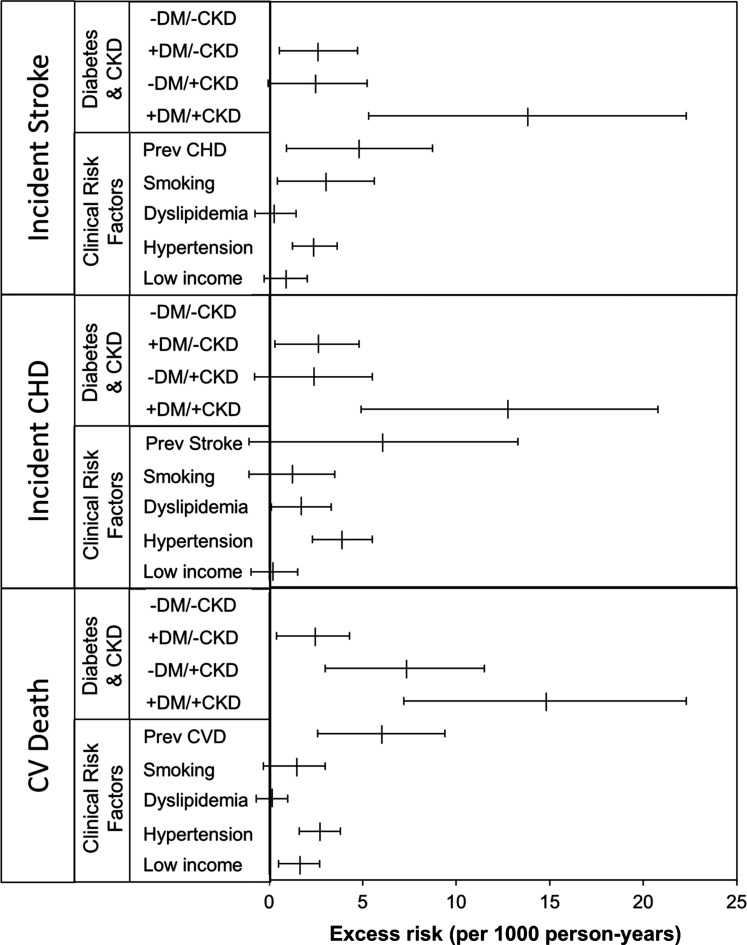
To compare the excess risk of the CV outcomes associated with diabetes and CKD with that associated with other CV risk factors (particularly prevalent CVD), we examined the risk differences for each risk factor in the fully adjusted model (model 2 in Table 3). However, given our focus on incident outcomes, prevalent CVD is defined differently for each outcome. For incident stroke, prevalent CVD consisted of prevalent CHD, because the participants with prevalent stroke had to be excluded. Similarly, for the outcome of incident CHD, prevalent CVD consisted of prevalent stroke. Only for the outcome of CV mortality did prevalent CVD include both prevalent stroke and CHD. In the fully adjusted model, the combination of diabetes and CKD was associated with greater excess risks for incident stroke, incident CHD, and CV death than other CV risk factors, including prevalent CVD, hyperlipidemia, hypertension, and smoking. Diabetes and CKD individually were associated with comparable excess rates of incident stroke and CHD, excess risks that were of similar magnitude to those for hypertension and smoking but less than those of prevalent CVD. However, CKD alone was associated with much higher rates of CV mortality than diabetes alone, an excess risk that was comparable with that of prevalent CVD (Figure 1).
Figure 1.
Risk differences for incident stroke, coronary heart disease (CHD), and cardiovascular mortality (per 1000 person-years) by clinical risk factor in the Jackson Heart Study. Incidence rates were calculated using Poisson regression. Absolute risk differences were estimated by comparing the incidence rates in each group with those in the reference group (participants with no diabetes or CKD) using Poisson regression and adjusted for age, age2, sex, hypertension, hyperlipidemia, smoking, and prevalent cardiovascular disease (CVD). Participants with a prior stroke were excluded from the analyses where the outcome was incident stroke; participants with prior CHD were excluded from the analyses where the outcome was incident CHD. CVD refers to a combination of stroke and CHD. CV death, cardiovascular death; DM, diabetes mellitus.
Discussion
In a black population with high incidence of CVD and mortality, diabetes alone was associated with excess risks of incident stroke, CHD, and CV mortality, whereas CKD alone was associated with excess risk for CV mortality. However, the combination of diabetes and kidney disease was associated with a greater excess risk for incident stroke, CHD, and CV mortality than established clinical risk factors, including a history of CVD.
Blacks, particularly in the southern low–income rural communities, have some of the highest rates of all-cause and CV mortality in the United States (2–4). Kidney disease and diabetes, both risk factors for CVD, are disproportionately more common in this population. CKD is associated with an excess risk of all-cause and CV mortality both in the general population (19–23) and among people with diabetes (5,6). In a nationally representative population, kidney disease captured a majority of the excess mortality risk associated with diabetes (9). Furthermore, absent kidney disease, diabetes was not associated with a large excess in mortality (9). Here, we extend that work by examining the association of diabetes and kidney disease with CV mortality as well as incident CV events in a high–risk black population. Risk differences were evaluated on an absolute scale to present the clinically relevant marginal risk associated with diabetes, kidney disease, or the combination over the baseline risk among the reference population without diabetes or CKD. Using this approach, we found a much larger excess risk for CV mortality in the presence of CKD than in the presence of diabetes, consistent with our findings in the NHANES III. Similarly, the combination of diabetes and CKD was associated with a much larger excess risk for CV mortality than either risk factor alone. Consistently, we found a trend toward an additive interaction between CKD and diabetes. However, except for incident stroke, this did not reach statistical significance.
On the other hand, although CKD alone conferred a significant excess risk for CV mortality, it was not associated with significant excess risk for incident stroke or CHD. The combination of diabetes and kidney disease was associated with a significant excess risk for both incident stroke and CHD. However, each risk factor alone was associated with a small excess risk for these outcomes, which in the case of kidney disease, was not robust to adjustment, suggesting an additive interaction between diabetes and CKD. This observation raises the possibility that, in absence of diabetes, CKD may contribute more to reduced survival after a CV event rather than a predisposition to it. In fact, presence and severity of kidney disease are associated with lower survival after CV events, including acute myocardial infarction, stroke, or congestive heart failure (24). In an interesting parallel, although blacks have comparable rates of CV events with whites (25,26), they experience higher CV mortality than whites (27–29). This suggests that the higher prevalence of kidney disease in blacks may contribute to the disparity in CV mortality in this population relative to whites.
The gap in socioeconomic status is believed to at least partly underlie the disparity in CV outcomes and mortality between blacks and whites (30–33). In the JHS, both diabetes and kidney disease were more prevalent in participants with lower socioeconomic status as assessed by income. Adjustment for age, sex, and socioeconomic status attenuated the excess risk for CV outcomes in the presence of the combination of diabetes and kidney disease. However, most of this attenuation was because of age (and not socioeconomic status). Furthermore, even after this adjustment, the excess risk for CV outcomes in the presence of concomitant diabetes and kidney disease remained sizable and significant. However, after adjustment for both diabetes and kidney disease, the excess risk associated with income was not significant for incident stroke and CHD but remained significant (although small) for CV mortality. These observations suggest that the previously observed associations between socioeconomic status and CVD outcomes may be mediated via higher rates of diabetes and CKD in people with lower socioeconomic status. They further suggest that socioeconomic status, similar to CKD, may have a greater influence on survival after a CV event than the predisposition to it.
Our findings are also relevant to the question of risk equivalence for the risk of CV outcomes associated with CKD compared with that from diabetes or prevalent CVD. Previous work in largely white populations has shown that CKD contributes significantly to CV risk stratification (34) and that the combination of diabetes and CKD is associated with comparable rates of incident myocardial infarction to preexisting CHD (35). Given our focus on incident outcomes (stroke and CHD), prevalent stroke (or CHD) could not be included in our assessment of risk factor for incident stroke (or CHD). As such, our findings address this question more fully for CV mortality, where prevalent CVD included prevalent stroke and CHD. Consistent with prior studies (34), we found that, in this black cohort, kidney disease, particularly in the presence of diabetes, was associated with a greater excess risk of CV outcomes and mortality than other clinically used CV risk factors, including preexisting CVD. This finding has direct clinical implications for CV risk stratification and intensive therapeutic targeting of the subpopulations at the highest risk for future CV events and mortality. For example, as pointed out in previous studies (34), full assessment of kidney function, including not only eGFR but also, urine albumin excretion, is likely to be beneficial for complete CV risk assessment.
Interestingly, the incidence of stroke was comparable with that of CHD in the JHS. This is counter to the general population, where incidence of CHD equals or exceeds that of all other CV events together (36,37). This may be related to the higher incidence of stroke noted in the southeastern United States compared with in the rest of the country (38,39). The higher rates of stroke observed in blacks versus whites may also contribute to this finding (40).
The observational nature of the study precludes differentiation of whether the combination of diabetes and kidney disease is causally related to CV outcomes, although this possibility is strongly supported by preexisting data (5,6,19–23). Additional limitations include assessment of kidney function and diabetes at one time point, the small number of events in some categories, and potential misclassification in determination of cause of mortality on the basis of International Classification of Diseases codes. The strengths of this study are use of a clinically relevant high–risk black population, use of physician–adjudicated CV outcomes, comparison with population internal references, uniform assessment of diabetes and kidney disease, and evaluation of associations on a clinically relevant additive scale.
In conclusion, this study highlights the significance of kidney disease as a risk factor for CVD and mortality in blacks, particularly in the presence of diabetes. Especially among blacks with diabetes, assessment of kidney function may be a helpful component of CV risk stratification for identification of a subpopulation for intensive risk modification.
Disclosures
None.
Supplementary Material
Acknowledgments
M.A. was supported by grants K23DK089017 and R01DK104706 from the National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) and a Norman S. Coplon Extramural Grant from Satellite Healthcare. R.K., I.H.d.B., and B.Y. were supported by National Institutes of Health NIDDK grant 1R01DK102134-01. I.H.d.B. was supported by grants R01DK087726 and R01DK088762 from the NIDDK. I.H.d.B. and B.Y. are also supported, in part, by funding from the Veterans Affairs Puget Sound Health Care System (Seattle, WA). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
M.A. is the guarantor of this study and takes responsibility for the findings and interpretations presented in this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13111215/-/DCSupplemental.
References
- 1.Ferdinand KC: Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol 97[2A]: 12A–19A, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Taylor HA, Hughes GD, Garrison RJ: Cardiovascular disease among women residing in rural America: Epidemiology, explanations, and challenges. Am J Public Health 92: 548–551, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor HA, Jr.: Establishing a foundation for cardiovascular disease research in an African-American community--the Jackson Heart Study. Ethn Dis 13: 411–413, 2003 [PubMed] [Google Scholar]
- 4.Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M: Eight Americas: Investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med 3: e260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, Rifkin DE, Sarnak MJ, Shlipak MG, Siscovick DS: Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 32: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J; ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C; FinnDiane Study Group : The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58: 1651–1658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orchard TJ, Secrest AM, Miller RG, Costacou T: In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: A report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53: 2312–2319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH: Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24: 302–308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention: National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, US Department of Health and Human Services, 2014
- 11.Centers for Disease Control and Prevention: National Diabetes Surveillance System, 2013. Atlanta, GA, US Department of Health and Human Services, 2014
- 12.Heron M: Deaths: Leading causes for 2013. National vital statistics reports, Vol 65, No 2. Hyattsville, MD, National Center for Health Statistics, 2016 [PubMed]
- 13.Taylor HA Jr.: The Jackson Heart Study: An overview. Ethn Dis 15[4 Suppl 6]: S6-1-3, 2005 [PubMed]
- 14.Taylor HA Jr.: The Jackson Heart Study of the future. Ethn Dis 12[3 Suppl 1]: S1-49-54, 2012 [PubMed]
- 15.International Expert Committee : International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association : Standards of medical care in diabetes--2010. Diabetes Care 33[Suppl 1]: S11–S61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D: Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 328: 131–144, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Rothman K: Modern Epidemiology, Boston, Little, Brown & Co, 1986
- 19.Szczech LA, Best PJ, Crowley E, Brooks MM, Berger PB, Bittner V, Gersh BJ, Jones R, Califf RM, Ting HH, Whitlow PJ, Detre KM, Holmes D; Bypass Angioplasty Revascularization Investigation (BARI) Investigators : Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation 105: 2253–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Astor BC, Hallan SI, Miller ER 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Bello AK, Hemmelgarn B, Lloyd A, James MT, Manns BJ, Klarenbach S, Tonelli M; Alberta Kidney Disease Network : Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol 6: 1418–1426, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M; Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Renal Data System : 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 25.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519–2527, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH: Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: A review. Diabetes Care 28: 2280–2288, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Dagogo-Jack S: Ethnic disparities in type 2 diabetes: Pathophysiology and implications for prevention and management. J Natl Med Assoc 95: 774–789, 2003 [PMC free article] [PubMed] [Google Scholar]
- 28.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB: State of disparities in cardiovascular health in the United States. Circulation 111: 1233–1241, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gentile NT, Seftchick MW: Poor outcomes in Hispanic and African American patients after acute ischemic stroke: Influence of diabetes and hyperglycemia. Ethn Dis 18: 330–335, 2008 [PubMed] [Google Scholar]
- 30.Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M: Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. Am J Epidemiol 141: 960–972, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Galobardes B, Smith GD, Lynch JW: Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol 16: 91–104, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ranjit N, Diez-Roux AV, Chambless L, Jacobs DR Jr., Nieto FJ, Szklo M: Socioeconomic differences in progression of carotid intima-media thickness in the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol 26: 411–416, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan GA, Keil JE: Socioeconomic factors and cardiovascular disease: A review of the literature. Circulation 88: 1973–1998, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J; CKD Prognosis Consortium : Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR; Alberta Kidney Disease Network : Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Gordon T, Kannel WB, Hjortland MC, McNamara PM: Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 89: 157–161, 1978 [DOI] [PubMed] [Google Scholar]
- 37.Lerner DJ, Kannel WB: Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J 111: 383–390, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Rich DQ, Gaziano JM, Kurth T: Geographic patterns in overall and specific cardiovascular disease incidence in apparently healthy men in the United States. Stroke 38: 2221–2227, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Glymour MM, Kosheleva A, Boden-Albala B: Birth and adult residence in the Stroke Belt independently predict stroke mortality. Neurology 73: 1858–1865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL: Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The Northern Manhattan Study. Circulation 111: 1327–1331, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.